MC TRIM Algorithm in Mandibula Phantom in Helium Therapy
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Bragg Curves
3.2. Recoils
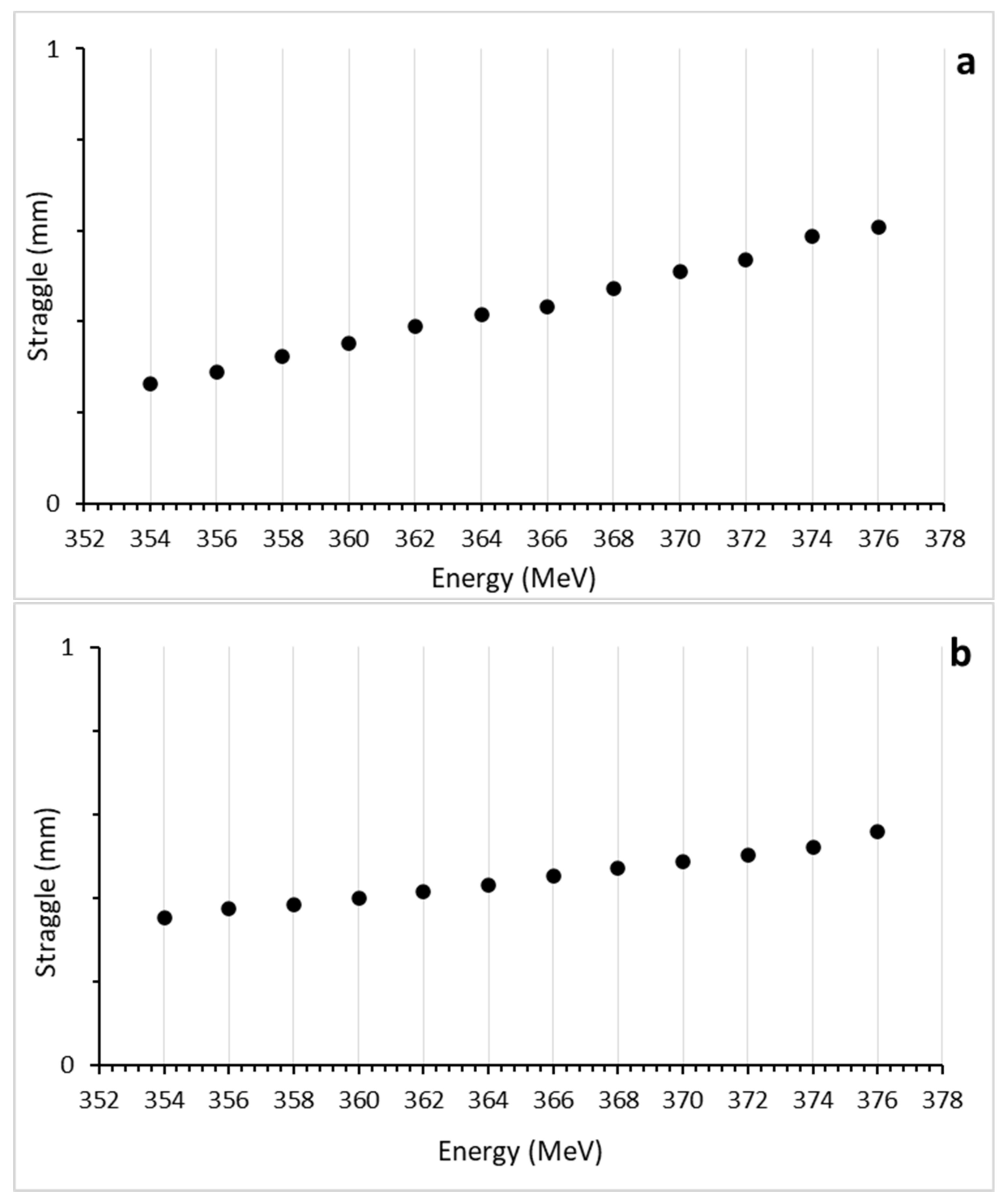
3.3. Lateral Straggle
4. Discussion
5. Conclusions
- Helium ions can be used as intermediate heavy ions in addition to proton and carbon ions.
- They have more LETs and less lateral scattering than the proton and also cost less than carbon.
- They have better performance than the proton in the treatment of dental tumors.
- The biophantom proposed in this study for calibration and dose calculations in dental tumors showed a realistic performance.
