Evaluation of Blood Cultures from SARS-CoV-2-Positive and Negative Adult Patients †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Blood Culture
2.2.1. Identification of Bacterial and Fungal Isolates
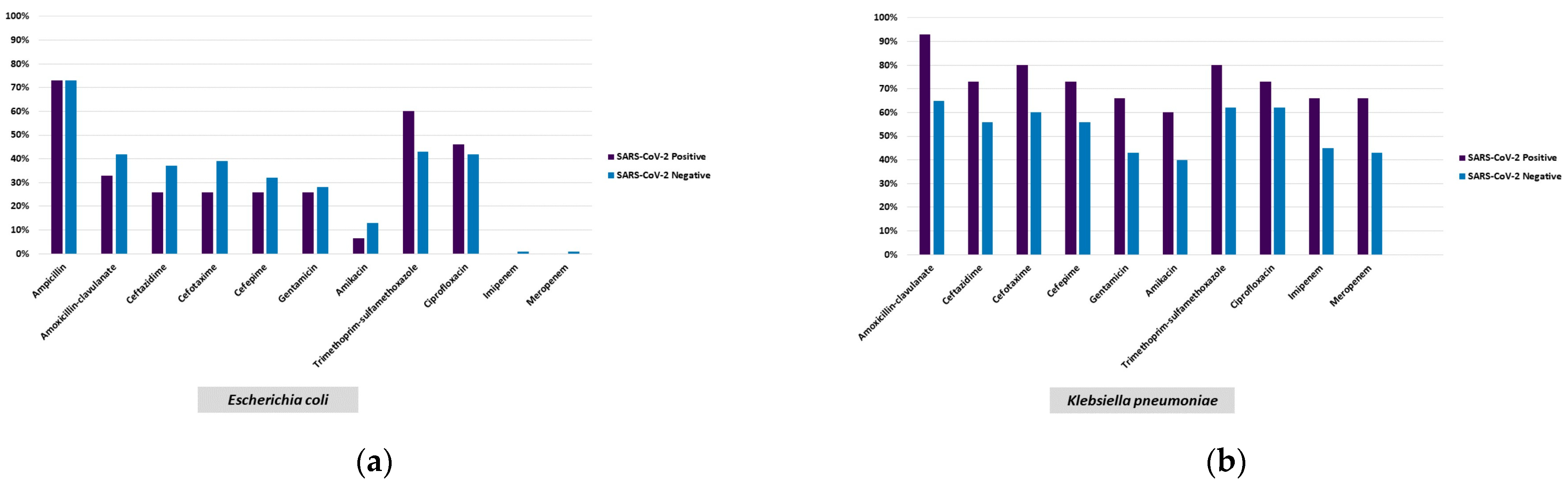
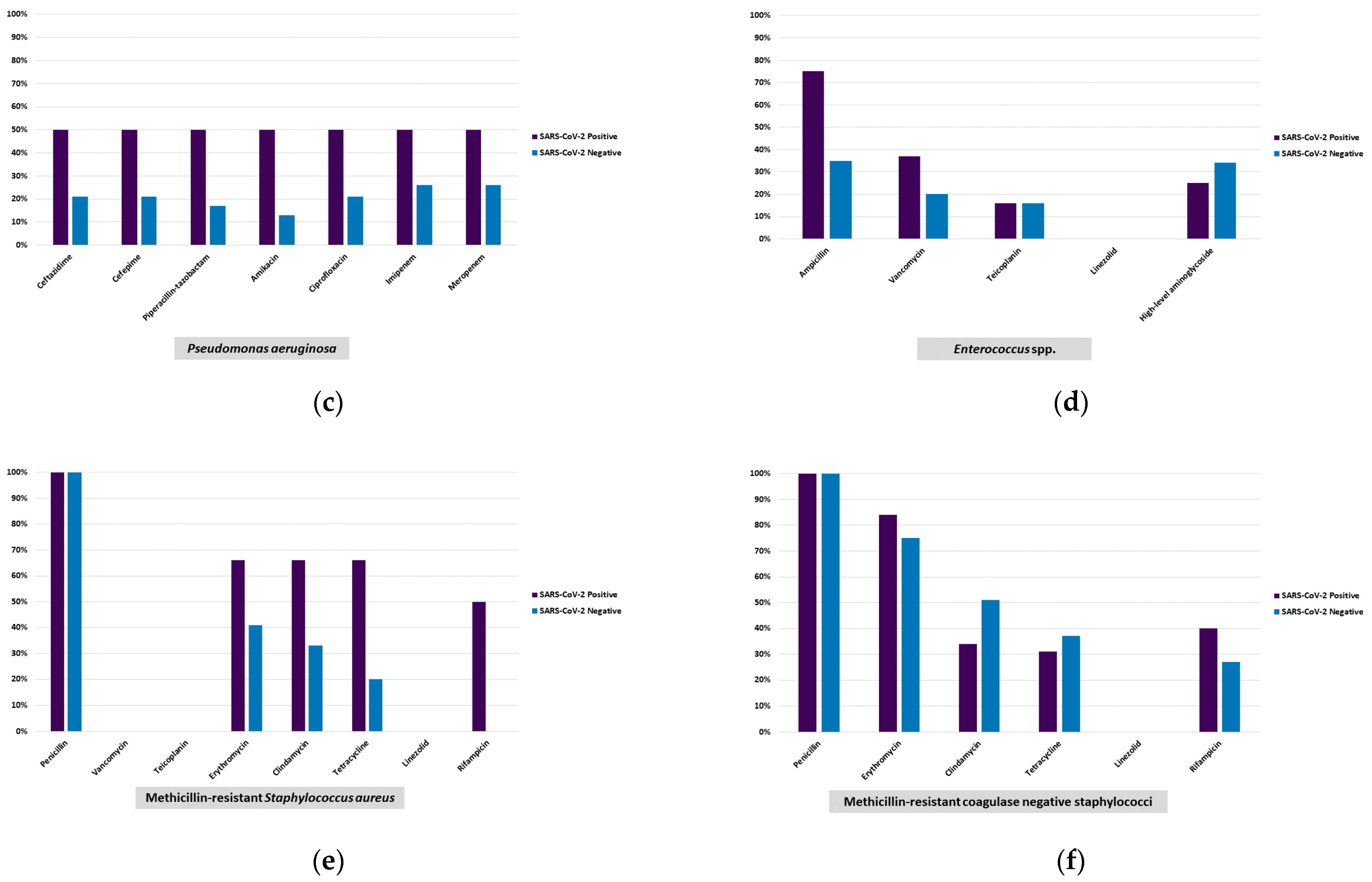
2.2.2. Antimicrobial Susceptibility Testing
2.3. The COVID-19 Diagnoses of the Patients
2.4. Statistical Analysis
2.5. Ethical Approval
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Manohar, P.; Loh, B.; Nachimuthu, R.; Hua, X.; Welburn, S.C.; Leptihn, S. Secondary bacterial infections in patients with viral pneumonia. Front. Med. 2020, 7, 420. [Google Scholar] [CrossRef]
- Bengoechea, J.A.; Bamford, C.G. SARS-CoV-2 bacterial co-infections and AMR: The deadly trio in COVID-19. EMBO. Mol. Med. 2020, 12, e12560. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Simmonds, A.; Annavajhala, M.K.; McConville, T.H.; Dietz, D.E.; Shoucri, S.M.; Laracy, J.C.; Rozenberg, F.D.; Nelson, B.; Greendyke, W.G.; Furuya, E.Y.; et al. Carbapenemase-producing Enterobacterales causing secondary infections during the COVID-19 crisis at a New York City hospital. J. Antimicrob. Chemother. 2021, 76, 380–384. [Google Scholar] [CrossRef] [PubMed]
- Gaibani, P.; Viciani, E.; Bartoletti, M.; Lewis, R.E.; Tonetti, T.; Lombardo, D.; Castagnetti, A.; Bovo, F.; Horna, C.S.; Ranieri, M.; et al. The lower respiratory tract microbiome of critically ill patients with COVID-19. Sci. Rep. 2021, 12, 10103. [Google Scholar] [CrossRef] [PubMed]
