Serum Amino Acid Profiling in Children with Autistic Spectrum Disorder: Insights from a Single-Center Study in Southern Romania
Abstract
:1. Introduction
2. Materials and Methods
2.1. Informed Consent
2.2. Study Groups
2.3. Screening
2.4. Collection and Testing of Laboratory Samples
2.5. Statistical Analysis
3. Results
3.1. Study Cohort: Individuals with Autism Spectrum Disorder (ASD) and Control Group Descriptive Data
3.1.1. Gender and Age Group Distribution
3.1.2. Environmental Distribution
3.1.3. Dietary Patterns and Nutritional Status
3.2. The Results for the Screening Questionnaire Social Communication Questionnaire (SCQ)
3.3. Results for the Serum Amino Acid Profile
3.3.1. Analysis of Amino Acids from the Study Group in Comparison to the Control Group
3.3.2. Analysis of Amino Acids Based on Gender in the Study Group Compared to the Control Group
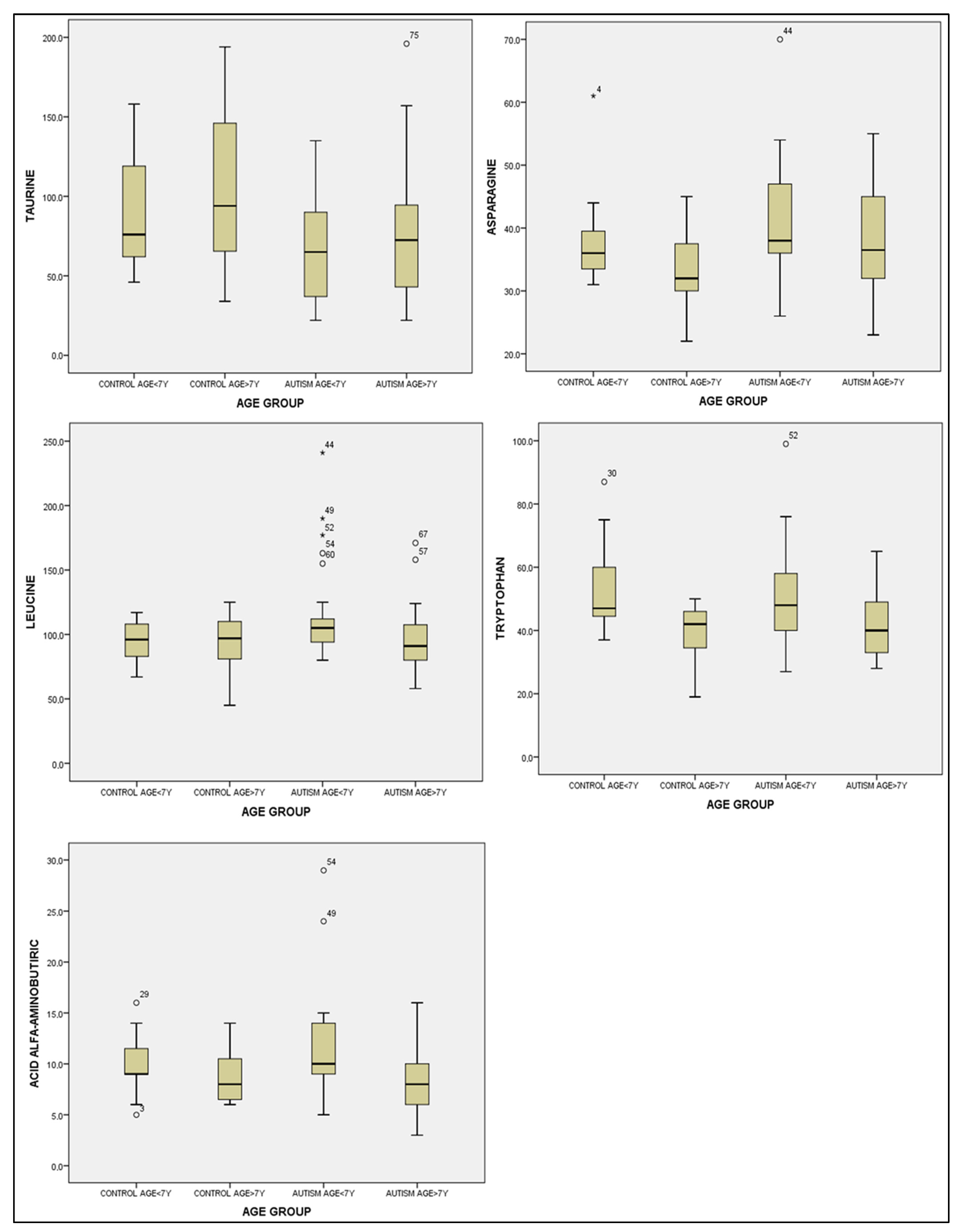
3.3.3. Statistical Analysis of Amino Acid Concentrations According to Age Groups
4. Discussion
4.1. Taurine’s Potential Role in Neurodevelopment
4.2. Gender and Amino Acid Levels
4.3. Age-Related Insights
4.4. Alpha-Aminobutyric Acid and Dietary Influences
4.5. Taurine and Amino Acid Imbalances
4.6. Asparagine and Future Directions
5. Limitations of this Study
6. Specific Next Steps
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Higher Autism Prevalence and COVID-19 Disruptions. Available online: https://www.cdc.gov/ncbddd/autism/features/new-autism-spectrum-disorder-report.html (accessed on 3 August 2023).