- The biomaterials that make up the biophantom created in this study gave results close to real tissues.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tommasino, F.; Scifoni, E.; Durante, M. New ions for therapy. Int. J. Part. Ther. 2015, 2, 428–438. [Google Scholar] [CrossRef]
- Ekinci, F.; Bostanci, E.; Güzel, M.S.; Dagli, O. Simulation based analysis of 4He, 7Li, 8Be and 10B ions for heavy ion therapy. Int. J. Radiat. Res. 2023, 21, 131–137. [Google Scholar]
- Ekinci, F.; Bostanci, E.; Güzel, M.S.; Dagli, O. Effect of different embolization materials on proton beam stereotactic radiosurgery Arteriovenous Malformation dose distributions using the Monte Carlo simulation code. J. Radiat. Res. Appl. Sci. 2022, 15, 191–197. [Google Scholar] [CrossRef]
- Castro, J.R.; Quivey, J.M.; Lyman, J.T.; Chen, G.T.; Phillips, T.L.; Tobias, C.A. Radiotherapy with heavy charged particles at Lawrence Berkeley Laboratory. J. Can. Assoc. Radiol. 1980, 31, 30–34. [Google Scholar]
- Mairani, A.; Mein, S.; Blakely, E.; Debus, J.; Durante, M.; Ferrari, A.; Fuchs, H.; Georg, D.; Grosshans, D.R.; Guan, F.; et al. Roadmap: Helium ion therapy. Phys. Med. Biol. 2022, 67, 15TR02. [Google Scholar] [CrossRef]
- Ekinci, F. Investigation of tissue equivalence of phantom biomaterials in 4He heavy ion therapy. Radiat. Eff. Defects Solids 2023, 178, 500–509. [Google Scholar] [CrossRef]
- Ekinci, F.; Bostancı, G.E.; Güzel, M.S.; Dağlı, Ö. Recoil analysis for heavy ion beams. Aksaray J. Sci. Eng. 2022, 6, 123–134. [Google Scholar] [CrossRef]
- Saunders, W.; Castro, J.R.; Chen, G.T.; Collier, J.M.; Zink, S.R.; Pitluck, S.; Phillips, T.L.; Char, D.; Gutin, P.; Gauger, G. Helium-ion radiation therapy at the Lawrence Berkeley Laboratory: Recent results of a Northern California Oncology Group clinical trial. Radiat. Res. 2006, 104, S227–S234. [Google Scholar]
- Ekinci, F.; Bostanci, E.; Güzel, M.S.; Dagli, Ö. A Monte Carlo Study for Soft Tissue Equivalency of Potential Polymeric Biomaterials Used in Carbon Ion Radiation Therapy. Nucl. Technol. 2023, 209, 1229–1239. [Google Scholar] [CrossRef]
- Jongen, Y.; Abs, M.; Blondin, A.; Kleeven, W.; Zaremba, S.; Vandeplassche, D.; Aleksandrov, V.; Gursky, S.; Karamyshev, O.; Karamysheva, G.; et al. Compact superconducting cyclotron C400 for hadron therapy. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2010, 624, 47–53. [Google Scholar] [CrossRef]
- Tessonnier, T.; Mairani, A.; Brons, S.; Sala, P.; Cerutti, F.; Ferrari, A.; Haberer, T.; Debus, J.; Parodi, K. Helium ions at the heidelberg ion beam therapy center: Comparisons between FLUKA Monte Carlo code predictions and dosimetric measurements. Phys. Med. Biol. 2017, 62, 6784. [Google Scholar] [CrossRef] [PubMed]
- Krämer, M.; Scifoni, E.; Schuy, C.; Rovituso, M.; Tinganelli, W.; Maier, A.; Kaderka, R.; Kraft-Weyrather, W.; Brons, S.; Tessonnier, T.; et al. Helium ions for radiotherapy? Physical and biological verifications of a novel treatment modality. Med. Phys. 2016, 43, 1995–2004. [Google Scholar] [CrossRef] [PubMed]
- Tessonnier, T.; Mairani, A.; Chen, W.; Sala, P.; Cerutti, F.; Ferrari, A.; Haberer, T.; Debus, J.; Parodi, K. Proton and helium ion radiotherapy for meningioma tumors: A Monte Carlo-based treatment planning comparison. Radiat. Oncol. 2018, 13, 2. [Google Scholar] [CrossRef] [PubMed]
- Senirkentli, G.B.; Ekinci, F.; Bostanci, E.; Güzel, M.S.; Dağli, Ö.; Karim, A.M.; Mishra, A. Proton Therapy for Mandibula Plate Phantom. Healthcare 2021, 9, 167. [Google Scholar] [CrossRef]
- Battistoni, G.; Cerutti, F.; Fassò, A.; Ferrari, A.; Muraro, S.; Ranft, J.; Roesler, S.; Sala, P.R. The FLUKA code: Description and benchmarking. AIP Conf. Proc. 2007, 896, 31–49. [Google Scholar]
- Böhlen, T.; Cerutti, F.; Chin, M.; Fassò, A.; Ferrari, A.; Ortega, P.; Mairani, A.; Sala, P.; Smirnov, G.; Vlachoudis, V. The FLUKA Code: Developments and Challenges for High Energy and Medical Applications. Nucl. Data Sheets 2014, 120, 211–214. [Google Scholar] [CrossRef]
- Battistoni, G.; Bauer, J.; Boehlen, T.T.; Cerutti, F.; Chin, M.P.W.; Augusto, R.D.S.; Ferrari, A.; Ortega, P.G.; Kozłowska, W.; Magro, G.; et al. The FLUKA Code: An Accurate Simulation Tool for Particle Therapy. Front. Oncol. 2016, 6, 116. [Google Scholar] [CrossRef]