- Bongiovanni, M.; Barda, B. Pseudomonas aeruginosa bloodstream infections in SARS-CoV-2 infected patients: A systematic review. J. Clin. Med. 2023, 12, 2252. [Google Scholar] [CrossRef]
- Wang, L.; Amin, A.K.; Khanna, P.; Aali, A.; McGregor, A.; Bassett, P.; Gopal Rao, G. An observational cohort study of bacterial coinfection and implications for empirical antibiotic therapy in patients presenting with COVID-19 to hospitals in North West London. J. Antimicrob. Chemother. 2021, 76, 796–803. [Google Scholar] [CrossRef]
- Yu, D.; Ininbergs, K.; Hedman, K.; Giske, C.G.; Stralin, K.; Özenci, V. Low prevalence of bloodstream infection and high blood culture contamination rates in patients with COVID-19. PLoS ONE 2020, 15, e0242533. [Google Scholar] [CrossRef]
- Nedel, W.; da Silveira, F.; da Silva, C.F.; Lisboa, T. Bacterial infection in coronavirus disease 2019 patients: Co-infection, super-infection and how it impacts on antimicrobial use. Curr. Opin. Crit. Care 2022, 28, 463–469. [Google Scholar] [CrossRef]
- Zhu, N.J.; Rawson, T.M.; Mookerjee, S.; Price, J.R.; Davies, F.; Otter, J.; Aylin, P.; Hope, R.; Gilchrist, M.; Shersing, Y.; et al. Changing patterns of bloodstream infections in the community and acute care across 2 Coronavirus disease 2019 epidemic waves: A retrospective analysis using data linkage. Clin. Infect. Dis. 2022, 24, e1082–e1091. