Comparison of Minimally Invasive Surgery with Open Surgery for Type II Endometrial Cancer: An Analysis of the National Cancer Database
Abstract
:1. Introduction
2. Materials and Methods
3. Statistical Analysis
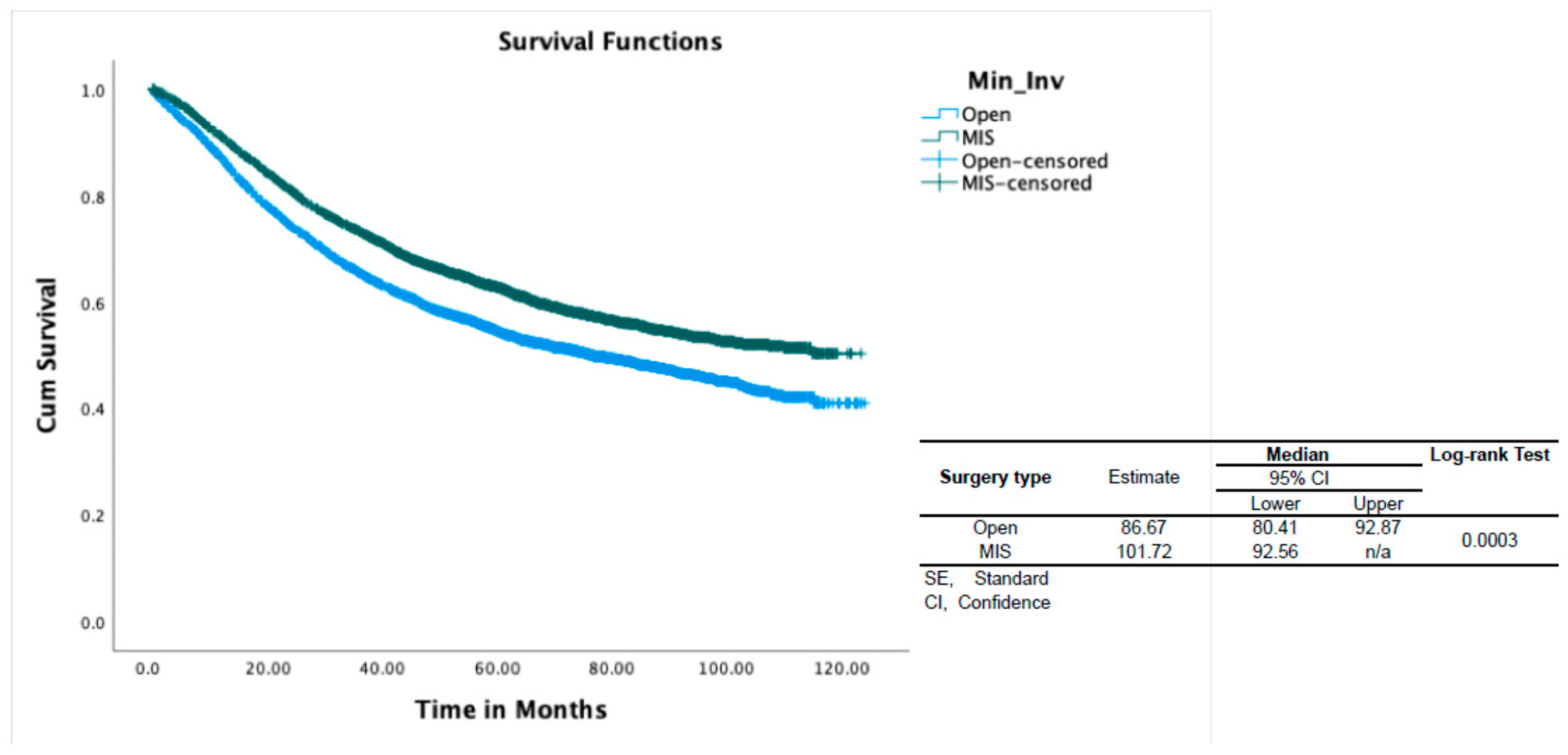
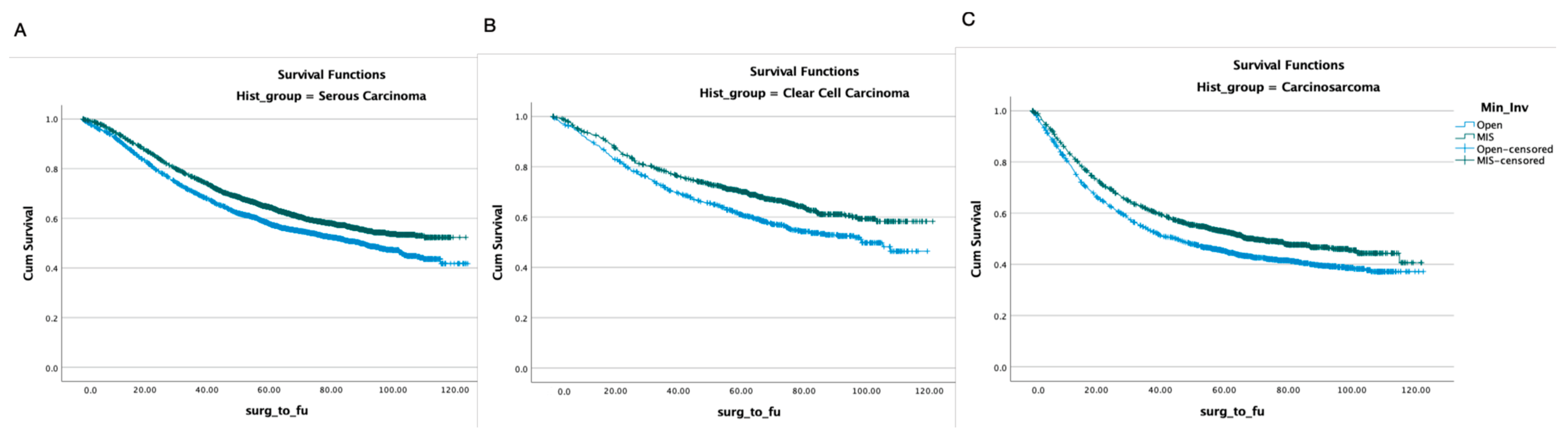
4. Results
4.1. Patient Characteristics
4.2. Survival Analysis
4.3. Perioperative Outcomes
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bockman, J.V. Two pathogenic types of endometrial carcinoma. Gynecol. Oncol. 1983, 15, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, C.A.; Kapp, D.S.; Chan, J.K. Clinical aspects of uterine papillary serous carcinoma. Curr. Opin. Obstet. Gynecol. 2008, 20, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.L.; Piedmonte, M.R.; Spirtos, N.M.; Eisenkop, S.M.; Schlaerth, J.B.; Mannel, R.S.; Spiegel, G.; Barakat, R.; Pearl, M.L.; Sharma, S.K. Laparoscopy compared with laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group Study LAP2. J. Clin. Oncol. 2009, 27, 5331–5336. [Google Scholar] [CrossRef] [PubMed]
- Janda, M.; Gebski, V.; Davies, L.C.; Forder, P.; Brand, A.; Hogg, R.; Jobling, T.W.; Land, R.; Manolitsas, T.; Nascimento, M.; et al. Effect of Total Laparoscopic Hysterectomy vs Total Abdominal Hysterectomy on Disease-Free Survival Among Women with Stage I Endometrial CancerA Randomized Clinical Trial. JAMA 2017, 317, 1224–1233. [Google Scholar] [CrossRef]
- Ramirez, P.T.; Frumovitz, M.; Pareja, R.; Lopez, A.; Vieira, M.; Ribeiro, R.; Buda, A.; Yan, X.; Shuzhong, Y.; Chetty, N.; et al. Minimally Invasive versus Abdominal Radical Hysterectomy for Cervical Cancer. N. Engl. J. Med. 2018, 379, 1895–1904. [Google Scholar] [CrossRef]
- Vergote, I.; Magrina, J.F.; Zanagnolo, V.; Magtibay, P.M.; Butler, K.; Gil-Moreno, A.; Feijoo, B.-D.; Kimmig, R.; Canis, M.; Bourdel, N.; et al. The LACC Trial and Minimally Invasive Surgery in Cervical Cancer. J. Minim. Invasive Gynecol. 2020, 27, 462–463. [Google Scholar] [CrossRef] [PubMed]