- Allison, J.; Amako, K.; Apostolakis, J.; Arce, P.; Asai, M.; Aso, T.; Bagli, E.; Bagulya, A.; Banerjee, S.; Barrand, G.; et al. Recent developments in Geant4. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2016, 835, 186–225. [Google Scholar] [CrossRef]
- Fatih, E.; Erkan, B.; Serdar, G.M.; Özlem, D. Analysing the effect of a cranium thickness on a Bragg peak range in the proton therapy: A TRIM and GEANT4 based study. Научнo-технические ведoмoсти Санкт-Петербургскoгo гoсударственнoгo пoлитехническoгo университета. Физикo-математические науки 2022, 15, 64–78. [Google Scholar]
- Sato, H.; Iwamoto, Y.; Hashimoto, S.; Ogawa, T.; Furuta, T.; Abe, S.I.; Kai, T.; Tsai, P.E.; Matsuda, N.; Iwase, H.; et al. Features of particle and heavy ion transport code system (PHITS) version 3.02. J. Nucl. Sci. Technol. 2018, 55, 684–690. [Google Scholar] [CrossRef]
- Lysakovski, P.; Ferrari, A.; Tessonnier, T.; Besuglow, J.; Kopp, B.; Mein, S.; Haberer, T.; Debus, J.; Mairani, A. Development and benchmarking of a monte carlo dose engine for proton radiation Therapy. Front. Phys. 2021, 9, 655. [Google Scholar] [CrossRef]
- Bölükdemir, M.H.; Ekinci, F. The Effect of the Second Peak formed in Biomaterials used in a Slab Head Phantom on the Proton Bragg Peak. Polite Derg. 2020, 23, 129–136. [Google Scholar]
- McDonald, M.W.; Liu, Y.; Moore, M.G.; Johnstone, P.A.S. Acute toxicity in comprehensive head and neck radiation for nasopharynx and paranasal sinus cancers: Cohort comparison of 3D conformal proton therapy and intensity modulated radiation therapy. Radiat. Oncol. 2016, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Romesser, P.B.; Cahlon, O.; Scher, E.; Zhou, Y.; Berry, S.L.; Rybkin, A.; Sine, K.M.; Tang, S.; Sherman, E.J.; Wong, R.; et al. Proton beam radiation therapy results in significantly reduced toxicity compared with intensity-modulated radiation therapy for head and neck tumors that require ipsilateral radiation. Radiother. Oncol. 2016, 118, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Parkins, G.E.; Armah, G.; Ampofo, P. Tumours and tumour-like lesions of the lower face at Korle Bu Teaching Hospital, Ghana—An eight year study. World J. Surg. Oncol. 2007, 5, 48. [Google Scholar] [CrossRef]
- Koichi, M.; Demizu, Y.; Hashimoto, N.; Mima, M.; Terashima, K.; Fujii, O.; Otsuki, N.; Murakami, M.; Fuwa, N.; Nibu, K.-I. Particle radiotherapy using protons or carbon ions for unresectable locally advanced head and neck cancers with skull base invasion. Jpn. J. Clin. Oncol. 2014, 44, 428–434. [Google Scholar]
- Emami, B.; Lyman, J.; Brown, A.; Coia, L.; Goitein, M.; Munzenrider, J.E.; Shank, B.; Solin, L.J.; Wesson, M. Tolerance of normal tissue to therapeutic irradiation. Int. J. Radiat. Oncol. Biol. Phys. 1991, 21, 109–122. [Google Scholar] [CrossRef]
- Fujita, M.; Hirokawa, Y.; Kashiwado, K.; Akagi, Y.; Kashimoto, K.; Kiriu, H.; Ohtani, K.; Wada, T. An analysis of mandibular bone complications in radiotherapy for T1 and T2 carcinoma of the oral tongue. Int. J. Radiat. Oncol. 1996, 34, 333–339. [Google Scholar] [CrossRef]
- Mosel, D.; Bauer, R.; Lynch, D.; Hwang, S. Oral complications in the treatment of cancer patients. Oral Dis. 2011, 17, 550–559. [Google Scholar] [CrossRef]
- Kumar, R.; Madanikia, S.; Starmer, H.; Yang, W.; Murano, E.; Alcorn, S.; McNutt, T.; Le, Y.; Quon, H. Radiation dose to the floor of mouth muscles predicts swallowing complications following chemoradiation in oropharyngeal squamous cell carcinoma. Oral Oncol. 2014, 50, 65–70. [Google Scholar] [CrossRef]
- Dirix, P.; Nuyts, S. Evidence-based organ-sparing radiotherapy in head and neck cancer. Lancet Oncol. 2010, 11, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Samson, D.O.; Jafri, M.Z.M.; Shukri, A.; Hashim, R.; Sulaiman, O.; Aziz, M.Z.A.; Yusof, M.F.M. Measurement of radiation attenuation parameters of modified defatted soy flour–soy protein isolate-based mangrove wood particleboards to be used for CT phantom production. Radiat. Environ. Biophys. 2020, 59, 483–501. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, J.F. SRIM: The Stopping and Range of Ion in Matter. Available online: https://www.srim.org (accessed on 13 March 2020).