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Bloodstream Infection Event (Central Line-Associated Bloodstream Infection and Non-Central Line-Associated Bloodstream Infection). 2021. Available online: https://www.cdc.gov/nhsn/pdfs/pscmanual/4psc_clabscurrent.pdf (accessed on 12 December 2022).
- Gilligan, P.H.; Alby, K.; York, M.K. Blood cultures. In Clinical Microbiology Procedures Handbook, 4th ed.; Leber, A.L., Ed.; ASM Press: Washington, DC, USA, 2016; pp. 3.4.1.1–3.4.1.25. [Google Scholar]
- Gilligan, P.H.; York, M.K. Brucellosis-Brucella spp. In Clinical Microbiology Procedures Handbook, 4th ed.; Leber, A.L., Ed.; ASM Press: Washington, DC, USA, 2016; pp. 16.6.1–16.6.12. [Google Scholar]
- CLSI. Epidemiological Cutoff Values for Antifungal Susceptibility Testing, 3rd ed.; CLSI supplement M59; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2020. [Google Scholar]
- Clinical and Laboratory Standards Institute. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; 4th Informational Supplement M27-S4; CLSI: Wayne, PA, USA, 2012. [Google Scholar]
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 10.0. 2020. Available online: http://www.eucast.org (accessed on 2 November 2020).
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 11.0. 2021. Available online: http://www.eucast.org (accessed on 1 January 2021).
- Centers for Disease Control and Prevention (CDC). Candida auris. Available online: https://www.cdc.gov/fungal/candida-auris/index.html (accessed on 16 February 2021).