- Volz, J.; Köster, S.; Spacek, Z.; Paweletz, N. The influence of pneumoperitoneum used in laparoscopic surgery on an intraabdominal tumor growth. Cancer 1999, 86, 770–774. [Google Scholar] [CrossRef]
- Mo, L.; Lin, F.; Pan, L.; Li, L.; Li, D. Effects of a simulated CO2 pneumoperitoneum environment on the proliferation, apoptosis, and metastasis of cervical cancer cells in vitro. Med. Sci. Monit. 2014, 20, 2497–2503. [Google Scholar] [CrossRef]
- Song, J.; Le, T.; Hopkins, L.; Fung-Kee-Fung, M.; Lupe, K.; Gaudet, M.; E, C.; Samant, R. A comparison of disease recurrence between robotic versus laparotomy approach in patients with intermediate-risk endometrial cancer. Int. J. Gynecol. Cancer 2020, 30, 160–166. [Google Scholar] [CrossRef]
- Monterossi, G.; Ghezzi, F.; Vizza, E.; Zannoni, G.F.; Uccella, S.; Corrado, G.; Restaino, S.; Quagliozzi, L.; Casarin, J.; Dinoi, G.; et al. Minimally Invasive Approach in Type II Endometrial Cancer. JMIG 2016, 24, 438–445. [Google Scholar]
- Scaletta, G.; Dinoi, G.; Capozzi, V.; Cianci, S.; Pelligra, S.; Ergasti, R.; Fagotti, A.; Scambia, G.; Fanfani, F. Comparison of minimally invasive surgery with laparotomic approach in the treatment of high-risk endometrial cancer: A systematic review. Eur. J. Surg. Oncol. 2020, 46, 782–788. [Google Scholar] [CrossRef] [PubMed]
- Berek, J.S.; Matias-Guiu, X.; Creutzberg, C.; Fotopoulou, C.; Gaffney, D.; Kehoe, S.; Lindemann, K.; Mutch, D.; Concin, N. FIGO staging of endometrial cancer: 2023. Int. J. Gynecol. Obstet. 2023, 162, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Fader, A.N.; Java, J.; Tenney, M.; Ricci, S.; Gunderson, C.C.; Temkin, S.M.; Spirtos, N.; Kushnir, C.L.; Pearl, M.L.; Zivanovic, O.; et al. Impact of histology and surgical approach on survival among women with early-stage, high-grade uterine cancer: An NRG Oncology/Gynecologic Oncology Group ancillary analysis. Gynecol. Oncol. 2016, 143, 460–465. [Google Scholar] [CrossRef]
