The Effect of Exercise on Cardiovascular Autonomic Nervous Function in Patients with Diabetes: A Systematic Review
Abstract
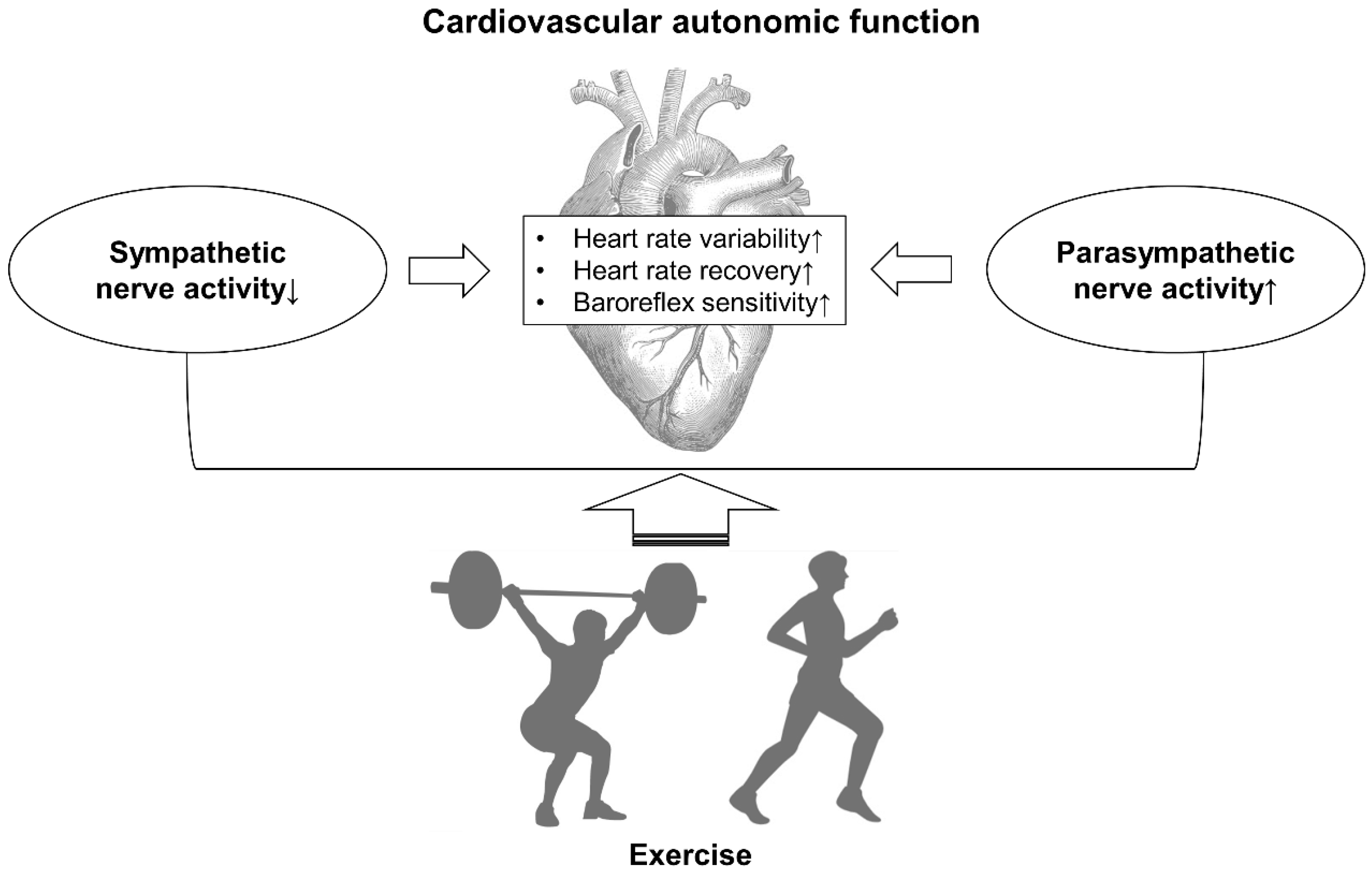
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Study Quality Assessment
3. Results
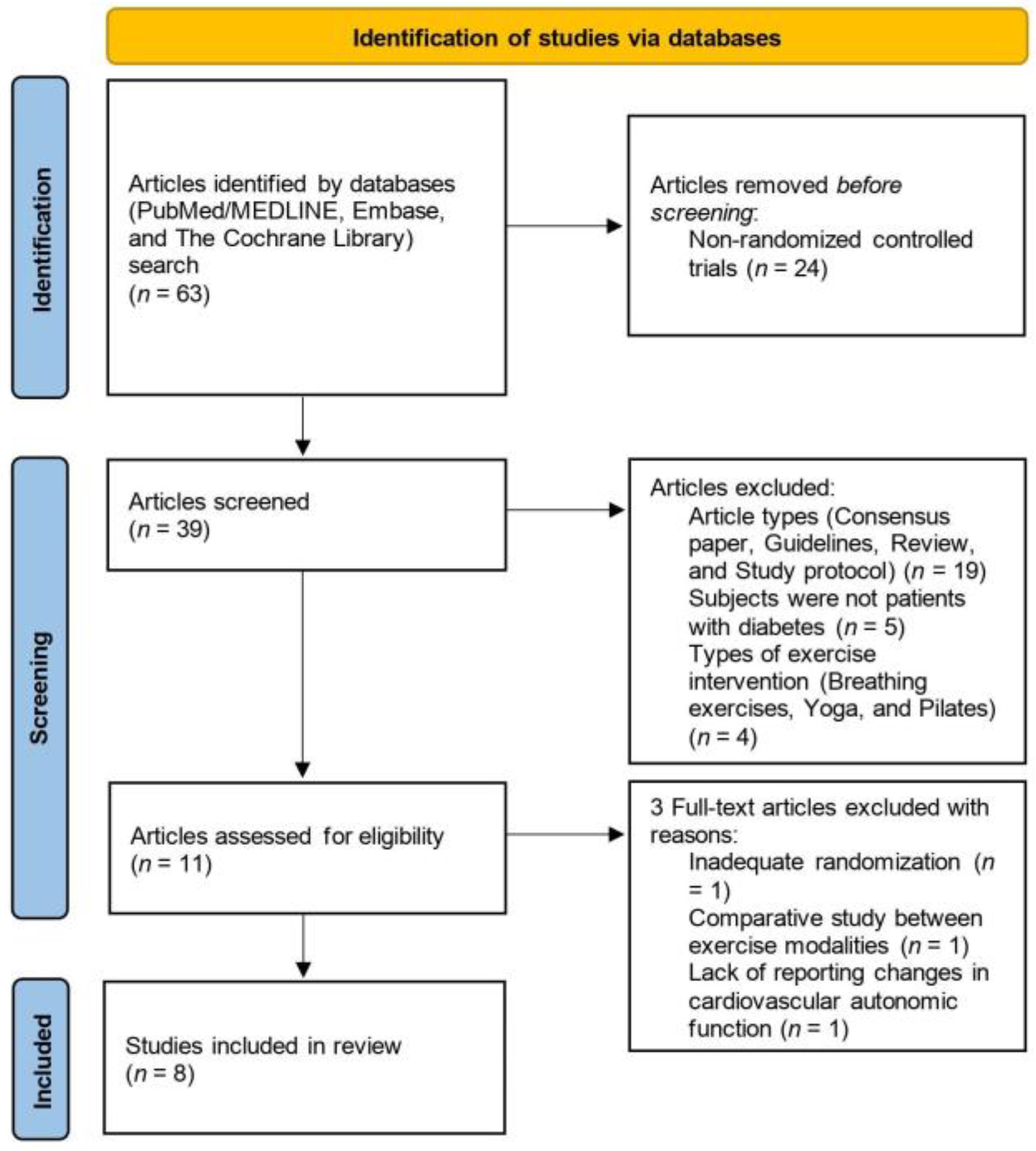
3.1. Study Selection
3.2. Study Characteristics
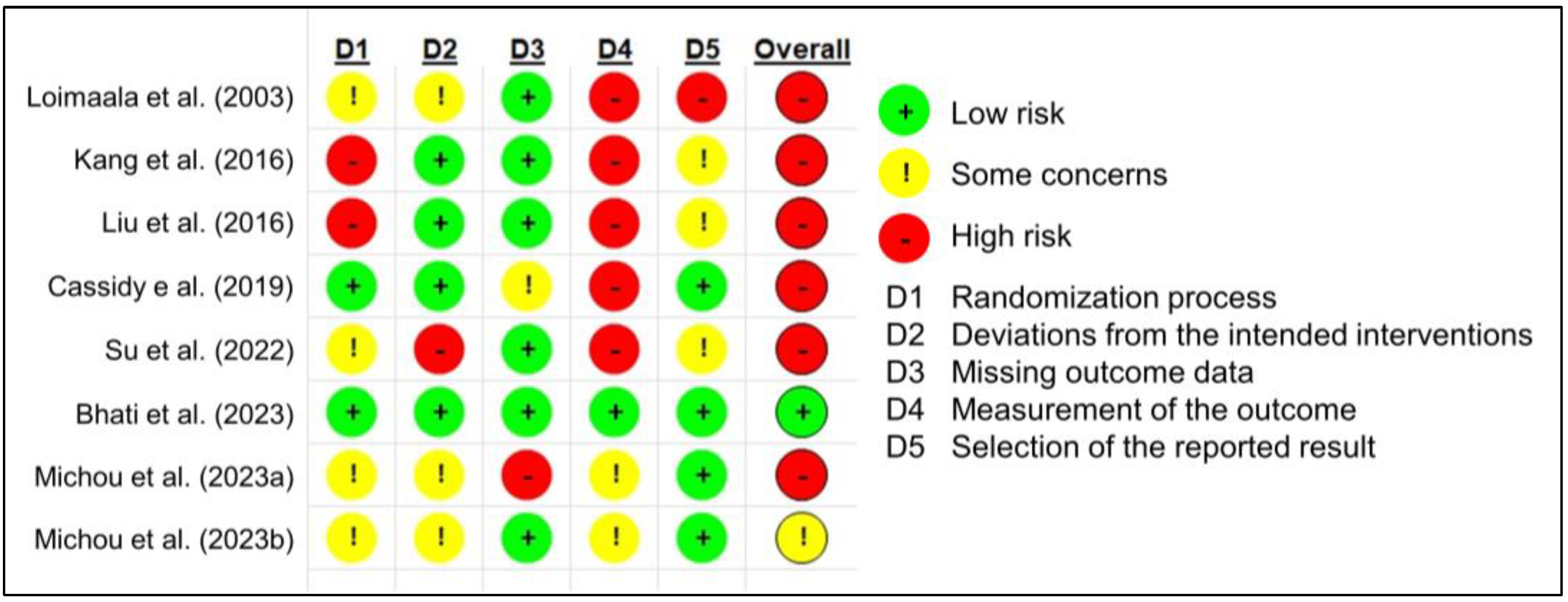
3.3. Study Quality
4. Discussion
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gibbons, C.H. Basics of autonomic nervous system function. Handb. Clin. Neurol. 2019, 160, 407–418. [Google Scholar] [PubMed]
- Aso, Y. Updates in diabetic neuropathy: A call for new diagnostic and treatment approaches. J. Diabetes Investig. 2022, 13, 432–434. [Google Scholar] [CrossRef] [PubMed]
- Spallone, V. Update on the Impact, Diagnosis and Management of Cardiovascular Autonomic Neuropathy in Diabetes: What Is Defined, What Is New, and What Is Unmet. Diabetes Metab. J. 2019, 43, 3–30. [Google Scholar] [CrossRef] [PubMed]
- Tesfaye, S.; Boulton, A.J.; Dyck, P.J.; Freeman, R.; Horowitz, M.; Kempler, P.; Lauria, G.; Malik, R.A.; Spallone, V.; Vinik, A.; et al. Diabetic neuropathies: Update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care 2010, 33, 2285–2293. [Google Scholar] [CrossRef] [PubMed]
- Spallone, V.; Ziegler, D.; Freeman, R.; Bernardi, L.; Frontoni, S.; Pop-Busui, R.; Stevens, M.; Kempler, P.; Hilsted, J.; Tesfaye, S.; et al. Cardiovascular autonomic neuropathy in diabetes: Clinical impact, assessment, diagnosis, and management. Diabetes Metab. Res. Rev. 2011, 27, 639–653. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, L.; Spallone, V.; Stevens, M.; Hilsted, J.; Frontoni, S.; Pop-Busui, R.; Ziegler, D.; Kempler, P.; Freeman, R.; Low, P.; et al. Methods of investigation for cardiac autonomic dysfunction in human research studies. Diabetes Metab. Res. Rev. 2011, 27, 654–664. [Google Scholar] [CrossRef] [PubMed]