- Bölükbaşı, Y.; Erköse, G.G.; Orhun, G.; Kuşkucu, M.A.; Çağatay, A.; Önel, M.; Öngen, B.; Ağaçfidan, A.; Esen, F.; Erturan, Z. First case of COVID-19 positive Candida auris fungemia in Turkey. Mikrobiyol. Bul. 2021, 55, 648–655. [Google Scholar] [CrossRef]
- Willan, J.; King, A.J.; Jeffery, K.; Bienz, N. Challenges for NHS hospitals during Covid-19 epidemic. BMJ 2020, 368, m1117. [Google Scholar] [CrossRef] [PubMed]
- Sepulveda, J.; Westblade, L.F.; Whittier, S.; Satlin, M.J.; Greendyke, W.G.; Aaron, J.G.; Zucker, J.; Dietz, D.; Sobieszczyk, M.; Choi, J.J.; et al. Bacteremia and blood culture utilization during COVID-19 surge in New York City. J. Clin. Microbiol. 2020, 58, e00875-20. [Google Scholar] [CrossRef] [PubMed]
- Hughes, S.; Troise, O.; Donaldson, H.; Mughal, N.; Moore, L.S.P. Bacterial and fungal coinfection among hospitalized patients with COVID-19: A retrospective cohort study in a UK secondary-care setting. Clin. Microbiol. Infect. 2020, 26, 1395–1399. [Google Scholar] [CrossRef] [PubMed]
- Bauer, K.A.; Puzniak, L.A.; Yu, K.C.; Finelli, L.; Moise, P.; Ai, C.; Watts, J.A.; Gupta, V. Epidemiology and outcomes of culture-positive bloodstream pathogens prior to and during the SARS-CoV-2 pandemic: A multicenter evaluation. BMC Infect. Dis. 2022, 22, 841. [Google Scholar] [CrossRef]
- Michailides, C.; Paraskevas, T.; Karalis, I.; Koniari, I.; Pierrakos, C.; Karamouzos, V.; Marangos, M.; Velissaris, D. Impact of bacterial infections on COVID-19 patients: Is timing important? Antibiotics 2023, 12, 379. [Google Scholar] [CrossRef]
- Bahceci, I.; Yildiz, I.E.; Duran, O.F.; Soztanaci, U.S.; Kirdi Harbawi, Z.; Senol, F.F.; Demiral, G. Secondary bacterial infection rates among patients with COVID-19. Cureus 2022, 14, e22363. [Google Scholar] [CrossRef]
- Segala, F.V.; Pafundi, P.C.; Masciocchi, C.; Fiori, B.; Taddei, E.; Antenucci, L.; De Angelis, G.; Guerriero, S.; Pastorino, R.; Damiani, A.; et al. Incidence of bloodstream infections due to multidrug-resistant pathogens in ordinary wards and intensive care units before and during the COVID-19 pandemic: A real-life, retrospective observational study. Infection 2023, 51, 1061–1069. [Google Scholar] [CrossRef]
- Silva, D.L.; Lima, C.M.; Magalhães, V.C.R.; Baltazar, L.M.; Peres, N.T.A.; Caligiorne, R.B.; Moura, A.S.; Fereguetti, T.; Martins, J.C.; Rabelo, L.F.; et al. Fungal and bacterial coinfections increase mortality of severely ill COVID-19 patients. J. Hosp. Infect. 2021, 113, 145–154. [Google Scholar] [CrossRef]
- Getahun, H.; Smith, I.; Trivedi, K.; Paulin, S.; Balkhy, H.H. Tackling antimicrobial resistance in the COVID-19 pandemic. Bull. World Health Organ. 2020, 98, 442. [Google Scholar] [CrossRef]
- Center for Disease Control and Prevention. COVID-19: US Impact on Antimicrobial Resistance, Special Report 2022; US Department of Health and Human Services, CDC: Atlanta, GA, USA, 2022. Available online: https://www.cdc.gov/drugresistance/covid19.html (accessed on 10 August 2023).