- American Psychiatric Association. Manual de Diagnostic şi Clasificare Statistică a Tulburărilor Mintale, 5th ed.; Medicală Cal-listo: București, Romania, 2016. [Google Scholar]
- Smith, A.M.; King, J.J.; West, P.R.; Ludwig, M.A.; Donley, E.L.; Burrier, R.E.; Amaral, D.G. Amino Acid Dysregulation Metabotypes: Potential Biomarkers for Diagnosis and Individualized Treatment for Subtypes of Autism Spectrum Disorder. Biol. Psychiatry 2018, 85, 345–354. [Google Scholar] [CrossRef]
- Hodges, H.; Fealko, C.; Soares, N. Autism spectrum disorder: Definition, epidemiology, causes, and clinical evaluation. Transl. Pediatr. 2020, 9, S55–S65. [Google Scholar] [CrossRef] [PubMed]
- Randazzo, M.; Prato, A.; Messina, M.; Meli, C.; Casabona, A.; Rizzo, R.; Barone, R. Neuroactive Amino Acid Profile in Autism Spectrum Disorder: Results from a Clinical Sample. Children 2023, 10, 412. [Google Scholar] [CrossRef] [PubMed]
- McPartland, J.C. Developing Clinically Practicable Biomarkers for Autism Spectrum Disorder. J. Autism Dev. Disord. 2017, 47, 2935–2937. [Google Scholar] [CrossRef] [PubMed]
- Klin, A. Biomarkers in Autism Spectrum Disorder: Challenges, Advances, and the Need for Biomarkers of Relevance to Public Health. Focus Am. Psychiatry Publ. 2018, 16, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Aldred, S.; Moore, K.M.; Fitzgerald, M.; Waring, R.H. Plasma amino acid levels in children with autism and their families. J. Autism Dev. Disord. 2003, 33, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-X.; Chen, Y.-R.; Peng, M.-Z.; Liu, X.; Cai, Y.-N.; Huang, Z.-F.; Yang, S.-Y.; Huang, J.-Y.; Wang, R.-H.; Yi, P.; et al. Plasma Amino Acid Profile in Children with Autism Spectrum Disorder in Southern China: Analysis of 110 Cases. J. Autism Dev. Disord. 2023; ahead of print. [Google Scholar] [CrossRef]
- Saleem, T.H.; Shehata, G.A.; Toghan, R.; Sakhr, H.M.; Bakri, A.H.; Desoky, T.; Hamdan, F.R.A.; Mohamed, N.F.; Hassan, M.H. Assessments of Amino Acids, Ammonia and Oxidative Stress Among Cohort of Egyptian Autistic Children: Correlations with Electroencephalogram and Disease Severity. Neuropsychiatr. Dis. Treat 2020, 16, 11–24. [Google Scholar] [CrossRef]
- Cai, J.; Ding, L.; Zhang, J.-S.; Xue, J.; Wang, L.-Z. Elevated plasma levels of glutamate in children with autism spectrum dis-orders. Neuroreport 2016, 27, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Patri, M. Synaptic Transmission and Amino Acid Neurotransmitters. In Neurochemical Basis of Brain Function and Dysfunction; IntechOpen: Rijeka, Croatia, 2019. [Google Scholar] [CrossRef]