- Chiva, L.; Zanagnolo, V.; Querleu, D.; Martin-Calvo, N.; Arévalo-Serrano, J.; Căpîlna, M.E.; Fagotti, A.; Kucukmetin, A.; Mom, C.; Chakalova, G.; et al. SUCCOR study: An international European cohort observational study comparing minimally invasive surgery versus open abdominal radical hysterectomy in patients with stage IB1 cervical cancer. Int. J. Gynecol. Cancer 2020, 30, 1269–1277. [Google Scholar] [CrossRef]
- Melamed, A.; Margul, D.J.; Chen, L.; Keating, N.L.; Del Carmen, M.G.; Yang, J.; Seagle, B.L.; Alexander, A.; Barber, E.L.; Rice, L.W.; et al. Survival after Minimally Invasive Radical Hysterectomy for Early-Stage Cervical Cancer. N. Engl. J. Med. 2018, 379, 1905–1914. [Google Scholar] [CrossRef]
- Hoegl, J.; Viveros-Carreño, D.; Palacios, T.; Gallego-Ardila, A.; Rauh-Hain, J.A.; Estrada, E.E.; Noll, F.; Krause, K.; Baiocchi, G.; Minig, L.; et al. Peritoneal carcinomatosis after minimally invasive surgery versus open radical hysterectomy: Systematic review and meta-analysis. Int. J. Gynecol. Cancer 2022, 32, 1497–1504. [Google Scholar] [CrossRef] [PubMed]
- Tewari, K.S.; Java, J.J.; Eskander, R.N.; Monk, B.J.; Burger, R.A. Early initiation of chemotherapy following complete resection of advanced ovarian cancer associated with improved survival: NRG Oncology/Gynecologic Oncology Group study. Ann. Oncol. 2016, 27, 114–121. [Google Scholar] [CrossRef]
- Joseph, N.; Clark, R.M.; Dizon, D.S.; Lee, M.S.; Goodman, A.; Boruta, D.; Schorge, J.O.; del Carmen, M.G.; Growdon, W.B. Delay in chemotherapy administration impacts survival in elderly patients with epithelial ovarian cancer. Gynecol. Oncol. 2015, 137, 401–405. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, P.; Xu, Y.; Yan, J.; Liu, Z.; Lau, W.B.; Lau, B.; Li, Y.; Zhao, X.; Wei, Y.; et al. Surgical stress and cancer progression: The twisted tango. Mol. Cancer 2019, 18, 132. [Google Scholar] [CrossRef]
