Potential Effect of Combined Exposure of Crystalline Silica Dust and Cigarette Smoking on the Incidence of Silicosis among Chinese Male Stone Processing Workers: A Cross-Sectional Study
Abstract
1. Introduction
2. Methods
2.1. Study Population
2.2. Silica Exposure Evaluation
2.3. Chest Radiographic Examination
2.4. Statistical Analysis
3. Results
3.1. Characteristic Profiles of Enrolled Participants
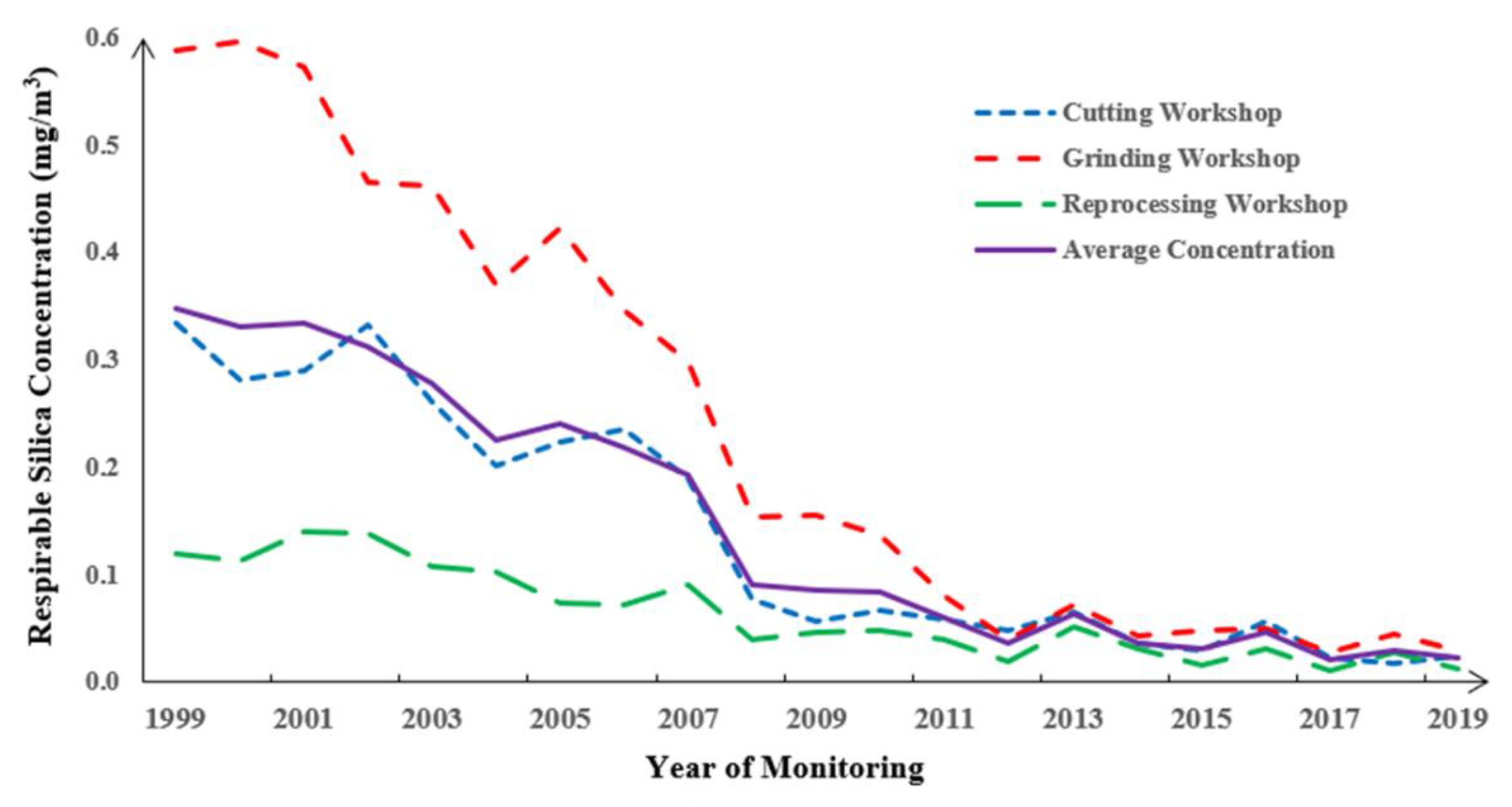
3.2. Annual Respirable Silica Concentration for the Plant
3.3. Association between Silicosis Prevalence and Silica Exposure
3.4. Association between Silicosis Prevalence and Cigarette Smoking
3.5. Combined Effect of Silica Exposure and Cigarette Smoking
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tolstoy, A.; Lesovik, V.; Fediuk, R.; Amran, M.; Gunasekaran, M.; Vatin, N.; Vasilev, Y. Production of Greener High-Strength Concrete Using Russian Quartz Sandstone Mine Waste Aggregates. Materials 2020, 13, 5575. [Google Scholar] [CrossRef] [PubMed]
- Rupani, M.P. Challenges and opportunities for silicosis prevention and control: Need for a national health program on silicosis in India. J. Occup. Med. Toxicol. 2023, 18, 11. [Google Scholar] [CrossRef]
- Zhou, Y.X.; Zhang, W.S.; Wu, D.; Fan, Y.D. The effect of silica exposure on the risk of lung cancer: A meta-analysis. J. Biochem. Mol. Toxicol. 2023, 37, 102024. [Google Scholar] [CrossRef]
- Hoy, R.F.; Jeebhay, M.F.; Cavalin, C.; Chen, W.H.; Cohen, R.A.; Fireman, E.; Go, L.H.T.; Leon-Jimenez, A.; Menendez-Navarro, A.; Ribeiro, M.; et al. Current global perspectives on silicosis-Convergence of old and newly emergent hazards. Respirology 2022, 27, 387–398. [Google Scholar] [CrossRef]
- Zhang, N.; Liu, K.L.; Wang, K.; Zhou, C.; Wang, H.J.; Che, S.S.; Liu, Z.H.; Yang, H.F. Dust induces lung fibrosis through dysregulated DNA methylation. Environ. Toxicol. 2019, 34, 728–741. [Google Scholar] [CrossRef]
- Sun, Y.; Bochmann, F.; Morfeld, P.; Ulm, K.; Liu, Y.W.; Wang, H.J.; Yang, L.; Chen, W.H. Change of Exposure Response over Time and Long-Term Risk of Silicosis among a Cohort of Chinese Pottery Workers. Int. J. Environ. Res. Public Health 2011, 8, 2923–2936. [Google Scholar] [CrossRef] [PubMed]