- Mahmoudi, H. Bacterial co-infections and antibiotic resistance in patients with COVID-19. GMS Hyg. Infect. Control 2020, 15, Doc35. [Google Scholar]
- Petrakis, V.; Panopoulou, M.; Rafailidis, P.; Lemonakis, N.; Lazaridis, G.; Terzi, I.; Papazoglou, D.; Panagopoulos, P. The impact of the Covıd-19 pandemic on antimicrobial resistance and management of bloodstream infections. Pathogens 2023, 12, 780. [Google Scholar] [CrossRef] [PubMed]
- Sinto, R.; Lie, K.C.; Setiati, S.; Suwarto, S.; Nelwan, E.J.; Djumaryo, D.H.; Karyanti, M.R.; Prayitno, A.; Sumariyono, S.; Moore, C.E.; et al. Blood culture utilization and epidemiology of antimicrobial-resistant bloodstream infections before and during the COVID-19 pandemic in the Indonesian national referral hospital. Antimicrob. Resist. Infect. Control 2022, 11, 73. [Google Scholar] [CrossRef] [PubMed]
- Chowdhary, A.; Tarai, B.; Singh, A.; Sharma, A. Multidrug-resistant Candida auris infections in critically ill coronavirus disease patients, India, April–July 2020. Emerg. Infect. Dis. 2020, 26, 2694–2696. [Google Scholar] [CrossRef] [PubMed]
- Arastehfar, A.; Carvalho, A.; Nguyen, M.H.; Hedayati, M.T.; Netea, M.G.; Perlin, D.S.; Hoenigl, M. COVID-19-associated candidiasis (CAC): An underestimated complication in the absence of immunological predispositions? J. Fungi 2020, 6, 211. [Google Scholar] [CrossRef]
- Rodriguez, J.Y.; Le Pape, P.; Lopez, O.; Esquea, K.; Labiosa, A.L.; Alvarez-Moreno, C. Candida auris: A latent threat to critically ill patients with Coronavirus disease 2019. Clin. Infect. Dis. 2020, 73, e2836-7. [Google Scholar] [CrossRef]
- Magnasco, L.; Mikulska, M.; Giacobbe, D.R.; Taramasso, L.; Vena, A.; Dentone, C.; Dettori, S.; Tutino, S.; Labate, L.; Di Pilato, V.; et al. Spread of carbapenem-resistant gram-negatives and Candida auris during the COVID-19 pandemic in critically ill patients: One step back in antimicrobial stewardship? Microorganisms 2021, 9, 95. [Google Scholar] [CrossRef]
- Çaklovica-Küçükkaya, İ.; Orhun, G.; Çağatay, A.A.; Kalaycı, S.; Esen, F.; Şahin, F.; Ağaçfidan, A.; Erturan, Z. P494 Comparison of Candida colonization in intensive care unit patients with and without COVID-19: First prospective cohort study from Turkey. Med. Mycol. 2022, 60 (Suppl. S1), myac072P494. [Google Scholar] [CrossRef]
| Clinics | Unit | SARS CoV-2 (+) (n:118) | SARS CoV-2 (−) (n:515) | Not Tested (n:19) |
|---|---|---|---|---|
| Inpatient | Surgical | 24 | 96 | - |
| Internal | 19 | 69 | 3 | |
| Outpatient | Surgical | - | 26 | - |
| Internal | 38 | 243 | 14 | |
| Intensive Care Unit | Surgical | 5 | 43 | 1 |