- Bakos, O.; Lawson, C.; Rouleau, S.; Tai, L.H. Combining surgery and immunotherapy: Turning an immunosuppressive effect into a therapeutic opportunity. J. Immunother. Cancer 2018, 6, 86. [Google Scholar] [CrossRef]
- Krall, J.A.; Reinhardt, F.; Mercury, O.A.; Pattabiraman, D.R.; Brooks, M.W.; Dougan, M.; Lambert, A.W.; Bierie, B.; Ploegh, H.L.; Dougan, S.K.; et al. The systemic response to surgery triggers the outgrowth of distant immune-controlled tumors in mouse models of dormancy. Sci. Transl. Med. 2018, 10, eaan3464. [Google Scholar] [CrossRef]
- Coffey, J.C.; Wang, J.H.; Smith, M.J.F.; Bouchier-Hayes, D.; Cotter, T.G.; Redmond, H.P. Excisional surgery for cancer cure: Therapy at a cost. Lancet Oncol. 2003, 4, 760–768. [Google Scholar] [CrossRef] [PubMed]
- Alexa, M.; Hasenburg, A.; Battista, M.J. The TCGA Molecular Classification of Endometrial Cancer and Its Possible Impact on Adjuvant Treatment Decisions. Cancers 2021, 13, 1478. [Google Scholar] [CrossRef] [PubMed]
- Gilks, C.B.; Oliva, E.; Soslow, R.A. Poor Interobserver Reproducibility in the Diagnosis of High-Grade Endometrial Carcinoma. Am. J. Surg. Pathol. 2013, 37, 874–881. [Google Scholar] [CrossRef]
- Han, G.; Sidhu, D.; Duggan, M.A.; Arseneau, J.; Cesari, M.; Clement, P.B.; Ewanowich, C.A.; Kalloger, S.E.; Köbel, M. Reproducibility of Histological Cell Type in High-Grade Endometrial Carcinoma. Mod. Pathol. 2013, 26, 1594–1604. [Google Scholar] [CrossRef]
- Douglas, A. Levine & The Cancer Genome Atlas Research Network. Integrated genomic characterization of endometrial carcinoma. Nature 2013, 497, 67–73. [Google Scholar]
- Whetstone, S.; Burke, W.; Sangini, S. Health disparities in endometrial cancer. Obstet. Gynecol. 2022, 139, 645–659. [Google Scholar] [CrossRef]
- Sherman, M.E.; Devesa, S.S. Analysis of racial differences in incidence, survival, and mortality for malignant tumors of the uterine corpus. Cancer 2003, 98, 176–186. [Google Scholar] [CrossRef]