- Zilliox, L.A.; Russell, J.W. Is there cardiac autonomic neuropathy in prediabetes? Auton. Neurosci. 2020, 229, 102722. [Google Scholar] [CrossRef]
- Chowdhury, M.; Nevitt, S.; Eleftheriadou, A.; Kanagala, P.; Esa, H.; Cuthbertson, D.J.; Tahrani, A.; Alam, U. Cardiac autonomic neuropathy and risk of cardiovascular disease and mortality in type 1 and type 2 diabetes: A meta-analysis. BMJ Open Diabetes Res. Care 2021, 9, e002480. [Google Scholar] [CrossRef]
- Kaze, A.D.; Yuyun, M.F.; Erqou, S.; Fonarow, G.C.; Echouffo-Tcheugui, J.B. Cardiac autonomic neuropathy and risk of incident heart failure among adults with type 2 diabetes. Eur. J. Heart Fail. 2022, 24, 634–641. [Google Scholar] [CrossRef]
- Lin, Y.K.; Fisher, S.J.; Pop-Busui, R. Hypoglycemia unawareness and autonomic dysfunction in diabetes: Lessons learned and roles of diabetes technologies. J. Diabetes Investig. 2020, 11, 1388–1402. [Google Scholar] [CrossRef]
- Jaiswal, M.; McKeon, K.; Comment, N.; Henderson, J.; Swanson, S.; Plunkett, C.; Nelson, P.; Pop-Busui, R. Association between impaired cardiovascular autonomic function and hypoglycemia in patients with type 1 diabetes. Diabetes Care 2014, 37, 2616–2621. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.N.; Duckworth, W.; Emanuele, N.; Hayward, R.A.; Wiitala, W.L.; Thottapurathu, L.; Reda, D.J.; Reaven, P.D.; Investigators of the Veterans Affairs Diabetes Trial. Effects of Severe Hypoglycemia on Cardiovascular Outcomes and Death in the Veterans Affairs Diabetes Trial. Diabetes Care 2019, 42, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Koivikko, M.L.; Salmela, P.I.; Airaksinen, K.E.; Tapanainen, J.S.; Ruokonen, A.; Mäkikallio, T.H.; Huikuri, H.V. Effects of sustained insulin-induced hypoglycemia on cardiovascular autonomic regulation in type 1 diabetes. Diabetes 2005, 54, 744–750. [Google Scholar] [CrossRef]