- Qi, M.; Yang, Q.; Chen, X.; Yang, J.D.L. Fast calculation of Mont Carlo ion transport code. J. Phys. Conf. Ser. 2021, 1739, 012030. [Google Scholar] [CrossRef]
- Was, S.G. Fundamentals of Radiation Materials Science. In Fundamentals of Radiation Materials Science Metals and Alloys; Springer: New York, NY, USA, 2017; ISBN 978-1-4939-3436-2/978-1-4939-3438-6. [Google Scholar] [CrossRef]
- Groom, D.E.; Klein, S.R. Passage of particles through matter. Eur. Phys. J. C 2000, 15, 163–173. [Google Scholar] [CrossRef]
- Volz, L.; Collins-Fekete, C.-A.; Piersimoni, P.; Johnson, R.P.; Bashkirov, V.; Schulte, R.; Seco, J. Stopping power accuracy and achievable spatial resolution of helium ion imaging using a prototype particle CT detector system. Curr. Dir. Biomed. Eng. 2017, 3, 401–404. [Google Scholar] [CrossRef]
- Parodi, K.; Polf, J.C. In vivo range verification in particle therapy. Med. Phys. 2018, 45, e1036–e1050. [Google Scholar] [CrossRef]
- Mein, S.; Kopp, B.; Tessonnier, T.; Ackermann, B.; Ecker, S.; Bauer, J.; Choi, K.; Aricò, G.; Ferrari, A.; Haberer, T.; et al. Dosimetric validation of Monte Carlo and analytical dose engines with raster-scanning 1H, 4He, 12C, and 16O ion-beams using an anthropomorphic phantom. Phys. Med. Eur. J. Med. Phys. 2019, 64, 123–131. [Google Scholar] [CrossRef]
- Hansen, D.; Sorensen, T.; Seco, J. WE-G-141-05: The ımage quality of ıon computed tomography at clinical ımaging dose levels. Med. Phys. 2013, 40, 508. [Google Scholar] [CrossRef]
- Martins, P.M.; Bello, R.D.; Rinscheid, A.; Roemer, K.; Werner, T.; Enghardt, W.; Pausch, G.; Seco, J. Prompt gamma spectroscopy for range control with CeBr3. Curr. Dir. Biomed. Eng. 2017, 3, 113–117. [Google Scholar] [CrossRef]
- Dal, B.R.; Magalhaes, M.P.; Seco, J. CeBr3 scintillators for 4He prompt gamma spectroscopy: Results from a Monte Carlo optimization study. Med. Phys. 2018, 45, 1622–1630. [Google Scholar]