- Doll, K.M.; Winn, A.N. Assessing endometrial cancer risk among US women: Long-term trends using hysterectomy-adjusted analysis. Am. J. Obstet. Gynecol. 2019, 221, 318.e1–318.e9. [Google Scholar] [CrossRef]
- Bach, P.B.; Schrag, D.; Brawley, O.W.; Galaznik, A.; Yakren, S.; Begg, C.B. Survival of Blacks and Whites after a cancer diagnosis. JAMA 2002, 287, 2106–2113. [Google Scholar] [CrossRef]
- Cote, M.L.; Ruterbusch, J.J.; Olson, S.H.; Lu, K.; Ali-Fehmi, R. The growing burden of endometrial cancer: A major racial disparity affecting Black women. Cancer Epidemiol. Biomark. Prev. 2015, 24, 1407–1415. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.D.; Fiorelli, J.; Schiff, P.B.; Burke, W.M.; Kansler, A.L.; Cohen, C.J.; Herzog, T.J. Racial disparities for uterine corpus tumors: Changes in clinical characteristics and treatment over time. Cancer 2009, 115, 1276–1285. [Google Scholar] [CrossRef]
- Fader, A.N.; Habermann, E.B.; Hanson, K.T.; Lin, J.F.; Grendys, E.C.; Dowdy, S.C. Disparities in treatment and survival for women with endometrial cancer: A contemporary National Cancer Database registry analysis. Gynecol. Oncol. 2016, 143, 98–104. [Google Scholar] [CrossRef]
- Madison, T.; Schottenfeld, D.; James, S.A.; Schwartz, A.G.; Gruber, S.B. Endometrial cancer: Socioeconomic status and racial/ethnic differences in stage at diagnosis, treatment, and survival. Am. J. Public Health 2004, 94, 2104–2111. [Google Scholar] [CrossRef] [PubMed]
- Rojas, C.; Tian, C.; Powell, M.A.; Chan, J.K.; Bateman, N.W.; Conrads, T.P.; Rocconi, R.P.; Jones, N.L.; Shriver, C.D.; Hamilton, C.A.; et al. Racial disparities in uterine and ovarian carcinosarcoma: A population-based analysis of treatment and survival. Gynecol. Oncol. 2020, 157, 67–77. [Google Scholar] [CrossRef] [PubMed]
| Unweighted | After IPTW | |||||
|---|---|---|---|---|---|---|
| Variables | Surgical Type | Surgical Type | ||||
| Open (n = 5782) | MIS (n = 7123) | p-value | Open (n = 5578) | MIS (n = 6867) | p-value | |
