Abstract
Improvements in medical care have turned severe diseases into chronic conditions, but often their treatment and the use of medical devices are related to specific complications. Here, we present a clinical case of a long-term dialysis patient who was infected with a rare opportunistic infectious agent—Gordonia sputi. In recent years, the incidence of Gordonia spp. infections in immunocompromised patients with central venous catheters (CVC) has appeared to rise. The isolation and identification of Gordonia spp. are challenging and require modern techniques. In addition, the treatment is usually persistent and often results in CVC extraction, which is associated with further risk and costs for the patient. We also studied the alterations in the immune status of the patient caused by long-term renal replacement therapy and persistent hepatitis C virus infection. Antibiotic therapy and immunostimulation with Inosine pranobex lead to successful eradication of the infection without the need for CVC replacement.
1. Introduction
Long-term hemodialysis is a life-supporting procedure, but it has many complications for the patient. Chronic inflammation due to the contact of blood with artificial materials and uremia affects the immune status of the patient. Consequently, the leading causes of mortality in the hemodialysis population are cardiovascular diseases, withdrawal from renal replacement treatment, and infections [1,2,3,4].
Here, we present a clinical case of a hemodialysis patient with bacteremia caused by the opportunistic pathogen Gordonia sputi. Furthermore, we tried to investigate if long-term renal replacement therapy (more than 35 years) affected the immune status of the patient.
Gordonia spp. are aerobic actinomycetes, found in soil and water, and were first described as a separate genus by Tsukamura in 1971 [5]. Some species are reported to cause infections in humans [6]. Even in rare incidences, their identification and treatment may be challenging. Colonization of medical devices appears to be a potential risk as some species are reported to adhere to and degrade rubber [7]. Often, successful treatment requires medical device extraction, which is related to higher risks and healthcare costs.
2. Case Presentation
We report a 59-year-old female dialysis patient with a double-lumen tunneled venous catheter. Her dialysis treatment was initiated in 1985 due to four hypotrophic kidneys with superposed chronic glomerulonephritis. After multiple blood transfusions back then, the patient was infected with the hepatitis C virus (HCV).
From November 2021, the patient reported malaise, weight loss, and febrile episodes of 37.5 °C to 38 °C the night after the dialysis procedure and the next day. No febrile episodes or chills during the procedure were noted. Multiple blood culture sets were tested, but no explicit bacterial agent was isolated. No leukocytosis was observed and only a slightly elevated C-reactive protein (CRP) was found. After every microbiology test, antibiotic treatment was applied, which resulted in temporary improvements in the symptoms. No clinical or laboratory findings, including echocardiography, were in favor of endocarditis. The patient refused a withdrawal of the dialysis catheter multiple times.
In September 2022, upon a new relapse of symptoms, another blood culture set was sent to the microbiology laboratory. On the fifth day of incubation, it yielded gracile Gram-positive rods, which grew on sheep blood agar as small non-hemolytic white colonies. Phenotypic identification was performed using 4 h semi-automated biochemical testing with RapID™ CB PLUS (Thermo Scientific, Waltham, MA, USA) and the microorganism was identified as Corynebacterium striatum. Since no leukocytosis was found, and CRP was 37 °C, another blood culture sample was taken to rule out contamination or to confirm the result. Upon 4 days of incubation of the new blood culture set, the aerobic blood culture vial became positive. Direct microscopy revealed midsized actinomycete-like rods and upon cultivation at 37 °C, in an aerobic environment, small white colonies on sheep blood agar appeared. They were subjected to Matrix-Assisted Laser Desorption Ionization–Time-of-Flight Mass Spectrometry (MALDI-TOF MS, Vitek MS, bioMerieux, France) identification and the protein profiles obtained were characteristic of Gordonia sputi (Figure 1). The antimicrobial susceptibility testing (AST) according to EUCAST revealed the isolate was susceptible to vancomycin, gentamicin, linezolid, imipenem, ceftriaxone, and ciprofloxacin. After antibiotic treatment with gentamicin and meropenem for 21 days, all symptoms disappeared, and the improvement in the patient’s condition remained constant. On the second and the fourth week after the end of the antibiotic course, blood culture samples were negative. Meanwhile, on 22 July 2022, Candida tropicalis from a throat swab was isolated. The candida infection persisted for about 6 months despite the peroral fluconazole therapy. A timeline of the events is presented in Figure 2.
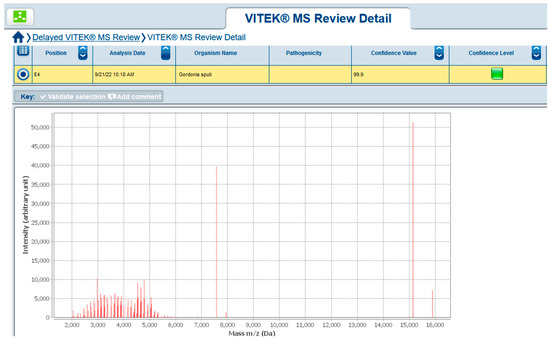
Figure 1.
Gordonia sputi MALDI-TOF MS result.
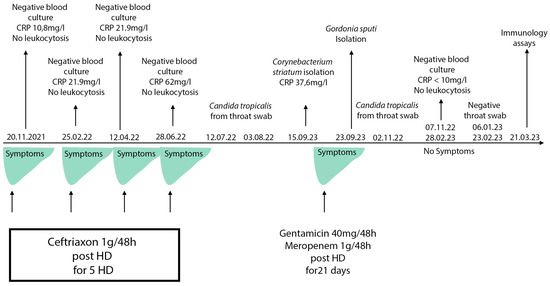
Figure 2.
Timeline of the clinical case events. HD: hemodialysis. CRP (C-reactive protein) reference range: 0–10 mg/L.
In March 2023, the patient was referred to the clinical immunology unit for further investigation of the immune status. For the assessment of humoral immunity, the levels of serum IgA, IgG, IgM, and complement fractions C3 and C4 were measured by an automated immunoturbidimetry analyzer (BA200, Biosystems, Barcelona, Spain). An internal quality control study (using two levels of control serum offered by the manufacturer) and calibration were performed according to the manufacturer’s instructions (Biosystems, Barcelona, Spain). The values of serum immunoglobulins are expressed in µg/mL and C3 and C4 levels are expressed in g/L (Table 1).