- Dal, B.R.; Martins, P.M.; Graça, J.; Hermann, G.; Kihm, T.; Seco, J. Results from the experimental evaluation of CeBr3 scintillators for 4He prompt gamma spectroscopy. Med. Phys. 2019, 46, 3615–3626. [Google Scholar]
- Gehrke, T.; Gallas, R.; Jäkel, O.; Martišíková, M. Proof of principle of helium-beam radiography using silicon pixel detectors for energy deposition measurement, identification, and tracking of single ions. Med. Phys. 2018, 45, 817–829. [Google Scholar] [CrossRef] [PubMed]
- Martišíková, M.; Gehrke, T.; Berke, S.; Aricò, G.; Jäkel, O. Helium ion beam imaging for image guided ion radiotherapy. Radiat. Oncol. 2018, 13, 109. [Google Scholar] [CrossRef] [PubMed]
- Dokic, I.; Mairani, A.; Niklas, M.; Zimmermann, F.; Chaudhri, N.; Krunic, D.; Tessonnier, T.; Ferrari, A.; Parodi, K.; Jäkel, O.; et al. Next generation multi-scale biophysical characterization of high precision cancer particle radiotherapy using clinical proton, helium-, carbon- and oxygen ion beams. Oncotarget 2016, 7, 56676–56689. [Google Scholar] [CrossRef] [PubMed]
- Mairani, A.; Dokic, I.; Magro, G.; Tessonnier, T.; Kamp, F.; Carlson, D.J.; Ciocca, M.; Cerutti, F.; Sala, P.R.; Ferrari, A.; et al. Biologically optimized helium ion plans: Calculation approach and its in vitro validation. Phys. Med. Biol. 2016, 61, 4283. [Google Scholar] [CrossRef]
- Mairani, A.; Magro, G.; Dokic, I.; Valle, S.M.; Tessonnier, T.; Galm, R.; Ciocca, M.; Parodi, K.; Ferrari, A.; Jäkel, O.; et al. Data-driven RBE parameterization for helium ion beams. Phys. Med. Biol. 2016, 61, 888–905. [Google Scholar] [CrossRef]
- Mein, S.; Klein, C.; Kopp, B.; Magro, G.; Harrabi, S.; Karger, C.P.; Haberer, T.; Debus, J.; Abdollahi, A.; Dokic, I.; et al. Assessment of Rbe-weighted dose models for carbon ıon therapy toward modernization of clinical practice at HIT: In vitro, in vivo, and in patients. Int. J. Radiat. Oncol. 2020, 108, 779–791. [Google Scholar] [CrossRef]