- Rao, A.D.; Bonyhay, I.; Dankwa, J.; Baimas-George, M.; Kneen, L.; Ballatori, S.; Freeman, R.; Adler, G.K. Baroreflex Sensitivity Impairment During Hypoglycemia: Implications for Cardiovascular Control. Diabetes 2016, 65, 209–215. [Google Scholar] [CrossRef]
- Picard, M.; Tauveron, I.; Magdasy, S.; Benichou, T.; Bagheri, R.; Ugbolue, U.C.; Navel, V.; Dutheil, F. Effect of exercise training on heart rate variability in type 2 diabetes mellitus patients: A systematic review and meta-analysis. PLoS ONE 2021, 16, e0251863. [Google Scholar] [CrossRef]
- Hamasaki, H. Effects of Resistance Training on Autonomic Nervous Function in Older Individuals. In Fitness Medicine; Sözen, H., Ed.; InTech: London, UK, 2016; pp. 111–127. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Riskofbias.Info. 2023. Available online: https://www.riskofbias.info/ (accessed on 30 June 2023).
- Sacre, J.W.; Jellis, C.L.; Jenkins, C.; Haluska, B.A.; Baumert, M.; Coombes, J.S.; Marwick, T.H. A six-month exercise intervention in subclinical diabetic heart disease: Effects on exercise capacity, autonomic and myocardial function. Metabolism 2014, 63, 1104–1114. [Google Scholar] [CrossRef]
- Bellavere, F.; Cacciatori, V.; Bacchi, E.; Gemma, M.L.; Raimondo, D.; Negri, C.; Thomaseth, K.; Muggeo, M.; Bonora, E.; Moghetti, P. Effects of aerobic or resistance exercise training on cardiovascular autonomic function of subjects with type 2 diabetes: A pilot study. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 226–233. [Google Scholar] [CrossRef]