- Dalangin, R.; Kim, A.; Campbell, R.E. The Role of Amino Acids in Neurotransmission and Fluorescent Tools for Their Detection. Int. J. Mol. Sci. 2020, 21, 6197. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.F.; Wang, W.Q.; Li, X.M.; Rauw, G.; Baker, G.B. Body fluid levels of neuroactive amino acids in autism spectrum disorders: A review of the literature. Amino Acids 2017, 49, 57–65. [Google Scholar] [CrossRef]
- Nisar, S.; Bhat, A.A.; Masoodi, T.; Hashem, S.; Akhtar, S.; Ali, T.A.; Amjad, S.; Chawla, S.; Bagga, P.; Frenneaux, M.P.; et al. Genetics of glutamate and its receptors in autism spectrum disorder. Mol. Psychiatry 2022, 27, 2380–2392. [Google Scholar] [CrossRef] [PubMed]
- Rutter, M.; Bailey, A.; Lord, C. (SCQ) The Social Communication Questionnaire Manual; Western Psychological Services: Los Angeles, CA, USA, 2003. [Google Scholar]
- Rubio-Casillas, A.; Redwan, E.M.; Uversky, V.N. On the Potential Therapeutic Roles of Taurine in Autism Spectrum Disorder. Neuroglia 2023, 4, 1–14. [Google Scholar] [CrossRef]
- Chen, C.; Xia, S.; He, J.; Lu, G.; Xie, Z.; Han, H. Roles of taurine in cognitive function of physiology, pathologies and toxication. Life Sci. 2019, 231, 116584. [Google Scholar] [CrossRef]
- Birdsall, T.C. Therapeutic applications of taurine. Altern. Med. Rev. 1998, 3, 128–136. [Google Scholar]
- Duszka, K. Versatile Triad Alliance: Bile Acid, Taurine and Microbiota. Cells 2022, 11, 2337. [Google Scholar] [CrossRef]
- Tochitani, S.; Furukawa, T.; Bando, R.; Kondo, S.; Ito, T.; Matsushima, Y.; Kojima, T.; Matsuzaki, H.; Fukuda, A. GABAA Receptors and Maternally Derived Taurine Regulate the Temporal Specification of Progenitors of Excitatory Glutamatergic Neurons in the Mouse Developing Cortex. Cereb. Cortex 2021, 31, 4554–4575. [Google Scholar] [PubMed]
- Bugajska, J.; Berska, J.; Wojtyto, T.; Bik-Multanowski, M.; Sztefko, K. The amino acid profile in blood plasma of young boys with autism. Psychiatr. Pol. 2017, 51, 359–368. [Google Scholar] [CrossRef]
- Naushad, S.M.; Jain, J.M.; Prasad, C.K.; Naik, U.; Akella, R.R. Autistic children exhibit distinct plasma amino acid profile. Indian J. Biochem. Biophys. 2013, 50, 474–478. [Google Scholar]
- Ghanizadeh, A. Increased Glutamate and Homocysteine and Decreased Glutamine Levels in Autism: A Review and Strategies for Future Studies of Amino Acids in Autism. Dis. Markers 2013, 35, 281–286. [Google Scholar] [CrossRef]
- Kałużna-Czaplińska, J.; Jóźwik-Pruska, J.; Chirumbolo, S.; Bjørklund, G. Tryptophan status in autism spectrum disorder and the influence of supplementation on its level. Metab. Brain Dis. 2017, 32, 1585–1593. [Google Scholar] [CrossRef]
- Lopez, M.J.; Mohiuddin, S.S. Biochemistry, Essential Amino Acids; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Park, E.; Cohen, I.; Gonzalez, M.; Castellano, M.R.; Flory, M.; Jenkins, E.C.; Brown, W.T.; Schuller-Levis, G. Is Taurine a Bi-omarker in Autistic Spectrum Disorder? Adv. Exp. Med. Biol. 2017, 975, 3–16. [Google Scholar] [PubMed]