| Internal | 32 | 38 | 1 |
| Microorganisms | Total n (%) | SARS- CoV-2 Positive n (%) | SARS- CoV-2 Negative n (%) | SARS- CoV-2 Non-Tested n (%) | Positive vs. Negative | |
|---|---|---|---|---|---|---|
| All microorganisms a | 671 (100) | 121 (18.0) | 531 (79.1) | 19 (2.8) | p-value | Differences of two proportions 95% CI j |
| Fermentative Gram-negative rods | 252 (37.5) | 34 (28,0) | 214 (40.3) | 4 (21) | 0.013 h | (3.2–21.2) |
| Escherichia coli | 131 (19.5) | 15 (12.4) | 113 (21.3) | 3 (15.8) | 0.026 h | (2.1–5.7) |
| Klebsiella pneumoniaeb/ CRKP c | 80/38 (11.9/5.6) | 15/9 (12.4/7.4) | 64/28 (12.05/5.3) | 1/1 (5.3/5.3) | 0.917 b,h 0.353 c,h | (−6.1–0.8) |
| (−2.9–0.5) | ||||||
| Klebsiella oxytoca | 5 (0.7) | 0 (0.0) | 5 (0.9) | - | 0.590 i | (0.1–1.7) |
| Enterobacter spp. | 7 (1.1) | 1 (0.8) | 6 (1.1) | - | 1.000 i | (−1.5–2.1) |
| Proteus mirabilis | 13 (1.9) | 1 (0.8) | 12 (2.3) | - | 0.480 i | (−0.5–3.5) |
| Non-Fermentative Gram-negative rods | 66 (9.8) | 16 (13.2) | 48 (9) | 2 (10.5) | 0.163 h | (−1.7–10.1) |
| Pseudomonas aeruginosa | 25 (3.7) | 2 (1.7) | 23 (4.3) | - | 0.199 i | (−0.2–5.5) |
| Pseudomonas spp. | 5 (0.7) | 2 (1.7) | 3 (0.6) | - | 0.233 i | (−0.7–2.8) |
| Acinetobacter baumannii | 14 (2.1) | 7 (5.8) | 6 (1.1) | 1 (5.3) | 0.004 i | (2.0–7.4) |
| Stenotrophomonas maltophilia | 7 (1.05) | 1 (0.8) | 6 (1.1) | - | 1.000 i | (−1.5–2.1) |
| Gram-positive cocci | 300 (44.7) | 59 (48.8) | 231 (40.1) | 10 (52.6) | 0.294 h | (−4.5–15.1) |
| MRSA d | 27 (4.0) | 3 (2.5) | 24 (4.5) | - | 0.309 h | (−1.3–5.3) |
| MSSA e | 53 (7.9) | 7 (5.8) | 43 (8.1) | 3 (15.8) | 0.388 h | (−2.5–7.1) |
| MR-CoNS f | 102 (15.2) | 26 (21.5) | 72 (13.5) | 4 (21.1) | 0.028 h | (1.0–15.0) |
| MS-CoNS g | 49 (7.3) | 12 (9.9) | 35 (6.6) | 2 (10.5) | 0.202 h | (−1.8–8.4) |
| Enterococcus faecalis | 12 (1.8) | 2 (1.7) | 10 (1.9) | - | 1.000 i | (−2.3–2.8) |
| Enterococcus faecium | 12 (1.8) | 3 (2.5) | 9 (1.7) | - | 0.474 i | (−1.9–3.5) |
| Enterococcus spp. | 16 (2.4) | 2 (1.7) | 14 (2.6) | - | 0.749 i | (−1.7–3.6) |
| Alpha hemolytic streptococci | 7 (1.05) | 0 (0.0) | 7 (1.3) | - | 0.359 i | (0.3–2.3) |
| Fungi | 38 (5.7) | 8 (6.6) | 27 (5.1) | 3 (15.8) | 0.501 h | (−3.0–6.0) |
| Candida albicans | 15 (2.2) | 2 (1.7) | 11 (2.1) | 2 (10.5) | 1.000 i | (−2.1–3.0) |
| Candida parapsilosis complex | 7 (1.05) | 1 (0.8) | 6 (1.1) | - | 1.000 i | (−1.5–2.1) |
| Candida tropicalis | 6 (0.9) | 0 (0.0) | 5 (0.9) | 1 (5.3) | 0.590 i | (0.1–1.7) |
| Fungi | Patients | Antifungal MIC, µg/mL | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Species (Tested n/Total n) | Strain No | SARS-CoV-2 Status | Fluconazole | Posaconazole | Voriconazole | Itraconazole | Amphotericin B | Caspofungin | Anidulafungin |