- Cruz, L.C.; Teixeira-Araujo, A.A.; Andrade, K.T.P.; Rocha, T.C.O.G.; Moreira, S.R. Low intensity resistance exercise attenuates the relationship between glucose and autonomic nervous system indicators during 24 h in women with type 2 diabetes. Sci. Sports 2018, 33, e75–e83. [Google Scholar] [CrossRef]
- Loimaala, A.; Huikuri, H.V.; Kööbi, T.; Rinne, M.; Nenonen, A.; Vuor, I. Exercise training improves baroreflex sensitivity in type 2 diabetes. Diabetes 2003, 52, 1837–1842. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.J.; Ko, K.J.; Baek, U.H. Effects of 12 weeks combined aerobic and resistance exercise on heart rate variability in type 2 diabetes mellitus patients. J. Phys. Ther. Sci. 2016, 28, 2088–2093. [Google Scholar] [CrossRef] [PubMed]
- Colberg, S.R.; Sigal, R.J.; Fernhall, B.; Regensteiner, J.G.; Blissmer, B.J.; Rubin, R.R.; Chasan-Taber, L.; Albright, A.L.; Braun, B.; American College of Sports Medicine; et al. Exercise and type 2 diabetes: The American College of Sports Medicine and the American Diabetes Association: Joint position statement. Diabetes Care 2010, 33, e147–e167. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, S.X.; Zheng, F.; Cai, Y.; Xie, K.L.; Zhang, W.L. Cardiovascular autonomic neuropathy in patients with type 2 diabetes. J. Diabetes Investig. 2016, 7, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Peçanha, T.; Silva-Júnior, N.D.; Forjaz, C.L. Heart rate recovery: Autonomic determinants, methods of assessment and association with mortality and cardiovascular diseases. Clin. Physiol. Funct. Imaging 2014, 34, 327–339. [Google Scholar] [CrossRef]
- Cassidy, S.; Vaidya, V.; Houghton, D.; Zalewski, P.; Seferovic, J.P.; Hallsworth, K.; MacGowan, G.A.; Trenell, M.I.; Jakovljevic, D.G. Unsupervised high-intensity interval training improves glycaemic control but not cardiovascular autonomic function in type 2 diabetes patients: A randomised controlled trial. Diab. Vasc. Dis. Res. 