- Roberts, N.L.S.; Mountjoy-Venning, W.C.; Anjomshoa, M.; Banoub, J.A.M.; Yasin, Y.J.; GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study. Lancet 2019, 393, E44. [Google Scholar]
- Smith, D.R.; Leggat, P.A. An international review of tobacco smoking in the medical profession: 1974–2004. BMC Public Health 2007, 7, 115. [Google Scholar] [CrossRef]
- Zhang, H.; Cai, B.Q. The impact of tobacco on lung health in China. Respirology 2003, 8, 17–21. [Google Scholar] [CrossRef]
- Hye-jung, J.; Koh, D.-H.; Sangjun, C.; Park, J.-H.; Kim, H.; Lee, S.G.; Park, D. Estimates of the Prevalence, Intensity and the Number of Workers Exposed to Cigarette Smoking across Occupations and Industries in Korea. J. Korean Med. Sci. 2019, 34, 1–14. [Google Scholar]
- Liu, Y.W.; Steenland, K.; Rong, Y.; Hnizdo, E.; Huang, X.J.; Zhang, H.; Shi, T.M.; Sun, Y.; Wu, T.C.; Chen, W.H. Exposure-Response Analysis and Risk Assessment for Lung Cancer in Relationship to Silica Exposure: A 44-Year Cohort Study of 34,018 Workers. Am. J. Epidemiol. 2013, 178, 1424–1433. [Google Scholar] [CrossRef]
- Sato, T.; Shimosato, T.; Klinman, D.M. Silicosis and lung cancer: Current perspectives. Lung Cancer-Targets Ther. 2018, 9, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Garcia, D.D.; Sultan, N.M.; Yerba, O.R.; Castro, S.J.; Palacios, E.A.; Cano, A.D. Silicosis and smoking: Intrinsic phenomenon in the respiratory system. Adv. Appl. Sociol. 2018, 8, 659. [Google Scholar] [CrossRef]
- Tse, L.A.; Yu, I.T.S.; Qiu, H.; Leung, C.C. Joint effects of smoking and silicosis on diseases to the lungs. PLoS ONE 2014, 9, e104494. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, M.I.; Waksman, J.; Curtis, J. Silicosis: A review. DM Disease-A-Month 2007, 53, 394–416. [Google Scholar] [CrossRef]
- Hessel, P.A.; Gamble, J.F.; Nicolich, M. Relationship between silicosis and smoking. Scand. J. Work Environ. Health 2003, 29, 329–336. [Google Scholar] [CrossRef]
- Brown, T. Silica exposure, smoking, silicosis and lung cancer-025EFcomplex interactions. Occup. Med. 2009, 59, 89–95. [Google Scholar] [CrossRef]
- Bag, R.; Suleman, N.; Guntupalli, K.K. Respiratory failure in interstitial lung disease. Curr. Opin. Pulm. Med. 2004, 10, 412–418. [Google Scholar] [CrossRef]