- Zaki, M.M.; Abdel-Al, H.; Al-Sawi, M. Assessment of plasma amino acid profile in autism using cation-exchange chromatog-raphy with postcolumn derivatization by ninhydrin. Turk. J. Med. Sci. 2017, 47, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Rizzari, C.; Lanvers-Kaminsky, C.; Valsecchi, M.G.; Ballerini, A.; Matteo, C.; Gerss, J.; Wuerthwein, G.; Silvestri, D.; Colombini, A.; Conter, V.; et al. Asparagine levels in the cerebrospinal fluid of children with acute lymphoblastic leukemia treated with pegylated-asparaginase in the induction phase of the AIEOP-BFM ALL 2009 study. Haematologica 2019, 104, 1812–1821. [Google Scholar] [CrossRef]
- Krall, A.S.; Xu, S.; Graeber, T.G.; Braas, D.; Christofk, H.R. Asparagine promotes cancer cell proliferation through use as an amino acid exchange factor. Nat. Commun. 2016, 7, 11457. [Google Scholar] [CrossRef] [PubMed]
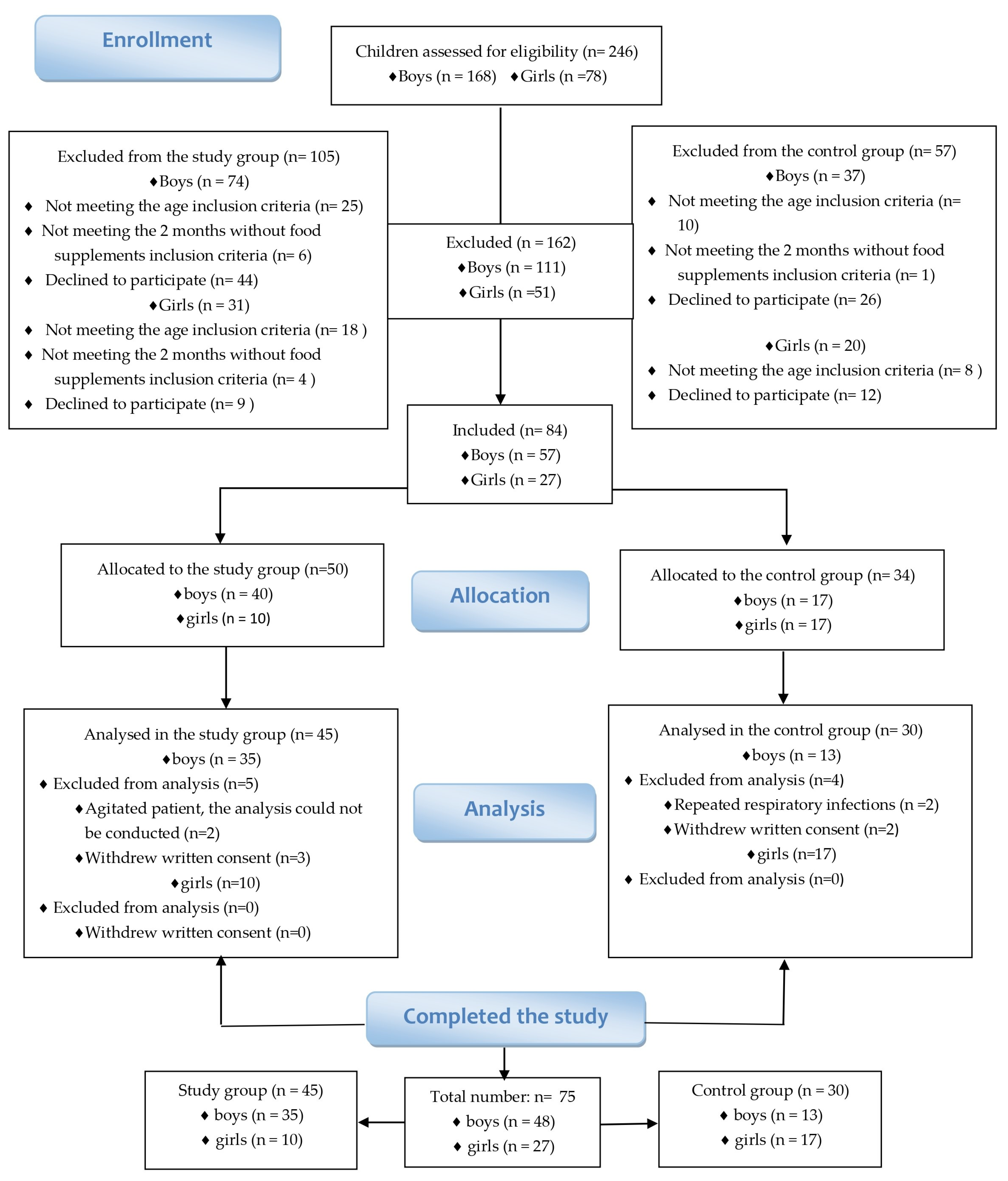

| Demographic Data Distribution | Study Group | Control Group | |
|---|---|---|---|
| Gender distribution | Boys | 35 | 13 |
| Girls | 10 | 17 | |
| Age group distribution | 2–6 years | 25 | 15 |
| 7–12 years | 20 | 15 | |
| Environment distribution | Urban | 35 | 26 |
| Rural | 10 | 4 | |
| Dietary types distribution | Restrictive diet | 35 | 0 |
| Diversified diet | 10 | 30 | |
| The nutritional status | Underweight | 18 | 6 |
| Normal weight | 20 | 22 | |
| Overweight | 5 | 2 | |
| Obese | 2 | 0 |
| SCQ Domains | Mean Score | Standard Deviation | p-Value (Kolmogorov—Smirnov Test *) |
|---|---|---|---|
| Social interaction | 6.73 | 2.81 | 0.052 |
| Communication | 5.78 | 1.78 | 0.000 |
| Restrictive and repetitive behavioral patterns | 5.44 | 1.68 | 0.008 |
| Amino Acids | Autism Group n = 45 | Control Group n = 30 | t-Test * | |
|---|---|---|---|---|
| Mean ± Standard Deviation (μmol/L) | Mean ± Standard Deviation (μmol/L) | t-Value | p-Value | |
| alpha-aminobutyric acid | 10.15 ± 4.86 | 9.43 ± 2.83 | 0.810 | 0.420 |
| arginine | 67.73 ± 17.33 | 67.40 ± 17.16 | −0.082 | 0.935 |
| asparagine | 39.75 ± 9.08 | 35.40 ± 7.10 | −2.323 | 0.023 |
| aspartic acid | 10.42 ± 3.75 | 9.63 ± 2.80 | −1.039 | 0.302 |
| citrulline | 34.64 ± 27.92 | 28.76 ± 8.80 | −1.317 | 0.193 |
| cysteine | 17.20 ± 11.91 | 22.03 ± 13.52 | −1.630 | 0.107 |
| glycine | 200.97 ± 41.03 | 195.90 ± 46.89 | −0.496 | 0.622 |
| glutamine | 632.75 ± 136.09 | 675.46 ± 124.15 | −1.404 | 0.165 |
| histidine | 68.06 ± 15.78 | 65.43 ± 15.44 | −0.714 | 0.477 |
| hydroxyproline | 14.71 ± 5.84 | 14.96 ± 4.18 | −0.221 | 0.826 |
| isoleucine | 54.911 ± 17.75 | 50.167 ± 9.68 | −1.491 | 0.140 |
| leucine | 108.71 ± 35.21 | 94.40 ± 19.20 | −2.267 | 0.026 |
| lysine | 132.84 ± 37.09 | 128.66 ± 30.65 | −0.531 | 0.597 |
| methionine | 26.667 ± 9.38 | 24.800 ± 6.46 | −1.019 | 0.311 |