| Candida albicans (7/15) | 1 | Positive | 2 (S) | 0.064 ᵃ (NWT) | 0.25 (I) | - | 0.5 ᵃ (WT) | 0.016 (S) | 0.012 (S) |
| 2 | Negative | 2 (S) | 0.064 ᵃ (NWT) | 0.047(S) | - | - | - | - | |
| 3 | Not tested | 0.75 (S) | - | - | - | - | 0.5 (I) | - | |
| 4 | Negative | 2 (S) | - | - | - | 0.25 ᵃ (WT) | 0.5 (I) | 0.012 (S) | |
| 5 | Positive | 1.5 (S) | - | - | - | - | - | - | |
| 6 | Negative | - | 0.25 ᵃ (NWT) | 1 (R) | - | - | 0.065 (S) | - | |
| 7 | Negative | 0.125 (S) | - | - | - | 0.047 ᵃ (WT) | 0.096 (S) | 0.003 (S) | |
| Candida parapsilosis complex (3/7) | 1 | Negative | 0.75 (S) | - | - | - | 0.25 ᵃ (WT) | 0.75 (S) | - |
| 2 | Negative | >256 (R) | 0.19 ᵃ (WT) | 0.5 (I) | 2 c | 0.75 ᵃ (WT) | 0.38 (S) | 0.75 (S) | |
| 3 | Negative | 24 (R) | 0.25 ᵃ (WT) | 0.75 (S) | |||||
| Candida tropicalis (2/6) | 1 | Negative | 0.5 (S) | - | 0.008 (S) | - | 0.25 ᵃ (WT) | 0.094 (S) | 0.008 (S) |
| 2 | Negative | 0.5 (S) | - | - | - | 0.25 ᵃ (WT) | - | 0.008 (S) | |
| Candida glabrata complex (1/2) | Positive | 1.5 (SDD) | - | 0.032 ᵃ (WT) | - | - | 0.25 (I) | - | |
| Candida auris (1/1) b | Positive | >256 (R) | 0.016c | 0.19 c | 0.19 c | 3 (R) | 1 (S) | 0.094 (S) | |
| Cryptococcus neoformans (1/1) | Negative | 8 ᵃ (WT) | - | - | - | 0.5 ᵃ (WT) | - | - | |
| Microorganisms (n) | |||||||
|---|---|---|---|---|---|---|---|
| Demographic Information | MR-CoNS a (n:102) | MS-CoNS b (n:49) | E. coli (n:131) | K. pneumoniae (n:80) | A. baumannii (n:14) | C. albicans (n:15) | C. glabrata complex (n:2) |
| SARS-CoV-2 (+) | 26 | 12 | 15 | 64 | 7 | 3 | 2 |
| Inpatient | 9 | 4 | 3 | 21 | 1 | 2 | 2 |
| Outpatient | 6 | 5 | 9 | 29 | 0 | 0 | 0 |
| ICU | 11 | 3 | 3 | 14 | 6 | 1 | 0 |
| SARS-CoV-2 (−) | 72 | 35 | 113 | 15 | 6 | 10 | 0 |
| Inpatient | 34 | 18 | 23 | 9 | 3 | 8 | 0 |
| Outpatient | 25 | 13 | 81 | 2 | 0 | 1 | 0 |
| ICU | 13 | 4 | 10 | 4 | 3 | 1 | 0 |
| SARS-CoV-2—not tested | 4 | 2 | 3 | 1 | 1 | 2 | 0 |
| Inpatient | 1 | 0 | 0 | 0 | 0 | 1 | 0 |
| Outpatient | 3 | 2 | 3 | 0 | 0 | 1 | 0 |
| ICU | 0 | 0 | 0 | 1 | 1 | 0 | 0 |
| Median age | 63.85 | 63.42 | 62.69 | 60.78 | 63.71 | 68.4 | 71 |
| Gender identity | |||||||
| Male | 44 | 25 | 74 | 44 | 10 | 8 | 1 |
| Female | 58 | 24 | 57 | 36 | 4 | 7 | 1 |
| Oncology patient | 39 | 11 | 40 | 26 | 3 | 6 | 0 |
| Hematologic malignancy | 7 | 6 | 13 | 3 | 2 | 2 | 0 |
| Hypertension | 33 | 17 | 36 | 33 | 6 | 6 | 1 |
| Diabetes mellitus | 22 | 11 | 27 | 19 | 3 | 3 | 1 |
| Coronary artery disease | 0 | 0 | 10 | 0 | 0 | 2 | 0 |