- Glass, D.S.; Grossfeld, D.; Renna, H.A.; Agarwala, P.; Spiegler, P.; DeLeon, J.; Reiss, A.B. Idiopathic pulmonary fibrosis: Current and future treatment. Clin. Respir. J. 2022, 16, 84–96. [Google Scholar] [CrossRef]
- Baues, M.; Dasgupta, A.; Ehling, J.; Prakash, J.; Boor, P.; Tacke, F.; Kiessling, F.; Lammers, T. Fibrosis imaging: Current concepts and future directions. Adv. Drug Deliv. Rev. 2017, 121, 9–26. [Google Scholar] [CrossRef]
- Wu, N.; Xue, C.; Yu, S.; Ye, Q. Artificial stone-associated silicosis in China: A prospective comparison with natural stone-associated silicosis. Respirology 2020, 25, 518–524. [Google Scholar] [CrossRef]
- Hanlon, J.; Galea, K.S.; Verpaele, S. Review of Published Laboratory-Based Aerosol Sampler Efficiency, Performance and Comparison Studies (1994–2021). Int. J. Environ. Res. Public Health 2022, 20, 267. [Google Scholar] [CrossRef] [PubMed]
- Shinohara, Y. Application of the Microwave Acid Digestion Technique to the Pyrophosphoric AcidMethod for Quantitative Analysis of Free Silica in Dust. Ind. Health 1993, 31, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Dahmann, D.; Taeger, D.; Kappler, M.; Büchte, S.; Morfeld, P.; Brüning, T.; Pesch, B. Assessment of exposure in epidemiological studies: The example of silica dust. J. Expo. Sci. Environ. Epidemiol. 2008, 18, 452–461. [Google Scholar] [CrossRef]
- Ribeiro, F.S.N.; de Camargo, E.A.; Filho, V.W. Design and validation of a job-exposure matrix to silica. Rev. Saude Publica 2005, 39, 18–26. [Google Scholar] [CrossRef] [PubMed][Green Version]
- GBZ 70-2015; Diagnosis of Occupational Pneumoconiosis. National Health Commission of the People’s Republic of China: Beijing, China, 2015.
- International Labour Office. International classification of radiographs of pneumoconiosis, revised. Occup. Saf. Health Ser. 2011, 22, Rev-2011. [Google Scholar]
- Knol, M.J.; van der Tweel, I.; Grobbee, D.E.; Numans, M.E.; Geerlings, M.I. Estimating interaction on an additive scale between continuous determinants in a logistic regression model. Int. J. Epidemiol. 2007, 36, 1111–1118. [Google Scholar] [CrossRef]
- GBZ 2.1-2019; Occupational Exposure Limits for Hazardous Agents in the Workplace Part I: Chemical Hazardous Agents. National Health Commission of the People’s Republic of China: Beijing, China, 2019.