- Horst, F.; Aricò, G.; Brinkmann, K.-T.; Brons, S.; Ferrari, A.; Haberer, T.; Mairani, A.; Parodi, K.; Reidel, C.-A.; Weber, U.; et al. Measurement of 4He charge- and mass-changing cross sections on H, C, O, and Si targets in the energy range 70–220 MeV/u for radiation transport calculations in ion-beam therapy. Phys. Rev. C 2019, 99, 014603. [Google Scholar] [CrossRef]
- Tessonnier, T.; Mairani, A.; Brons, S.; Haberer, T.; Debus, J.; Parodi, K. Experimental dosimetric comparison of 1H, 4He, 12C and 16O scanned ion beams. Phys. Med. Biol. 2017, 62, 3958. [Google Scholar] [CrossRef]
- Mein, S.; Choi, K.; Kopp, B.; Tessonnier, T.; Bauer, J.; Ferrari, A.; Haberer, T.; Debus, J.; Abdollahi, A.; Mairani, A. Fast robust dose calculation on GPU for high-precision 1H, 4He, 12C and 16O ion therapy: The FRoG platform. Sci. Rep. 2018, 8, 14829. [Google Scholar] [CrossRef]

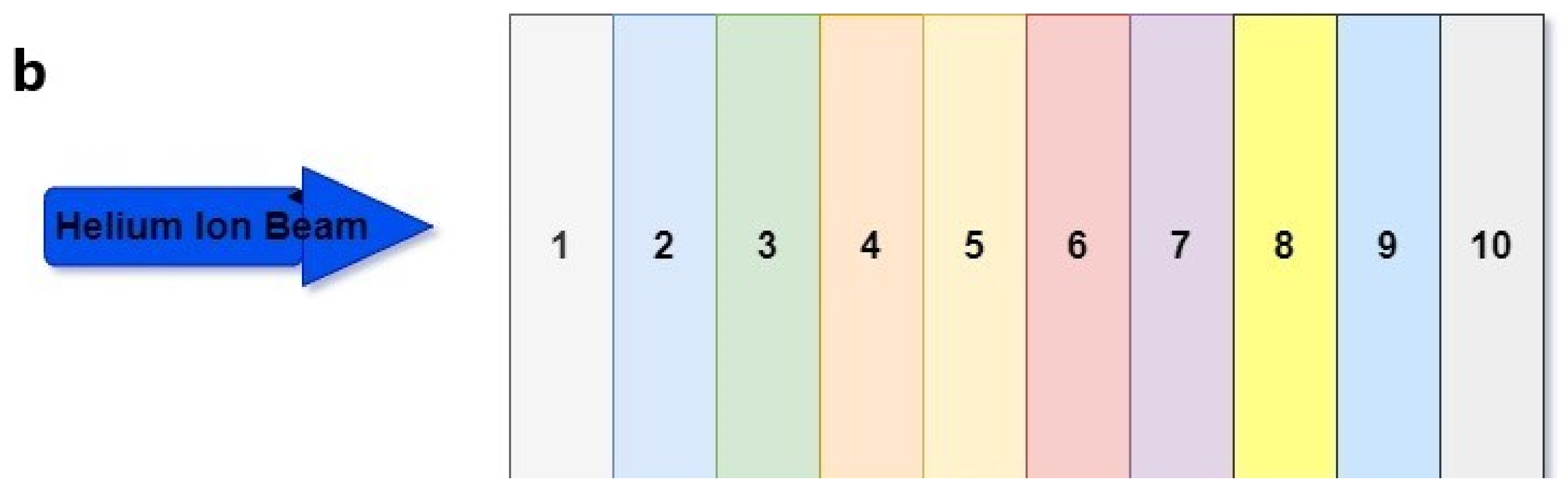
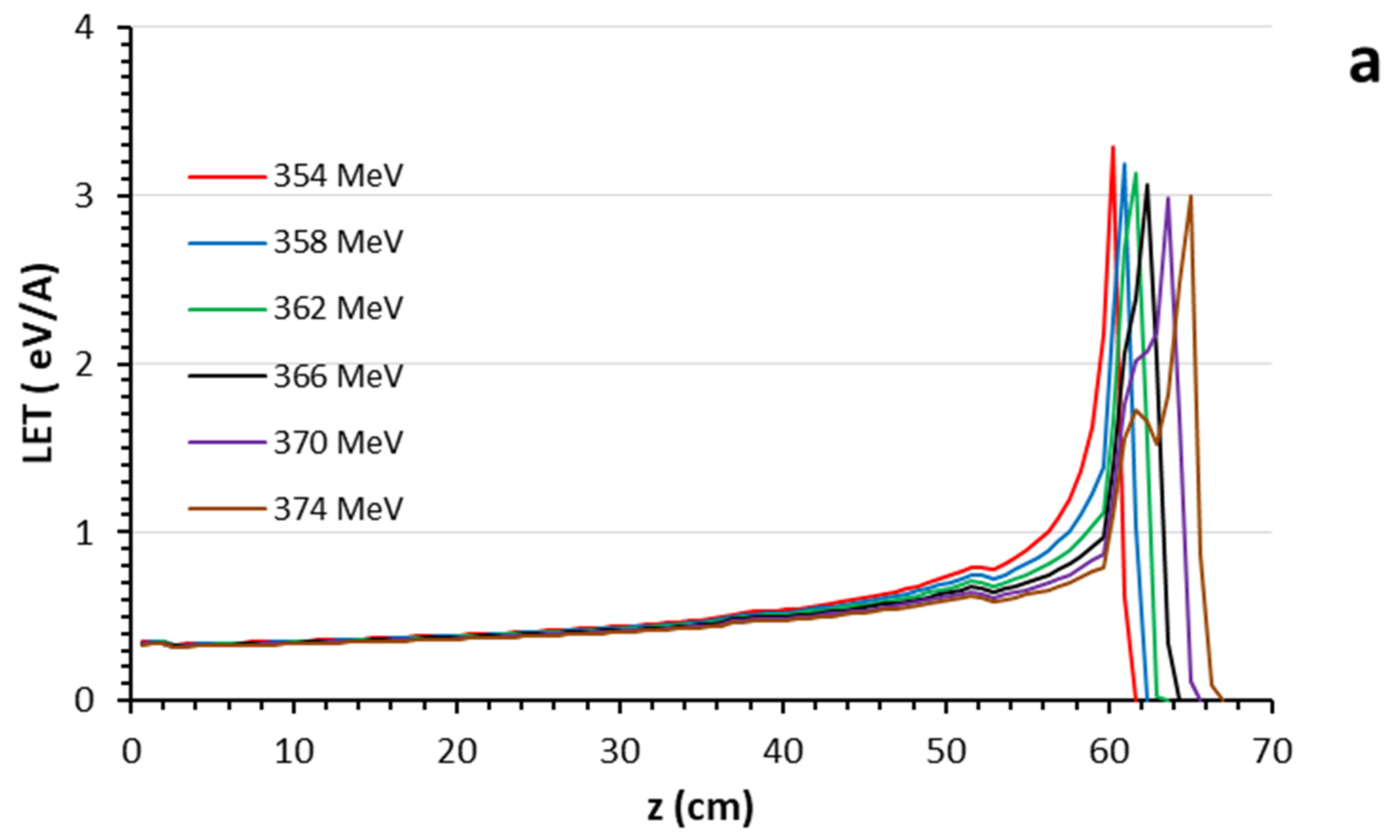
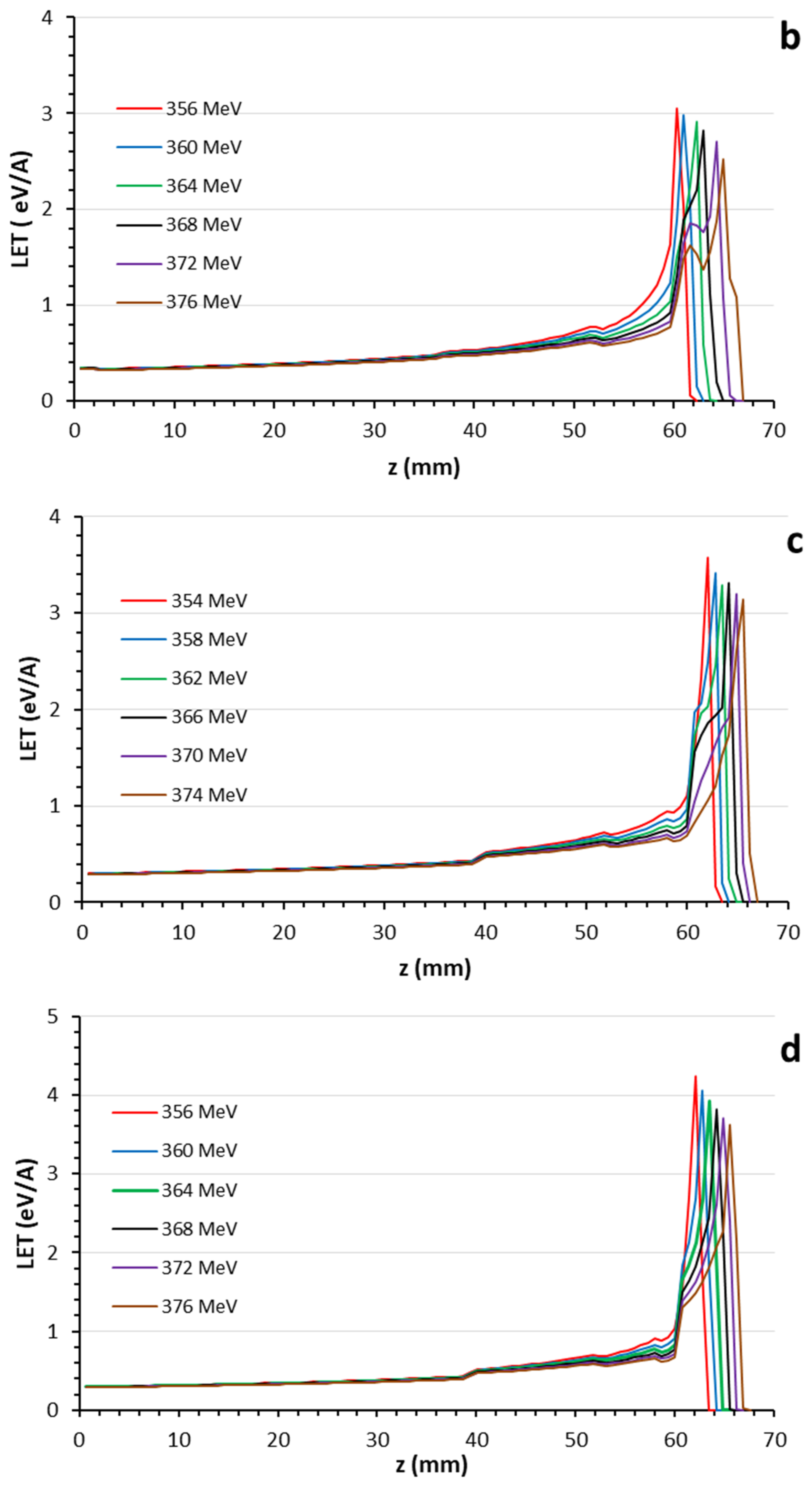
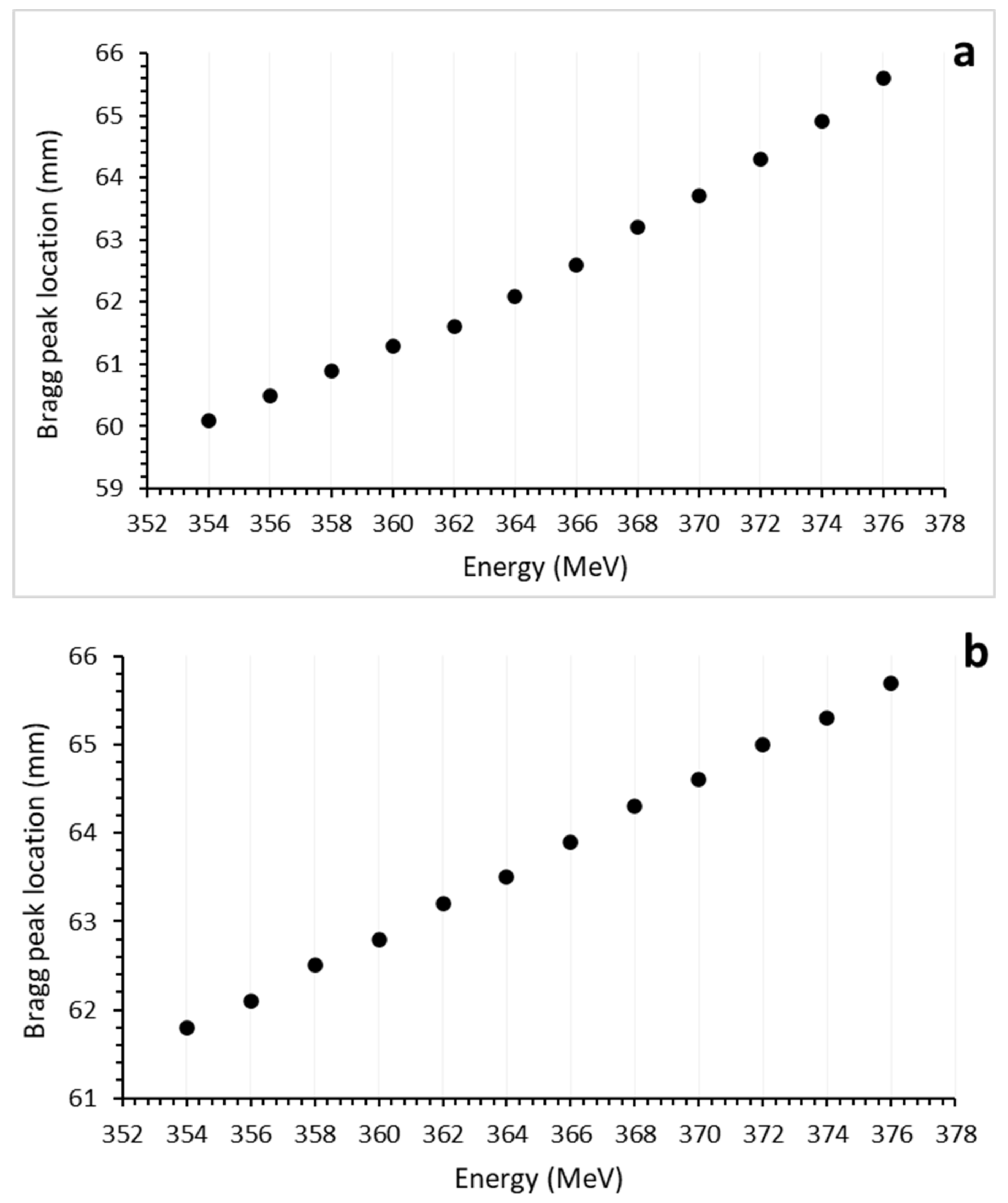
| Layer | Biomaterial | Chemical Composition (%) | Atomic Density (1022 atom/cm3) | Mass Density (g/cm3) |
|---|---|---|---|---|
| 1 | Skin | H:10.0, O:59.4, C:25.0, N:4.6, S:0.3, Cl:0.3, P:10.3, Na:0.2, K:0.1 | 9.88 | 1.02 |
| PMMA | H:53.3, C:33.3, O:13.3 | 8.57 | 0.95 | |
| 2 | Parotid gland | H:62.5, C:16.4, N:1.27, O:19.6, S: 0.037, Cl:0.016, Na:0.025, P:0.019 | 10.32 | 1.02 |
| PMMA | H:53.3, C:33.3, O:13.3 | 8.57 | 0.95 | |
| 3 | SMAS | H:58.3, C:37.4, N:1.45, O:1.89, F:0.532, Ca:0.266 | 10.65 | 1.027 |
| PMMA | H:53.3, C:33.3, O:13.3 | 8.57 | 0.95 | |
| 4 | Masseter muscle | H:52.6, C:8.9, N:1.6, O:26.6, S:5.85, Cl:1.76, K:0.64, P:0.404 | 10.11 | 1.05 |
| Paralene_N | H:50, C:50 | 10.26 | 1.11 | |
| 5 | Buccal Fat | H:63.4, C:28.4, N:0.304, O:7.77, Cl:0.018, Na:0.011 | 10.35 | 0.92 |
| Polyethylene | H:66.6, C:33.4 | 12.23 | 0.95 | |