| ornithine | 49.11 ± 20.10 | 52.00 ± 20.36 | −0.600 | 0.546 |
| phenylalanine | 46.467 ± 13.15 | 42.433 ± 9.40 | −1.547 | 0.126 |
| proline | 152.35 ± 60.85 | 147.66 ± 61.32 | −0.325 | 0.746 |
| serine | 115.82 ± 31.55 | 103.80 ± 31.87 | −1.607 | 0.113 |
| taurine | 72.08 ± 37.75 | 100.16 ± 45.27 | −2.912 | 0.005 |
| threonine | 114.75 ± 39.21 | 104.30 ± 30.91 | −1.287 | 0.202 |
| tyrosine | 58.867 ± 20.00 | 55.267 ± 16.72 | −0.843 | 0.402 |
| valine | 195.33 ± 63.28 | 176.43 ± 48.35 | −1.463 | 0.148 |
| Amino Acids | Autism Group n = 45 Mean ± Standard Deviation | Control Group n = 30 Mean ± Standard Deviation | p-Value | ||
|---|---|---|---|---|---|
| Male (n = 35) | Female (n = 10) | Male (n = 13) | Female (n = 17) | ||
| alpha-aminobutyric acid | 9.88 ± 4.49 | 11.10 ± 6.17 | 8.46 ± 2.50 | 10.17 ± 2.92 | 0.492 |
| arginine | 64.42 ± 17.15 | 65.30 ± 18.64 | 69.92 ± 17.12 | 65.47 ± 17.46 | 1 |
| asparagine | 39.77 ± 8.56 | 39.70 ± 9.26 | 35.15 ± 1.51 | 35.58 ± 8.29 | 1 |
| aspartic acid | 10.28 ± 3.41 | 10.90 ± 4.62 | 9.84 ± 2.85 | 9.47 ± 2.85 | 0.736 |
| citrulline | 36.31 ± 30.96 | 28.80 ± 11.89 | 29.61 ± 6.87 | 28.11 ± 10.20 | 0.551 |
| cystine | 15.77 ± 11.31 | 22.20 ± 13.20 | 24.07 ± 13.73 | 20.47 ± 13.56 | 1 |
| glutamine | 637.17 ± 139.81 | 617.30 ± 130.47 | 653.41 ± 96.86 | 692.41 ± 142.12 | 0.448 |
| glycine | 206.08 ± 42.37 | 183.10 ± 31.49 | 199.38 ± 55.22 | 193.23 ± 41.00 | 0.466 |
| histidine | 68.42 ± 15.10 | 66.80 ± 18.80 | 65.76 ± 12.63 | 65.17 ± 17.67 | 1 |
| hydroxyproline | 14.80 ± 6.13 | 14.40 ± 4.94 | 15.30 ± 3.90 | 14.70 ± 4.96 | 0.981 |
| isoleucine | 56.25 ± 18.22 | 50.20 ± 15.93 | 50.23 ± 6.27 | 50.11 ± 11.84 | 0.397 |
| leucine | 111.05 ± 35.90 | 100.50 ± 33.07 | 95.38 ± 15.81 | 93.64 ± 21.89 | 0.178 |
| lisine | 136.68 ± 38.91 | 119.40 ± 27.34 | 125.61 ± 31.75 | 131.00 ± 30.55 | 1 |
| methionine | 27.88 ± 10.04 | 22.40 ± 4.90 | 24.53 ± 5.37 | 25.00 ± 7.34 | 0.234 |
| methylhistidine | 2.22 ± 1.11 | 2.00 ± 1.76 | 2.23 ± 0.83 | 2.05 ± 0.89 | 1 |
| ornithine | 49.62 ± 19.35 | 47.30 ± 23.57 | 53.30 ± 26.34 | 51.00 ± 15.11 | 1 |
| phenylalanine | 47.05 ± 13.90 | 44.40 ± 10.50 | 42.84 ± 8.33 | 42.11 ± 10.39 | 0.486 |
| proline | 156.34 ± 65.93 | 138.40 ± 37.44 | 146.23 ± 46.68 | 148.76 ± 71.96 | 0.855 |
| serine | 117.34 ± 34.10 | 110.50 ± 20.79 | 105.84 ± 24.50 | 102.23 ± 37.22 | 0.400 |
| taurine | 75.51 ± 35.99 | 60.10 ± 43.21 | 109.15 ± 50.57 | 93.29 ± 41.00 | 0.034 |
| threonina | 119.60 ± 41.91 | 97.80 ± 21.64 | 96.46 ± 28.08 | 110.29 ± 32.45 | 0.145 |
| tyrosine | 60.94 ± 21.14 | 51.60 ± 13.82 | 51.84 ± 13.45 | 57.88 ± 18.82 | 0.344 |
| valine | 196.91 ± 62.49 | 189.80 ± 69.14 | 176.84 ± 29.27 | 176.11 ± 59.96 | 0.577 |
| Amino Acids | Autism Group n = 45 Mean ± Standard Deviation | Control Group n = 30 Mean ± Standard Deviation | ||||