- Chen, W.H.; Liu, Y.W.; Wang, H.J.; Hnizdo, E.; Sun, Y.; Su, L.P.; Zhang, X.K.; Weng, S.F.; Bochmann, F.; Hearl, F.J.; et al. Long-Term Exposure to Silica Dust and Risk of Total and Cause-Specific Mortality in Chinese Workers: A Cohort Study. PLoS Med. 2012, 9, e1001206. [Google Scholar] [CrossRef]
- Parimon, T.; Hohmann, M.S.; Yao, C.F. Cellular Senescence: Pathogenic Mechanisms in Lung Fibrosis. Int. J. Mol. Sci. 2021, 22, 6214. [Google Scholar] [CrossRef]
- Tiwari, R.R. Agreement between chest radiography and high-resolution computed tomography in diagnosing dust-related interstitial lung fibrosis. Toxicol. Ind. Health 2015, 31, 235–238. [Google Scholar] [CrossRef]
- Sun, J.K.; Weng, D.; Jin, C.S.; Yan, B.; Xu, G.H.; Jin, B.; Xia, S.N.; Chen, J. The Value of High Resolution Computed Tomography in the Dianostics of Small Opacities and Complications of Silicosis in Mine Machinery Manufacturing Workers, Compared to Radiography. J. Occup. Health 2008, 50, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Silica, A.V. Silicosis, and Cancer: Controversy in Occupational Medicine; Goldsmith, D., Winn, D., Shy, C., Eds.; Prager: New York, NY, USA, 1986; Volume 375, p. 384. [Google Scholar]
- Zhang, M.; Zheng, Y.D.; Du, X.Y.; Lu, Y.; Li, W.J.; Qi, C.; Wu, Z.L. Silicosis in Automobile Foundry Workers: A 29-Year Cohort Study. Biomed. Environ. Sci. 2010, 23, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Guarnieri, G.; Mauro, S.; Lucernoni, P.; Sbaraglia, M.; Putzu, M.G.; Zuliani, P.; Rossi, F.; Vio, S.; Bianchi, L.; Martinelli, A.; et al. Silicosis in finishing workers in quartz conglomerates processing. Med. Del Lavoro 2020, 111, 99–106. [Google Scholar] [CrossRef]
- Li, J.H.; Cone, J.E.; Brackbill, R.M.; Giesinger, I.; Yung, J.; Farfel, M.R. Pulmonary Fibrosis among World Trade Center Responders: Results from the WTC Health Registry Cohort. Int. J. Environ. Res. Public Health 2019, 16, 825. [Google Scholar] [CrossRef]
- Baumgartner, K.B.; Samet, J.M.; Coultas, D.B.; Stidley, C.A.; Hunt, W.C.; Colby, T.V.; Waldron, J.A. Occupational and environmental risk factors for idiopathic pulmonary fibrosis: A multicenter case-control study. Am. J. Epidemiol. 2000, 152, 307–315. [Google Scholar] [CrossRef]
- Johnson, C.Y.; Rocheleau, C.M.; Grajewski, B.; Howards, P.P. Structure and Control of Healthy Worker Effects in Studies of Pregnancy Outcomes. Am. J. Epidemiol. 2019, 188, 562–569. [Google Scholar] [CrossRef] [PubMed]
- Andersson, M.; Blanc, P.D.; Toren, K.; Jarvholm, B. Smoking, occupational exposures, and idiopathic pulmonary fibrosis among Swedish construction workers. Am. J. Ind. Med. 2021, 64, 251–257. [Google Scholar] [CrossRef]
- Sohrabi, Y.; Sabet, S.; Yousefinejad, S.; Rahimian, F.; Aryaie, M.; Soleimani, E.; Jafari, S. Pulmonary function and respiratory symptoms in workers exposed to respirable silica dust: A historical cohort study. Heliyon 2022, 8, e11642. [Google Scholar] [CrossRef]