| 6 | Mucosa | H:10.1, C:77.5, N:3.50, O:5.23, F:1.74, Ca:1.83 | 5.24 | 1.028 |
| PMMA | H:53.3, C:33.3, O:13.3 | 8.57 | 0.95 | |
| 7 | Saliva | H:66.6, O:33.3 | 10.02 | 1 |
| Water | H:66.6, O:33.3 | 10.02 | 1 | |
| 8 | Gum | H:52.6, C:32.9, N:0.862, O:7.89, Cl:1.72, Mg:3.63 | 8.88 | 1 |
| PMMA | H:53.3, C:33.3, O:13.3 | 8.57 | 0.95 | |
| 9 | Cortical bone | H:39.2, C:15.0, N:3.48, O:31.6, S:0.108, P:3.86, Ca:6.53, Mg:9.57 | 9.94 | 1.92 |
| Teflon | C:33.3, F:66.6 | 7.95 | 2.2 | |
| 10 | Cancellous bone | H:57.7, C:23.0, N:1.36, O:15.7, S:4.27, P:0.752, Ca:1.26, Fe:1.23 | 10.42 | 1.18 |
| Teflon | C:33.3, F:66.6 | 7.95 | 2.2 |
| Phantom | Energy | Total Recoil | Contributions to Recoils of Atoms (%) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H | C | N | O | S | Cl | Na | K | P | Mg | Ca | Fe | F | |||
| Tissue | 354 | 2.272 | 23.21 | 26.13 | 3.15 | 26.17 | 0.13 | 0.02 | 0.02 | 0.02 | 7.31 | 3.21 | 10.67 | 0.02 | |
| 356 | 2.521 | 13.28 | 15.38 | 3.21 | 41.98 | 0.21 | 0.02 | 0.02 | 0.02 | 6.62 | 0.11 | 19.18 | 0.02 | ||
| 358 | 2.275 | 14.71 | 16.12 | 3.14 | 39.52 | 0.03 | 0.03 | 0.03 | 0.03 | 8.08 | 0.17 | 18.12 | 0.03 | ||
| 360 | 2.541 | 15.72 | 17.12 | 3.68 | 39.29 | 0.24 | 0.02 | 0.02 | 0.02 | 7.46 | 0.14 | 16.28 | 0.02 | ||
| 362 | 2.332 | 12.51 | 19.51 | 2.78 | 42.32 | 0.33 | 0.05 | 0.05 | 0.05 | 7.15 | 0.09 | 15.19 | 0.05 | ||
| 364 | 2.439 | 18.41 | 27.21 | 2.55 | 31.46 | 0.17 | 0.02 | 0.02 | 0.02 | 6.21 | 0.08 | 13.82 | 0.03 | ||
| 366 | 1.783 | 31.24 | 32.48 | 2.03 | 28.12 | 0.14 | 0.01 | 0.01 | 0.01 | 1.72 | 0.01 | 4.22 | 0.04 | ||
| 368 | 1.730 | 30.25 | 34.28 | 2.64 | 27.12 | 0.01 | 0.01 | 0.01 | 0.01 | 1.54 | 0.01 | 4.11 | 0.04 | ||
| 370 | 1.702 | 26.23 | 33.11 | 6.76 | 24.39 | 0.07 | 0.01 | 0.01 | 0.01 | 5.26 | 0.01 | 4.08 | 0.07 | ||
| 372 | 1.724 | 30.66 | 31.08 | 1.88 | 29.09 | 0.14 | 0.01 | 0.01 | 0.01 | 2.77 | 0.01 | 4.31 | 0.03 | ||