|---|---|---|---|---|---|---|
| Age <7 Ani (n = 25) | Age >7 Ani (n = 20) | Age <7 Ani (n = 15) | Age >7 Ani (n = 15) | f(3) | p-Value | |
| alpha-aminobutyric acid | 11.76 ± 5.40 | 8.15 ± 3.21 | 10.06 ± 2.89 | 8.80 ± 2.73 | 3.517 | 0.019 |
| asparagine | 40.76 ± 9.54 | 38.50 ± 8.53 | 37.80 ± 7.55 | 33.00 ± 5.90 | 2.780 | 0.047 |
| arginine | 64.84 ± 17.08 | 71.35 ± 71.35 | 69.66 ± 17.72 | 65.66 ± 16.88 | 0.70 | 0.554 |
| aspartic acid | 11.40 ± 4.39 | 9.20 ± 2.33 | 10.40 ± 2.19 | 8.86 ± 3.20 | 5.86 | 0.119 |
| alanine | 286.04 ± 105.22 | 294.05 ± 76.80 | 294.20 ± 101.58 | 282.00 ± 117.69 | 0.62 | 0.980 |
| betha-alanine | 3.80 ± 1.35 | 3.20 ± 1.10 | 3.40 ± 0.91 | 3.40 ± 1.12 | 1.922 | 0.589 |
| citrulline | 39.68 ± 36.28 | 28.35 ± 8.27 | 32.13 ± 10.33 | 25.40 ± 5.43 | 1.624 | 0.191 |
| cysteine | 14.44 ± 10.32 | 20.65 ± 13.09 | 17.33 ± 12.77 | 26.73 ± 12.98 | 3.420 | 0.022 |
| glutamic acid | 21.72 ± 10.42 | 18.20 ± 7.14 | 27.86 ± 24.88 | 17.66 ± 8.05 | 1.87 | 0.142 |
| glutamine | 621.20 ± 165.67 | 647.200 ± 88.46 | 698.66 ± 140.66 | 652.26 ± 104.83 | 1.080 | 0.363 |
| glycine | 202.24 ± 45.98 | 199.40 ± 34.97 | 203.33 ± 37.54 | 188.46 ± 55.02 | 0.384 | 0.764 |
| histidine | 71.24 ± 18.34 | 64.10 ± 11.02 | 70.66 ± 14.66 | 60.20 ± 14.84 | 2.172 | 0.099 |
| hydroxyproline | 15.32 ± 6.22 | 13.95 ± 5.38 | 15.66 ± 4.16 | 14.26 ± 4.23 | 0.440 | 0.725 |
| isoleucine | 58.56 ± 20.02 | 50.35 ± 13.56 | 51.00 ± 8.06 | 49.33 ± 11.29 | 1.758 | 0.163 |
| leucine | 116.72 ± 38.95 | 98.70 ± 27.65 | 94.66 ± 16.19 | 94.13 ± 22.39 | 5.333 | 0.149 |
| lysine | 133.20 ± 44.07 | 132.40 ± 27.06 | 134.66 ± 27.41 | 122.66 ± 33.43 | 0.382 | 0.766 |
| methionine | 27.24 ± 10.60 | 25.95 ± 7.81 | 26.40 ± 6.62 | 23.20 ± 6.10 | 0.749 | 0.527 |
| methylhystidine | 2.32 ± 1.37 | 2.00 ± 1.12 | 1.80 ± 0.67 | 2.46 ± 0.91 | 4.007 | 0.261 |
| ornithine | 43.44 ± 17.81 | 56.20 ± 20.97 | 55.93 ± 19.90 | 48.06 ± 20.72 | 2.083 | 0.110 |
| phenylalanine | 49.48 ± 15.43 | 42.70 ± 8.56 | 43.46 ± 8.33 | 41.40 ± 10.56 | 3.967 | 0.265 |
| proline | 162.44 ± 62.61 | 139.75 ± 57.63 | 160.46 ± 75.60 | 134.86 ± 41.51 | 0.999 | 0.399 |
| sarcosine | 1.68 ± 0.69 | 1.20 ± 0.41 | 1.60 ± 0.73 | 1.33 ± 0.48 | 7.435 | 0.059 |
| serine | 118.64 ± 29.30 | 112.30 ± 34.60 | 110.20 ± 38.84 | 97.40 ± 22.54 | 1.414 | 0.246 |
| threonine | 117.36 ± 40.38 | 111.50 ± 38.47 | 108.93 ± 28.19 | 99.66 ± 33.74 | 0.751 | 0.525 |
| taurine | 67.12 ± 32.68 | 78.30 ± 43.32 | 91.86 ± 37.65 | 108.46 ± 51.77 | 3.518 | 0.019 |
| tryptophan | 51.04 ± 16.23 | 42.40 ± 11.22 | 53.53 ± 13.59 | 39.13 ± 9.21 | 4.542 | 0.006 |
| tyrosine | 57.84 ± 21.19 | 60.15 ± 18.86 | 59.60 ± 16.40 | 50.93 ± 16.43 | 0.807 | 0.494 |
| valine | 213.96 ± 71.41 | 172.05 ± 42.49 | 180.33 ± 52.13 | 172.53 ± 45.74 | 7.647 | 0.054 |