- Samarelli, A.V.; Masciale, V.; Aramini, B.; Colo, G.P.; Tonelli, R.; Marchioni, A.; Bruzzi, G.; Gozzi, F.; Andrisani, D.; Castaniere, I.; et al. Molecular Mechanisms and Cellular Contribution from Lung Fibrosis to Lung Cancer Development. Int. J. Mol. Sci. 2021, 22, 12179. [Google Scholar] [CrossRef]
- Lai, H.P.; Liu, Y.W.; Zhou, M.; Shi, T.M.; Zhou, Y.; Weng, S.F.; Chen, W.H. Combined effect of silica dust exposure and cigarette smoking on total and cause-specific mortality in iron miners: A cohort study. Environ. Health 2018, 17, 46. [Google Scholar] [CrossRef]
- Chen, M. Developing Prediction Models for Determining the Most Optimal Intervals of Chest Radiographic Examinations and Cost-Effectiveness Analyses for Workers Exposed to Silica Dust. Doctoral Dissertation, Chinese University of Hong Kong, Hong Kong, China, 2012. [Google Scholar]

| Characteristics | Total Workers (N = 1688) | Levels of Cumulative Exposure to Silica # | |||
|---|---|---|---|---|---|
| First Quarter (N = 460) | Second Quarter (N = 395) | Third Quarter (N = 403) | Fourth Quarter (N = 430) | ||
| Age (N (%)) | |||||
| <35 years old | 613 (36.32) | 379 (82.39) | 184 (46.58) | 48 (11.91) | 2 (0.47) |
| 35–45 years old | 802 (47.51) | 77 (16.74) | 189 (47.85) | 324 (80.40) | 212 (49.30) |
| ≥45 years old | 273 (16.17) | 4 (0.87) | 22 (5.57) | 31 (7.69) | 216 (50.23) |
| Age (years) | 37.56 ± 6.86 | 31.08 ± 4.38 | 35.62 ± 4.75 | 38.79 ± 3.95 | 45.14 ± 4.66 |
| Marriage (N (%)) | |||||
| Married | 1540 (91.23) | 364 (79.13) | 363 (91.90) | 390 (96.77) | 423 (98.37) |
| Unmarried | 148 (8.77) | 96 (20.87) | 32 (8.10) | 13 (3.23) | 7 (1.63) |
| Division (N (%)) | |||||
| Frontline Workers | 1269 (75.18) | 364 (79.13) | 316 (80.00) | 308 (76.43) | 281 (65.35) |
| Auxiliary Workers | 419 (24.82) | 96 (20.87) | 79 (20.00) | 95 (23.57) | 149 (34.65) |
| Cumulative Concentration of Silica Exposure (mg/m3-y) | 1.54 ± 0.52 | 0.61± 0.24 | 1.35 ± 0.29 | 1.99 ± 0.32 | 3.22 ± 0.57 |
| Average Concentration of Silica Exposure (mg/m3) | 0.15 ± 0.10 | 0.12 ± 0.08 | 0.14 ± 0.06 | 0.16 ± 0.10 | 0.19 ± 0.15 |
| Duration of Exposure (years) | 10.84 ± 3.01 | 4.74 ± 1.53 | 9.71 ± 1.34 | 12.57 ± 1.81 | 17.20 ± 2.77 |
| Status of Smoking (N (%)) | |||||
| Never Smoking | 834 (49.41) | 255 (55.43) | 180 (45.57) | 215 (53.35) | 184 (42.79) |
| Ever Smoking | 854 (50.59) | 205 (44.57) | 215 (54.43) | 188 (46.65) | 246 (57.21) |
| Daily Cigarettes * | 11.68 ± 5.07 | 10.21 ± 4.48 | 10.34 ± 3.89 | 11.52 ± 4.67 | 14.20 ± 5.75 |
| Duration of Smoking (years) * | 13.11 ± 6.89 | 9.06 ± 3.81 | 10.53 ± 4.90 | 12.85 ± 5.52 | 18.94 ± 7.44 |
| Pack Years of Smoking * | 7.35 ± 5.52 | 4.54 ± 2.73 | 5.06 ± 3.94 | 6.48 ± 3.42 | 12.84 ± 8.93 |
| Silicosis (N (%)) | |||||
| Negative | 1444 (85.55) | 419 (91.09) | 340 (86.08) | 345 (85.61) | 340 (79.07) |