| 374 | 1.890 | 30.48 | 32.22 | 1.68 | 30.42 | 0.14 | 0.01 | 0.01 | 0.01 | 1.62 | 0.01 | 3.32 | 0.13 | ||
| 376 | 0.656 | 47.71 | 22.11 | 1.58 | 20.72 | 0.13 | 0.01 | 0.01 | 0.01 | 1.26 | 0.01 | 6.43 | 0.02 | ||
| Standard deviation | 0.51 | 9.90 | 6.90 | 1.32 | 6.98 | 0.08 | 0.01 | 0.01 | 2.61 | 0.87 | 5.95 | 0.03 | |||
| Biomaterial | 354 | 1.700 | 29.23 | 59.19 | 11.16 | 0.46 | |||||||||
| 356 | 2.516 | 28.81 | 50.52 | 10.24 | 10.42 | ||||||||||
| 358 | 1.927 | 24.82 | 45.24 | 17.32 | 12.62 | ||||||||||
| 360 | 1.831 | 20.72 | 44.62 | 10.34 | 24.32 | ||||||||||
| 362 | 2.995 | 25.24 | 42.12 | 6.86 | 25.78 | ||||||||||
| 364 | 1.859 | 24.22 | 48.32 | 6.14 | 21.32 | ||||||||||
| 366 | 2.675 | 22.16 | 39.46 | 16.16 | 22.22 | ||||||||||
| 368 | 1.829 | 19.14 | 46.22 | 9.12 | 25.52 | ||||||||||
| 370 | 2.501 | 21.42 | 42.24 | 14.86 | 21.52 | ||||||||||
| 372 | 2.056 | 18.48 | 44.72 | 12.32 | 24.48 | ||||||||||
| 374 | 2.725 | 13.97 | 52.32 | 17.42 | 16.28 | ||||||||||
| 376 | 1.979 | 32.52 | 40.24 | 7.91 | 19.32 | ||||||||||
| Standard deviation | 4.97 | 5.39 | 3.81 | 7.26 | |||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ekinci, F.; Acici, K.; Asuroglu, T.; Emek Soylu, B. MC TRIM Algorithm in Mandibula Phantom in Helium Therapy. Healthcare 2023, 11, 2523. https://doi.org/10.3390/healthcare11182523
Ekinci F, Acici K, Asuroglu T, Emek Soylu B. MC TRIM Algorithm in Mandibula Phantom in Helium Therapy. Healthcare. 2023; 11(18):2523. https://doi.org/10.3390/healthcare11182523
Chicago/Turabian StyleEkinci, Fatih, Koray Acici, Tunc Asuroglu, and Busra Emek Soylu. 2023. "MC TRIM Algorithm in Mandibula Phantom in Helium Therapy" Healthcare 11, no. 18: 2523. https://doi.org/10.3390/healthcare11182523
APA StyleEkinci, F., Acici, K., Asuroglu, T., & Emek Soylu, B. (2023). MC TRIM Algorithm in Mandibula Phantom in Helium Therapy. Healthcare, 11(18), 2523. https://doi.org/10.3390/healthcare11182523