| Taurine | ||||||
|---|---|---|---|---|---|---|
| (I) Age Group | (J) Age Group | Mean Difference (I-J) | Std. Error | Sig. | 95% Confidence Interval | |
| Lower Bound | Upper Bound | |||||
| Control age < 7 years | Control age > 7 years | −16.600 | 14.930 | 1.000 | −57.123 | 23.923 |
| Autism age < 7 years | 24.746 | 13.354 | 0.408 | −11.499 | 60.992 | |
| Autism age > 7 years | 13.566 | 13.965 | 1.000 | −24.340 | 51.473 | |
| Control age > 7 years | Control age < 7 years | 16.600 | 14.930 | 1.000 | −23.923 | 57.123 |
| Autism age < 7 years | 41.346 * | 13.354 | 0.017 | 5.101 | 77.592 | |
| Autism age > 7 years | 30.166 | 13.965 | 0.205 | −7.740 | 68.073 | |
| Autism age < 7 years | Control age < 7 years | −24.746 | 13.354 | 0.408 | −60.992 | 11.499 |
| Control age > 7 years | −41.346 * | 13.354 | 0.017 | −77.592 | −5.101 | |
| Autism age > 7 years | −11.180 | 12.266 | 1.000 | −44.473 | 22.113 | |
| Autism age > 7 years | Control age < 7 years | −13.566 | 13.965 | 1.000 | −51.473 | 24.340 |
| Control age > 7 years | −30.166 | 13.965 | 0.205 | −68.073 | 7.740 | |
| Autism age < 7 years | 11.180 | 12.266 | 1.000 | −22.113 | 44.473 | |
| Asparagine | ||||||
| Control age < 7 years | Control age > 7 years | 4.800 | 3.021 | 0.700 | −3.401 | 13.001 |
| Autism age < 7 years | −2.960 | 2.702 | 1.000 | −10.296 | 4.376 | |
| Autism age > 7 years | −0.700 | 2.826 | 1.000 | −8.372 | 6.972 | |
| Control age > 7 years | Control age < 7 years | −4.800 | 3.021 | 0.700 | −13.001 | 3.401 |
| Autism age < 7 years | −7.760 * | 2.702 | 0.032 | −15.096 | −.424 | |
| Autism age > 7 years | −5.500 | 2.826 | 0.334 | −13.172 | 2.172 | |
| Autism age < 7 years | Control age < 7 years | 2.960 | 2.702 | 1.000 | −4.376 | 10.296 |
| Control age > 7 years | 7.760 * | 2.702 | 0.032 | 0.424 | 15.096 | |
| Autism age > 7 years | 2.260 | 2.482 | 1.000 | −4.478 | 8.998 | |
| Autism age > 7 years | Control age < 7 years | 0.700 | 2.826 | 1.000 | −6.972 | 8.372 |
| Control age > 7 years | 5.500 | 2.826 | .334 | −2.172 | 13.172 | |
| Autism age < 7 years | −2.260 | 2.482 | 1.000 | −8.998 | 4.478 | |
| Tryptophan | ||||||
| Control age < 7 years | Control age > 7 years | 14.400 * | 4.844 | 0.024 | 1.251 | 27.549 |
| Autism age < 7 years | 2.493 | 4.333 | 1.000 | −9.267 | 14.254 | |
| Autism age > 7 years | 11.133 | 4.531 | 0.099 | −1.166 | 23.433 | |
| Control age > 7 years | Control age < 7 years | −14.400 * | 4.844 | 0.024 | −27.549 | −1.251 |
| Autism age < 7 years | −11.906 * | 4.333 | 0.046 | −23.667 | −0.146 | |
| Autism age > 7 years | −3.266 | 4.531 | 1.000 | −15.566 | 9.033 | |
| Autism age < 7 years | Control age < 7 years | −2.493 | 4.333 | 1.000 | −14.254 | 9.267 |
| Control age > 7 years | 11.906 * | 4.333 | 0.046 | 0.146 | 23.667 | |