| Positive | 244 (14.45) | 41 (8.91) | 55 (13.92) | 58 (14.39) | 90 (20.93) |
| Stratification | ORs and 95% CIs for Silicosis | |||||
|---|---|---|---|---|---|---|
| Continuous Cumulative Exposure to Silica | Higher vs. Lower Exposed a | Categorical Cumulative Exposure to Silica b | p Value for Trend c | |||
| Second Quarter | Third Quarter | Fourth Quarter | ||||
| Not Stratified d | 1.070 (1.022, 1.120) | 1.739 (1.253, 2.413) | 1.632 (1.072, 2.484) | 1.654 (1.041, 2.629) | 4.142 (1.444, 11.884) | 0.001 |
| By Age e | ||||||
| <35 years old | 1.071 (0.923, 1.243) | 4.796 (2.410, 9.545) | 1.417 (0.777, 2.582) | 5.307 (2.643, 10.655) | 9.667 (1.561, 36.505) | <0.001 |
| 35–45 years old | 1.090 (1.014, 1.171) | 1.166 (0.735, 1.851) | 0.837 (0.443, 1.580) | 1.625 (0.909, 2.905) | 1.010 (0.496, 2.057) | 0.210 |
| ≥45 years old | 1.065 (0.986, 1.150) | 1.660 (0.884, 3.117) | 1.289 (0.422, 5.933) | 1.301 (0.588, 2.879) | 3.758 (1.270, 8.124) | 0.039 |
| By Division f | ||||||
| Frontline Workers | 1.063 (1.013, 1.116) | 1.481 (1.046, 2.097) | 1.515 (0.982, 2.338) | 1.366 (0.833, 2.239) | 2.247 (0.678, 7.442) | 0.039 |
| Auxiliary Workers | 1.127 (0.920, 1.382) | 6.547 (2.292, 18.702) | 1.081 (0.447, 2.613) | 1.472 (1.097, 2.296) | 1.639 (1.079, 3.408) | <0.001 |
| By Smoking Status g | ||||||
| Never Smoking | 1.063 (0.991, 1.139) | 2.088 (1.264, 3.448) | 1.827 (0.978, 3.410) | 2.048 (0.999, 4.197) | 13.783 (1.382, 137.463) | 0.002 |
| Ever Smoking | 1.086 (1.015, 1.162) | 1.513 (0.982, 2.332) | 1.489 (0.841, 2.638) | 1.395 (0.760, 2.558) | 2.711 (0.776, 9.466) | 0.055 |
| Stratification | ORs and 95% CIs for Silicosis | |||||
|---|---|---|---|---|---|---|
| Continuous Pack Years of Smoking a | Smokers vs. Non-Smoker | Categorical Pack Years of Smoking b | p Value for Trend c | |||
| <5 Pack Years | 5–10 Pack Years | ≥10 Pack Years | ||||
| Not Stratified d | 1.011 (0.986, 1.037) | 1.239 (0.939, 1.635) | 0.975 (0.623, 1.527) | 1.423 (0.985, 2.055) | 1.276 (0.880, 1.851) | 0.159 |
| By Age e | ||||||
| <35 years old | 1.024 (0.923, 1.136) | 1.095 (0.617, 1.945) | 0.841 (0.408, 1.737) | 1.276 (0.579, 2.813) | 1.996 (0.676, 5.895) | 0.192 |
| 35–45 years old | 0.968 (0.920, 1.020) | 1.249 (0.845, 1.845) | 1.245 (0.674, 2.301) | 1.592 (1.002, 2.529) | 0.844 (0.469, 1.519) | 0.673 |
| ≥45 years old | 1.027 (0.994, 1.062) | 1.299 (0.741, 2.277) | 0.406 (0.048, 3.426) | 0.665 (0.203, 2.174) | 1.536 (0.859, 2.748) | 0.092 |
| By Division f | ||||||
| Frontline Workers | 0.994 (0.960, 1.029) | 1.135 (0.822, 1.567) | 0.798 (0.469, 1.357) | 1.461 (0.963, 2.216) | 1.125 (0.723, 1.751) | 0.489 |
| Auxiliary Workers | 1.036 (0.994, 1.079) | 1.449 (0.832, 2.525) | 1.965 (0.839, 4.602) | 1.111 (0.494, 2.499) | 1.480 (0.720, 3.044) | 0.336 |
| By Exposure Status g | ||||||
| Lower Exposed | 1.007 (0.977, 1.038) | 1.348 (0.973, 1.866) | 1.157 (0.690, 1.942) | 1.607 (1.041, 2.481) | 1.276 (0.824, 1.976) | 0.230 |