| Follow-up in months Median(IQR) | 55.9 (23–78.9) | 57.2 (26–74.6) | ||||
| Age | 67 (61–74) | 68 (62–74) | <0.001 | 68 (62–74) | 67 (62–74) | 0.965 |
| Race, n (%) | ||||||
| White | 3967 (68.1) | 5450 (75.7) | 4037 (72.4) | 4970 (72.4) | 0.985 | |
| Black | 1559 (26.8) | 1378 (19.1) | 1257 (22.5) | 1546 (22.5) | ||
| Asian/Pacific Islander | 178 (3.1) | 218 (3) | <0.001 | 168 (3) | 207 (3) | |
| Other | 63 (1.1) | 78 (1.1) | 61 (1.1) | 74 (1.1) | ||
| Unknown | 60 (1) | 73 (1) | 56 (1) | 69 (1) | ||
| Ethnicity, n (%) | ||||||
| Non-Hispanic | 5277 (90.6) | 6574 (91.3) | 5056 (90.6) | 6276 (91.4) | 0.13 | |
| Hispanic | 367 (6.3) | 444 (6.2) | 0.072 | 358 (6.4) | 417 (6.1) | |
| Unknown | 183 (3.1) | 179 (2.5) | 164 (2.9) | 174 (2.5) | ||
| Insurance, n (%) | ||||||
| No | 219 (3.8) | 141 (2) | <0.001 | 194 (3.5) | 147 (2.1) | 0.001 |
| Private | 1968 (33.8) | 2541 (35.3) | 1836 (32.9) | 2478 (36.1) | ||
| Medicaid/Medicare/Other Public | 3541 (60.8) | 4456 (61.9) | 3463 (62.1) | 4187 (61) | ||
| Unknown | 99 (1.7) | 59 (0.8) | 85 (1.5) | 55 (0.8) | ||
| Median Income Quartiles, n (%) | ||||||
| <USD 30,000 | 885 (17) | 840 (13.1) | <0.001 | 794 (16) | 860 (14.1) | <0.001 |
| USD 30,000–USD 34,999 | 934 (17.9) | 1007 (15.7) | 860 (17.3) | 967 (15.8) | ||
| USD 35,000–USD 45,999 | 1381 (26.5) | 1721 (26.9) | 1330 (26.7) | 1642 (26.9) | ||
| USD 46,000 | 2018 (38.7) | 2826 (44.2) | 1992 (40) | 2647 (43.3) | ||
| Charlson Comorbidity Index, n (%) | ||||||
| 0 | 4117 (70.7) | 5200 (72.3%) | 0.164 | 3949 (70.8) | 4935 (71.9) | 0.162 |
| 1 | 1336 (22.9) | 1584 (22%) | 1269 (22.7) | 1522 (22.2) | ||
| 2 | 293 (5) | 317 (4.4%) | 281 (5) | 317 (4.6) | ||
| 3 | 81 (1.4) | 96 (1.3%) | 79 (1.4) | 93 (1.4) | ||
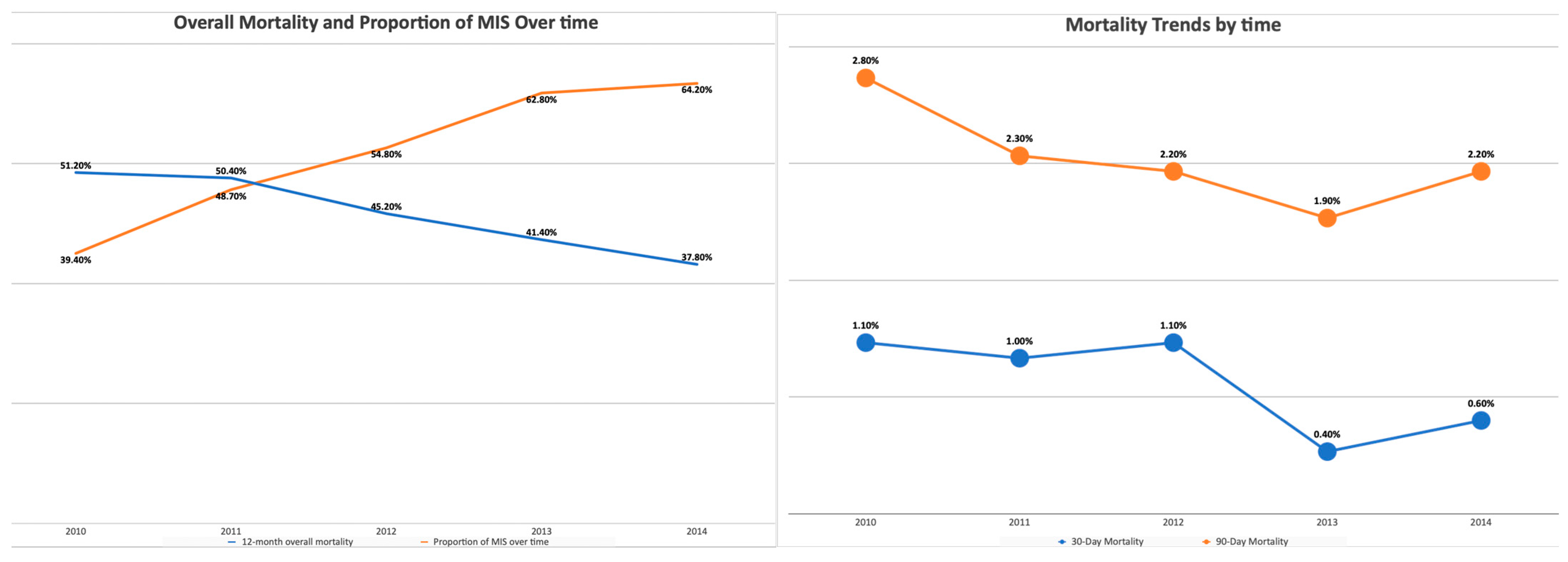
| Year of diagnosis, n (%) | ||||||
| 2010 | 1267 (21.7) | 823 (11.4%) | <0.001 | 1217 (21.8) | 790 (11.5) | <0.001 |
| 2011 | 1228 (21.1) | 1163 (16.2%) | 1179 (21.1) | 1119 (16.3) | ||
| 2012 | 1140 (19.6) | 1383 (19.2%) | 1088 (19.5) | 1317 (19.2) | ||