2019, 16, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; He, J.; Cui, J.; Li, H.; Men, J. The effects of aerobic exercise combined with resistance training on inflammatory factors and heart rate variability in middle-aged and elderly women with type 2 diabetes mellitus. Ann. Noninvasive Electrocardiol. 2022, 27, e12996. [Google Scholar] [CrossRef] [PubMed]
- Bhati, P.; Hussain, M.E.; Deepak, K.K.; Masood, S.; Anand, P. Progressive resistance training ameliorates deteriorating cardiac autonomic dysfunction, subclinical inflammation and endothelial dysfunction in type 2 diabetes mellitus: A randomized control trial. Diabetes Metab. Syndr. 2023, 17, 102778. [Google Scholar] [CrossRef]
- Ewing, D.J.; Martyn, C.N.; Young, R.J.; Clarke, B.F. The value of cardiovascular autonomic function tests: 10 years experience in diabetes. Diabetes Care 1985, 8, 491–498. [Google Scholar] [CrossRef]
- Araujo, C.G.; Laukkanen, J.A. Heart and Skeletal Muscles: Linked by Autonomic Nervous System. Arq. Bras. Cardiol. 2019, 112, 747–748. [Google Scholar] [CrossRef]
- Michou, V.; Liakopoulos, V.; Roumeliotis, S.; Roumeliotis, A.; Anifanti, M.; Tsamos, G.; Papagianni, A.; Zempekakis, P.; Deligiannis, A.; Kouidi, E. Effects of Home-Based Exercise Training on Cardiac Autonomic Neuropathy and Metabolic Profile in Diabetic Hemodialysis Patients. Life 2023, 13, 232. [Google Scholar] [CrossRef] [PubMed]
- Michou, V.; Nikodimopoulou, M.; Liakopoulos, V.; Anifanti, M.; Papagianni, A.; Zembekakis, P.; Deligiannis, A.; Kouidi, E. Home-Based Exercise Training and Cardiac Autonomic Neuropathy in Kidney Transplant Recipients with Type-II Diabetes Mellitus. Life 2023, 13, 1394. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov. Available online: https://classic.clinicaltrials.gov/ct2/home (accessed on 30 June 2023).