| Higher Exposed | 1.011 (0.963, 1.062) | 0.875 (0.508, 1.509) | 0.585 (0.236, 1.452) | 0.961 (0.478, 1.932) | 1.041 (0.499, 2.170) | 0.838 |
| Figure Stratification | ORs and 95% CIs for Silicosis a | Interaction Effect for Additivity (RERI) e | Interaction Factor on Multiplicative Scale f | |
|---|---|---|---|---|
| Lower Exposed | Higher Exposed | |||
| Unstratified b | ||||
| Never Smoking | 1.000 (Reference) | 2.159 (1.328, 3.510) | 0.512 (0.078, 3.417) | 0.690 (0.369, 1.291) |
| Ever Smoking | 1.367 (0.988, 1.892) | 3.038 (1.311, 4.168) | ||
| <35 years old c | ||||
| Never Smoking | 1.000 (Reference) | 4.562 (1.620, 12.847) | 2.698 (0.642, 9.887) | 1.091 (0.288, 4.132) |
| Ever Smoking | 1.072 (0.552, 2.082) | 7.332 (2.230, 12.749) | ||
| 35–45 years old c | ||||
| Never Smoking | 1.000 (Reference) | 1.637 (0.840, 3.192) | −0.799 (−2.554, 0.956) | 0.543 (0.221, 1.336) |
| Ever Smoking | 1.460 (0.925, 2.305) | 1.298 (0.667, 2.527) | ||
| ≥45 years old c | ||||
| Never Smoking | 1.000 (Reference) | 2.161 (0.817, 5.714) | 0.452 (0.248, 5.796) | 0.654 (0.196, 2.185) |
| Ever Smoking | 1.482 (0.754, 2.913) | 3.095 (0.930, 4.719) | ||
| Frontline Workers d | ||||
| Never Smoking | 1.000 (Reference) | 1.737 (1.037, 2.912) | 1.646 (0.145, 3.832) | 0.752 (0.378, 1.497) |
| Ever Smoking | 1.244 (0.840, 1.844) | 3.627 (1.003, 2.639) | ||
| Auxiliary Workers d | ||||
| Never Smoking | 1.000 (Reference) | 2.131 (0.567, 6.904) | 3.575 (0.658, 12.091) | 0.982 (0.322, 2.992) |
| Ever Smoking | 1.646 (0.930, 2.915) | 6.352 (1.961, 16.573) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, Y.; Miao, L.; Xu, P.; Yang, X.; Qu, M.; Lai, H. Potential Effect of Combined Exposure of Crystalline Silica Dust and Cigarette Smoking on the Incidence of Silicosis among Chinese Male Stone Processing Workers: A Cross-Sectional Study. Healthcare 2023, 11, 2260. https://doi.org/10.3390/healthcare11162260
Xue Y, Miao L, Xu P, Yang X, Qu M, Lai H. Potential Effect of Combined Exposure of Crystalline Silica Dust and Cigarette Smoking on the Incidence of Silicosis among Chinese Male Stone Processing Workers: A Cross-Sectional Study. Healthcare. 2023; 11(16):2260. https://doi.org/10.3390/healthcare11162260
Chicago/Turabian StyleXue, Yu, Long Miao, Ping Xu, Xinglong Yang, Man Qu, and Hanpeng Lai. 2023. "Potential Effect of Combined Exposure of Crystalline Silica Dust and Cigarette Smoking on the Incidence of Silicosis among Chinese Male Stone Processing Workers: A Cross-Sectional Study" Healthcare 11, no. 16: 2260. https://doi.org/10.3390/healthcare11162260
APA StyleXue, Y., Miao, L., Xu, P., Yang, X., Qu, M., & Lai, H. (2023). Potential Effect of Combined Exposure of Crystalline Silica Dust and Cigarette Smoking on the Incidence of Silicosis among Chinese Male Stone Processing Workers: A Cross-Sectional Study. Healthcare, 11(16), 2260. https://doi.org/10.3390/healthcare11162260