| 2013 | 1064 (18.3) | 1811 (25.2%) | 1023 (18.3) | 1724 (25.1) | ||
| 2014 | 1128 (19.4) | 2017 (28%) | 1070 (19.2) | 1917 (27.9) | ||
| Histology, n (%) | ||||||
| Serous Carcinoma | 3288 (52.9) | 4539 (63.7%) | <0.001 | 3395 (60.9) | 4182 (60.9) | 0.958 |
| Clear-Cell Carcinoma | 708 (12.2) | 901 (12.6%) | 694 (12.4) | 855 (12.5) | ||
| Carcinosarcoma | 1786 (30.9) | 1683 (23.6%) | 1489 (26.7) | 1830 (26.6) | ||
| Lymph Node Dissection | 4937 (85.4%) | 6380 (89.6%) | <0.001 | 4767 (85.5) | 6139 (89.4) | <0.001 |
| Tumor Size (mm) | 59 (32–120) | 45 (27–92) | <0.001 | 55 (30–115) | 48 (29–92) | 0.99 |
| FIGO stage, n (%) | ||||||
| IC | 2988 (51.7%) | 4646 (65.2%) | <0.001 | 3318 (59.5) | 4085 (59.5) | 0.991 |
| IIC | 571 (9.9%) | 525 (7.4%) | 473 (8.5) | 581 (8.5) | ||
| III | 2223 (38.4%) | 1952 (27.4%) | 1786 (32) | 2201 (32) | ||
| Chemotherapy, n (%) | 2156 (37.6%) | 2407 (33.9%) | <0.001 | 2004 (35.9) | 2461 (35.8) | 0.912 |
| Days from surgery to chemotherapy | 41 (30–56) | 38 (29–51) | <0.001 | 41 (30–56) | 38 (29–51) | <0.001 |
| Radiation therapy, n (%) | 2101 (37%) | 2813 (40.1%) | <0.001 | 2191 (39.3) | 2697 (39.3) | 0.992 |
| Length of Hospital Stay | 4 (3–5) | 1 (1–2) | <0.001 | 4 (3–5) | 1 (1–2) | <0.001 |
| Months from Surgery to Last visit | 53.55 (21.4–77.48) | 58.63 (29.21–75.55) | <0.001 | 55.94 (23.57–78.94) | 57.22 (26.07–74.63) | 0.805 |
| Death, n (%) | 2839 (48.7%) | 2902 (40.3%) | <0.001 | 2607 (46.7) | 2932 (42.7) | <0.001 |
| 30 Day Readmission, n (%) | 289 (5.0%) | 177 (2.5%) | 266 (4.8) | 175 (2.6) | <0.001 | |
| 30 Day Mortality, n (%) | 76 (1.3%) | 37 (0.5%) | <0.001 | 64 (1.2) | 37 (0.5) | <0.001 |
| 90 Day Mortality, n (%) | 207 (3.6%) | 109 (1.5%) | <0.001 | 165 (3) | 112 (1.6) | <0.001 |
| HR | 95% CI | Sig. | ||
|---|---|---|---|---|
| Variables | Lower | Upper | ||
| Age | 1.032 | 1.029 | 1.035 | <0.001 |
| Race | ||||
| White | ref. | ref. | ref. | ref. |