| Autism age > 7 years | 8.640 | 3.980 | 0.200 | −2.163 | 19.443 | |
| Autism age > 7 years | Control age < 7 years | −11.133 | 4.531 | 0.099 | −23.433 | 1.166 |
| Control age > 7 years | 3.266 | 4.531 | 1.000 | −9.033 | 15.566 | |
| Autism age < 7 years | −8.640 | 3.980 | 0.200 | −19.443 | 2.163 | |
| Alpha-amino | butiric | acid | ||||
| Control age < 7 years | Control age > 7 years | 1.266 | 1.449 | 1.000 | −2.667 | 5.200 |
| Autism age < 7 years | −1.693 | 1.296 | 1.000 | −5.212 | 1.825 | |
| Autism age > 7 years | 1.916 | 1.355 | 0.971 | −1.763 | 5.596 | |
| Control age > 7 years | Control age < 7 years | −1.266 | 1.449 | 1.000 | −5.200 | 2.667 |
| Autism age < 7 years | −2.960 | 1.296 | 0.152 | −6.478 | 0.558 | |
| Autism age > 7 years | 0.650 | 1.355 | 1.000 | −3.030 | 4.330 | |
| Autism age < 7 years | Control age < 7 years | 1.693 | 1.296 | 1.000 | −1.825 | 5.212 |
| Control age > 7 years | 2.960 | 1.296 | 0.152 | −0.558 | 6.478 | |
| Autism age > 7 years | 3.610 * | 1.190 | 0.020 | 0.378 | 6.842 | |
| Autism age > 7 years | Control age < 7 years | −1.916 | 1.355 | 0.971 | −5.596 | 1.763 |
| Control age > 7 years | −0.650 | 1.355 | 1.000 | −4.330 | 3.030 | |
| Autism age < 7 years | −3.610 * | 1.190 | 0.020 | −6.842 | −0.378 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anastasescu, C.M.; Gheorman, V.; Popescu, F.; Stoicănescu, E.-C.; Gheorman, V.; Riza, A.-L.; Badea, O.; Streață, I.; Militaru, F.; Udriștoiu, I. Serum Amino Acid Profiling in Children with Autistic Spectrum Disorder: Insights from a Single-Center Study in Southern Romania. Healthcare 2023, 11, 2487. https://doi.org/10.3390/healthcare11182487
Anastasescu CM, Gheorman V, Popescu F, Stoicănescu E-C, Gheorman V, Riza A-L, Badea O, Streață I, Militaru F, Udriștoiu I. Serum Amino Acid Profiling in Children with Autistic Spectrum Disorder: Insights from a Single-Center Study in Southern Romania. Healthcare. 2023; 11(18):2487. https://doi.org/10.3390/healthcare11182487
Chicago/Turabian StyleAnastasescu, Cătălina Mihaela, Veronica Gheorman, Florica Popescu, Eugen-Cristi Stoicănescu, Victor Gheorman, Anca-Lelia Riza, Oana Badea, Ioana Streață, Felicia Militaru, and Ion Udriștoiu. 2023. "Serum Amino Acid Profiling in Children with Autistic Spectrum Disorder: Insights from a Single-Center Study in Southern Romania" Healthcare 11, no. 18: 2487. https://doi.org/10.3390/healthcare11182487
APA StyleAnastasescu, C. M., Gheorman, V., Popescu, F., Stoicănescu, E.-C., Gheorman, V., Riza, A.-L., Badea, O., Streață, I., Militaru, F., & Udriștoiu, I. (2023). Serum Amino Acid Profiling in Children with Autistic Spectrum Disorder: Insights from a Single-Center Study in Southern Romania. Healthcare, 11(18), 2487. https://doi.org/10.3390/healthcare11182487