- Wang, X.; Ji, X. Sample Size Estimation in Clinical Research: From Randomized Controlled Trials to Observational Studies. Chest 2020, 158, S12–S20. [Google Scholar] [CrossRef] [PubMed]
- Zoppini, G.; Cacciatori, V.; Gemma, M.L.; Moghetti, P.; Targher, G.; Zamboni, C.; Thomaseth, K.; Bellavere, F.; Muggeo, M. Effect of moderate aerobic exercise on sympatho-vagal balance in Type 2 diabetic patients. Diabet. Med. 2007, 24, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Voulgari, C.; Pagoni, S.; Vinik, A.; Poirier, P. Exercise improves cardiac autonomic function in obesity and diabetes. Metabolism 2013, 62, 609–621. [Google Scholar] [CrossRef] [PubMed]
- Colberg, S.R.; Grieco, C.R.; Somma, C.T. Exercise effects on postprandial glycemia, mood, and sympathovagal balance in type 2 diabetes. J. Am. Med. Dir. Assoc. 2014, 15, 261–266. [Google Scholar] [CrossRef]
- Besnier, F.; Labrunée, M.; Pathak, A.; Pavy-Le Traon, A.; Galès, C.; Sénard, J.M.; Guiraud, T. Exercise training-induced modification in autonomic nervous system: An update for cardiac patients. Ann. Phys. Rehabil. Med. 2017, 60, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.P.; Zheng, H. Central neural control of sympathetic nerve activity in heart failure following exercise training. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H527–H537. [Google Scholar] [CrossRef]
- Assmann, T.S.; Brondani, L.A.; Bouças, A.P.; Rheinheimer, J.; de Souza, B.M.; Canani, L.H.; Bauer, A.C.; Crispim, D. Nitric oxide levels in patients with diabetes mellitus: A systematic review and meta-analysis. Nitric Oxide 2016, 61, 1–9. [Google Scholar] [CrossRef]
- Shi, Y.; Vanhoutte, P.M. Macro- and microvascular endothelial dysfunction in diabetes. J. Diabetes 2017, 9, 434–449. [Google Scholar] [CrossRef]
- Qiu, S.; Cai, X.; Yin, H.; Sun, Z.; Zügel, M.; Steinacker, J.M.; Schumann, U. Exercise training and endothelial function in patients with type 2 diabetes: A meta-analysis. Cardiovasc. Diabetol. 2018, 17, 64. [Google Scholar] [CrossRef] [PubMed]
- Kiviniemi, A.M.; Tulppo, M.P.; Eskelinen, J.J.; Savolainen, A.M.; Kapanen, J.; Heinonen, I.H.; Huikuri, H.V.; Hannukainen, J.C.; Kalliokoski, K.K. Cardiac autonomic function and high-intensity interval training in middle-age men. Med. Sci. Sports Exerc. 2014, 46, 1960–1967. [Google Scholar] [CrossRef] [PubMed]
- Shimojo, G.; Joseph, B.; Shah, R.; Consolim-Colombo, F.M.; De Angelis, K.; Ulloa, L. Exercise activates vagal induction of dopamine and attenuates systemic inflammation. Brain Behav. Immun. 2019, 75, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Sparks, J.R.; Kishman, E.E.; Sarzynski, M.A.; Davis, J.M.; Grandjean, P.W.; Durstine, J.L.; Wang, X. Glycemic variability: Importance, relationship with physical activity, and the influence of exercise. Sports Med. Health Sci. 2021, 3, 183–193. [Google Scholar] [CrossRef]
- Cockcroft, E.J.; Narendran, P.; Andrews, R.C. Exercise-induced hypoglycaemia in type 1 diabetes. Exp. Physiol. 2020, 105, 590–599. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; Wiley: Chichester, UK, 2019. [Google Scholar]
- Eizirik, D.L.; Pasquali, L.; Cnop, M. Pancreatic β-cells in type 1 and type 2 diabetes mellitus: Different pathways to failure. Nat. Rev. Endocrinol. 2020, 16, 349–362. [Google Scholar] [CrossRef] [PubMed]
- Genua, I.; Franch-Nadal, J.; Navas, E.; Mata-Cases, M.; Giménez-Pérez, G.; Vlacho, B.; Mauricio, D.; Goday, A. Obesity and related comorbidities in a large population-based cohort of subjects with type 1 diabetes in Catalonia. Front. Endocrinol. 2022, 13, 1015614. [Google Scholar] [CrossRef] [PubMed]
- Nianogo, R.A.; Arah, O.A. Forecasting Obesity and Type 2 Diabetes Incidence and Burden: The ViLA-Obesity Simulation Model. Front. Public Health 2022, 10, 818816. [Google Scholar] [CrossRef]
- Fang, P.; Dong, J.; Zeng, F.; Tang, Z. Analysis of the association between glucose profiles and β-cell function for diabetic cardiovascular autonomic neuropathy in China. J. Diabetes Investig. 2017, 8, 354–362. [Google Scholar] [CrossRef]
- World Health Organization. Ageing Data. Available online: https://platform.who.int/data/maternal-newborn-child-adolescent-ageing/ageing-data (accessed on 4 July 2023).