| Black | 1.261 | 1.184 | 1.343 | <0.001 |
| Asian/Pacific Islander | 0.871 | 0.73 | 1.038 | 0.123 |
| Other | 1.067 | 0.819 | 1.39 | 0.63 |
| Unknown | 1.126 | 0.861 | 1.474 | 0.386 |
| Histology | ||||
| Serous carcinoma | ref. | ref. | ref. | ref. |
| Clear-cell carcinoma | 0.886 | 0.81 | 0.969 | 0.008 |
| Carcinosarcoma | 1.53 | 1.442 | 1.624 | <0.001 |
| FIGO stage | ||||
| IC | ref. | ref. | ref. | ref. |
| IIC | 1.775 | 1.609 | 1.957 | <0.001 |
| III | 3.107 | 2.933 | 3.291 | <0.001 |
| MIS | 0.905 | 0.857 | 0.956 | <0.001 |
| Chemotherapy | 0.77 | 0.725 | 0.817 | <0.001 |
| Radiation therapy | 0.801 | 0.756 | 0.848 | <0.001 |
| Lymph node dissection | 0.586 | 0.545 | 0.631 | <0.001 |
| Tumor Size (10 mm increment) | 0.998 | 0.998 | 0.999 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Silver, M.; Chen, Y.-J.; Wolf, J.; Hayek, J.; Alagkiozidis, I. Comparison of Minimally Invasive Surgery with Open Surgery for Type II Endometrial Cancer: An Analysis of the National Cancer Database. Healthcare 2023, 11, 3122. https://doi.org/10.3390/healthcare11243122
Zhang Q, Silver M, Chen Y-J, Wolf J, Hayek J, Alagkiozidis I. Comparison of Minimally Invasive Surgery with Open Surgery for Type II Endometrial Cancer: An Analysis of the National Cancer Database. Healthcare. 2023; 11(24):3122. https://doi.org/10.3390/healthcare11243122
Chicago/Turabian StyleZhang, Qi, Michael Silver, Yi-Ju Chen, Jennifer Wolf, Judy Hayek, and Ioannis Alagkiozidis. 2023. "Comparison of Minimally Invasive Surgery with Open Surgery for Type II Endometrial Cancer: An Analysis of the National Cancer Database" Healthcare 11, no. 24: 3122. https://doi.org/10.3390/healthcare11243122
APA StyleZhang, Q., Silver, M., Chen, Y.-J., Wolf, J., Hayek, J., & Alagkiozidis, I. (2023). Comparison of Minimally Invasive Surgery with Open Surgery for Type II Endometrial Cancer: An Analysis of the National Cancer Database. Healthcare, 11(24), 3122. https://doi.org/10.3390/healthcare11243122