| COPD c | 9 | 2 | 0 | 0 | 0 | 3 | 0 |
| COVID-19 pneumonia | 22 | 14 | 24 | 12 | 5 | 2 | 0 |
| Clinic/Units | Microorganism | ||
|---|---|---|---|
| SARS-CoV-2 (+) [n:8] | Inpatient (n:3) | Surgical (n:1) | Candida krusei, Kodamea ohmeri |
| Internal (n:2) | Proteus mirabilis, Escherichia coli | ||
| Klebsiella pneumoniae, Candida glabrata complex | |||
| Outpatient (n:4) | Surgical (n:1) | Candida glabrata complex, Candida albicans | |
| Internal (n:3) | Streptococcus pneumoniae, Escherichia coli | ||
| Candida kefyr, Enterococcus gallinarum, Enterococcus faecium | |||
| Escherichia coli, Enterobacter spp. | |||
| Intensive Care Unit (n:1) | Surgical (n:0) | - | |
| Internal (n:1) | Enterococcus spp., Candida albicans | ||
| SARS-CoV-2 (-) [n:9] | Inpatient (n:3) | Surgical (n:2) | Candida albicans, Candida parapsilosis |
| Escherichia coli, Candida parapsilosis | |||
| Internal (n:1) | Pseudomonas aeruginosa, Acinetobacter spp. | ||
| Outpatient (n:5) | Surgical (n:1) | Citrobacter spp., Klebsiella oxytoca | |
| Internal (n:4) | Raoultella planticola, Escherichia coli | ||
| Klebsiella pneumoniae, Enterococcus spp. | |||
| Enterococcus spp., Escherichia coli, MSSA b | |||
| Enterococcus spp., Escherichia coli | |||
| Intensive Care Unit (n:1) | Surgical (n:0) | - | |
| Internal (n:1) | Proteus mirabilis, Klebsiella pneumoniae | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akgün Karapınar, B.; Çaklovica Küçükkaya, İ.; Bölükbaşı, Y.; Küçükkaya, S.; Erköse Genç, G.; Erturan, Z.; Ağaçfidan, A.; Öngen, B. Evaluation of Blood Cultures from SARS-CoV-2-Positive and Negative Adult Patients. Healthcare 2023, 11, 2581. https://doi.org/10.3390/healthcare11182581
Akgün Karapınar B, Çaklovica Küçükkaya İ, Bölükbaşı Y, Küçükkaya S, Erköse Genç G, Erturan Z, Ağaçfidan A, Öngen B. Evaluation of Blood Cultures from SARS-CoV-2-Positive and Negative Adult Patients. Healthcare. 2023; 11(18):2581. https://doi.org/10.3390/healthcare11182581
Chicago/Turabian StyleAkgün Karapınar, Bahar, İlvana Çaklovica Küçükkaya, Yasemin Bölükbaşı, Sertaç Küçükkaya, Gonca Erköse Genç, Zayre Erturan, Ali Ağaçfidan, and Betigül Öngen. 2023. "Evaluation of Blood Cultures from SARS-CoV-2-Positive and Negative Adult Patients" Healthcare 11, no. 18: 2581. https://doi.org/10.3390/healthcare11182581
APA StyleAkgün Karapınar, B., Çaklovica Küçükkaya, İ., Bölükbaşı, Y., Küçükkaya, S., Erköse Genç, G., Erturan, Z., Ağaçfidan, A., & Öngen, B. (2023). Evaluation of Blood Cultures from SARS-CoV-2-Positive and Negative Adult Patients. Healthcare, 11(18), 2581. https://doi.org/10.3390/healthcare11182581