- Serhiyenko, V.A.; Serhiyenko, A.A. Cardiac autonomic neuropathy: Risk factors, diagnosis and treatment. World J. Diabetes 2018, 9, 1–24. [Google Scholar] [CrossRef]
- Qureshi, M.S.; Iqbal, M.; Zahoor, S.; Ali, J.; Javed, M.U. Ambulatory screening of diabetic neuropathy and predictors of its severity in outpatient settings. J. Endocrinol. Investig. 2017, 40, 425–430. [Google Scholar] [CrossRef]
- Vágvölgyi, A.; Ábrahám, J.E.; Máthéné Köteles, É.; Korom, A.; Barnai, M.; Szűcs, M.; Orosz, A.; Kempler, P.; Menyhárt, A.; Nemes, A.; et al. A three-month physical training program improves cardiovascular autonomic function in patients with metabolic syndrome with and without diabetes—A pilot study. Front. Endocrinol. 2023, 14, 1224353. [Google Scholar] [CrossRef]
- Tang, Y.; Ang, L.; Jaiswal, M.; Dillon, B.R.; Esfandiari, N.H.; Shah, H.S.; Spino, C.; Plunkett, C.; Perkins, B.A.; Busui, R.P.; et al. Cardiovascular autonomic neuropathy and risk of kidney function decline in type 1 and type 2 diabetes: Findings from the PERL and ACCORD cohorts. Diabetes 2023, db230247. [Google Scholar] [CrossRef]
- Gad, H.; Elgassim, E.; Mohammed, I.; Alhaddad, A.Y.; Aly, H.A.H.Z.; Cabibihan, J.J.; Al-Ali, A.; Sadasivuni, K.K.; Petropoulos, I.N.; Ponirakis, G.; et al. Cardiovascular autonomic neuropathy is associated with increased glycemic variability driven by hyperglycemia rather than hypoglycemia in patients with diabetes. Diabetes Res. Clin. Pract. 2023, 200, 110670. [Google Scholar] [CrossRef]
- Huang, Y.; Xie, P.; Zhang, S.; Liu, M.; Huang, R.; Xiong, Z.; Zhong, X.; Lin, Y.; Zhou, Z.; Zhang, W.; et al. Intensive Glycemic Therapy in Type 2 Diabetes Patients with Cardiac Autonomic Dysfunction: The ACCORD Trial. Mayo Clin. Proc. 2023. [Google Scholar] [CrossRef] [PubMed]
| Reference | Country | Study Design | Study Period | Subjects (Baseline Characteristics) | Primary Study Outcomes | Intervention/Control | Results |
|---|---|---|---|---|---|---|---|
| Loimaala et al. (2003) [23] | Finland | Randomized, controlled, parallel-group trial | 12 months | 50 male patients with T2D, 1 dropout Intervention group: Age: 53.6 ± 6.2 years, BMI: 29 3 ± 3.7 kg/m2, HbA1c: 8.2 ± 2.1% Control group: Age: 54.0 ± 5.0 years, BMI: 29.8 ± 3.6 kg/m2, HbA1c: 8.0 ± 1.3% | Baroreflex sensitivity HRV | AE + RT/conventional therapy | Baroreflex sensitivity↑, resting heart rate↓ SDNN→, pNN50→, HF→, LF→, HF/LF ratio→ VO2max↑, muscle strength↑ HbA1c↓, systolic blood pressure↓ Extracellular water↑ |
| Kang et al. (2016) [24] | South Korea | Randomized, controlled, parallel-group trial | 12 weeks | 16 female patients with T2D, no dropouts Intervention group: Age: 56.0 ± 7.4 years, BMI: 23.9 ± 2.9 kg/m2, HbA1c: 6.4 ± 0.6% Control group: Age: 57.5 ± 4.6 years, BMI: 225.5 ± 3.1 kg/m2, HbA1c: 6.4 ± 0.5% | HRV | AE + RT/unclear | SDNN→, rMSSD→, LF→, HF→, LF/HF ratio→ VO2max↑, grip strength↑ Weight↓, waist circumference↓, body fat percentage↓ HbA1c↓, insulin↓, HOMA-IR↓, systolic blood pressure↓, diastolic blood pressure↓ |
| Liu et al. (2016) [26] | China | Randomized, controlled, parallel-group trial | 12 weeks | 123 patients with T2D in the cross-sectional analysis 42 patients with T2D Intervention group (12 men and 10 women): Age: 52.6 ± 8.1 years, BMI: unclear, HbA1c: 6.50 ± 0.96% Control group (11 men and 9 women): Age: 53.5 ± 10.7 years, BMI: unclear, HbA1c: 6.91 ± 0.62% | HRR | AE + RT/diet therapy + metformin | HRR↑, resting heart rate↓, max heart rate↓ Fasting blood glucose↓, postprandial glucose↓, HbA1c↓ Fasting insulin↓, postprandial insulin↓ Triglycerides↓, low-density lipoprotein cholesterol↓ |
| Cassidy et al. (2019) [28] | United Kingdom | Randomized, controlled, open-label, parallel-group trial | 12 weeks | 28 patients with T2D, 6 dropouts Intervention group (9 men and 2 women): Age: 60 ± 3 years, BMI: 31.2 ± 1.70 kg/m2, HbA1c: 7.13 ± 0.31% Control group (8 men and 3 women): Age: 59 ± 3 years, BMI: 32.0 ± 1.65 kg/m2, HbA1c: 7.18 ± 0.17% | HRV Baroreflex sensitivity Blood pressure variability | HIIT/usual care | R-R intervals→, SDNN→, LF→, HF→, LH/FH ratio→ Baroreflex sensitivity→ Systolic blood pressure HF↓ HbA1c↓ |
| Su et al. (2022) [29] | China | Randomized, controlled, parallel-group trial | 12 weeks | 30 female patients with T2D, 3 dropouts Intervention group (n = 14): Age: 64.01 ± 1.98 years, BMI: 24.90 ± 0.67 kg/m2, HbA1c: unclear Control group (n = 13): Age: 63.61 ± 2.56 years, BMI: 24.90 ± 0.67 kg/m2, HbA1c: unclear | HRV Inflammatory markers | AE + RT/regular treatment | SDNN↑, rMSSD↑, HF↑, LF/HF ratio↑, heart rate↓ IL-6↓, CRP↓ Fasting blood glucose↓, 2-h postprandial glucose↓ |
| Bhati et al. (2023) [30] | India | Randomized, controlled, single blinded, parallel-group trial | 12 weeks | 56 T2D patients with CAN, no dropout Intervention group (15 men and 13 women): Age: 52.8 ± 6.82 years, BMI: 28.4 ± 3.35 kg/m2, HbA1c: 8.4 ± 1.52% Control group (17 men and 11 women): Age: 54.0 ± 8.18 years, BMI: 28.0 ± 3.82 kg/m2, HbA1c: 8.2 ± 1.73% | Cardiovascular autonomic reflex tests HRV HRR Baroreflex sensitivity | RT/usual care | SDNN↑, rMSSD↑, pNN50↑, SDSD↑, HF↑, LF/HF ratio↑ Fasting blood glucose↓, postprandial glucose↓, HbA1c↓ IL-6↓, IL-18↓, eNOS↑ |
| Michou et al. (2023) [33] | Greece | Randomized, controlled, parallel-group trial | 6 months | 28 T2D patients with diabetic kidney disease, 13 dropouts Intervention group (10 men and 5 woman): Age: 62.06 ± 6.34 years, BMI: 28.28 ± 6.22 kg/m2, HbA1c: 6.85 ± 0.69% Control group (7 men and 6 women): Age: 63.30 ± 8.50 years, BMI: 29.05 ± 5.71 kg/m2, HbA1c: 7.01 ± 1.20% | HRV, Heart rate turbulence Metabolic parameters Cardiorespiratory fitness | AE + RT/usual care | SDNN↑, rMSSD↑, pNN50↑, LF↓ High-density lipoprotein cholesterol↑, HbA1c↓ Resting heart rate↓, METs↑, VO2peak↑, systolic blood pressure↓ |
| Michou et al. (2023) [34] | Greece | Randomized, controlled, parallel-group trial | 6 months | 25 T2D patients with CAN, 5 dropouts Intervention group (10 men and 3 women): Age: 54.9 ± 9.9 years, BMI: 24.8 ± 3.9 kg/m2, HbA1c: 6.8 ± 0.2% Control group (9 men and 3 women): Age: 54.0 ± 12.7 years, BMI: 25.6 ± 2.0 kg/m2, HbA1c: 6.6 ± 1.0% | HRV, Heart rate turbulence Cardiorespiratory fitness Functional capacity | AE + RT/usual care | SDNN↑, SDANN↑, rMSSD↑, pNN50↑, HF↑, TS↑, LF↓, VLF↓, LF/HF ratio↓ Exercise time↑, METs↑, VO2peak↑, exercise HR↑, systolic blood pressure↓ 30 s STS↑, upper limb strength↑, lower limb strength↑ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamasaki, H. The Effect of Exercise on Cardiovascular Autonomic Nervous Function in Patients with Diabetes: A Systematic Review. Healthcare 2023, 11, 2668. https://doi.org/10.3390/healthcare11192668
Hamasaki H. The Effect of Exercise on Cardiovascular Autonomic Nervous Function in Patients with Diabetes: A Systematic Review. Healthcare. 2023; 11(19):2668. https://doi.org/10.3390/healthcare11192668
Chicago/Turabian StyleHamasaki, Hidetaka. 2023. "The Effect of Exercise on Cardiovascular Autonomic Nervous Function in Patients with Diabetes: A Systematic Review" Healthcare 11, no. 19: 2668. https://doi.org/10.3390/healthcare11192668
APA StyleHamasaki, H. (2023). The Effect of Exercise on Cardiovascular Autonomic Nervous Function in Patients with Diabetes: A Systematic Review. Healthcare, 11(19), 2668. https://doi.org/10.3390/healthcare11192668