Table 1.
Results from the evaluation of the serum immunoglobulins and complement fractions.
Laboratory investigations showed normal levels of the three immunoglobulins and the complement fractions. For the evaluation of the cellular immune status, lymphocyte subpopulation counts (LSc) were measured by 6-color TBNK reagent using Trucount Absolute Counting Tubes (BD, New Jersey, USA) in a peripheral venous blood sample within 2 h of blood draw by BD FACSCanto II, BD, USA, and the kit consisted of the following monoclonal antibodies: CD3-FITC (clone SK7), CD16-PE (clone B73.1), CD56-PE (clone NCAM16.2), CD45-PerCP-Cy5.5 (clone 2D1), CD4-PE-Cy7 (clone SK3), CD19-allophycocyanin (APC) (clone SJ25C1), and CD8-APC-Cy7 (clone SK1) (Figure 3).

Figure 3.
Flow cytometry gating strategy: FACS dot plot on BD FACS Canto II gating CD4 and CD8 T cells. Panel (A) depicts CD45 + lymphocytes detected in the dot plot of CD45 PerCP-Cy5.5-A vs. SSC-A. Panel (B) shows the CD19 APC-A vs. SSC-A dot plot with BD Trucount absolute count bead events. Panel (C) depicts CD3 + T cells in the dot plot of CD3 FITC-A vs. SSC-A. In the CD8 APC-Cy7-A vs. CD4 PE-Cy7-A dot plot, panel (D) depicts suppressor/cytotoxic (CD4-CD8 +) and helper/inducer (CD4 + CD8 -) T lymphocytes. Panel (E) illustrates the natural killer subset (NK cells) identified as CD3– and CD16+ and/or CD56+.
Flowcytometric testing revealed an impairment of the cell-mediated immunity with lymphopenia and a decreased absolute number of immunocompetent CD3+ T cells, helper/inducer CD4+ T cells, and cytotoxic/suppressor CD8+ T cells, and borderline low B and NK (natural killer) cells. The CD4/CD8 T cell ratio was normal. (Table 2).

Table 2.
Results from immunophenotyping of lymphocyte subpopulations performed on FACSCanto II Clinical Flow Cytometry System, situated in Medical Microbiology and Immunology Department of Medical University-Plovdiv according to a standardized procedure and using commercial TBNK-multitest reagent kit and national age-adjusted reference ranges of lymphocyte subsets.
Additionally, cytokine analysis was conducted using a human Th1/Th2/Th17 cytokine cytometric bead array kit (Cytometric Bead Array (CBA) Human Th1/Th2/Th17 Cytokine Kit, BD, USA), which allowed for the simultaneous detection of the IL-2, IL-4, IL-6, IL-10, TNF-a, IFN-ɣ, and IL-17A cytokines in serum. The altered function of immune cells resulted in an intriguing dysregulation of cytokine production characterized by elevated levels of Th2 cytokines (IL-4, IL-6, and IL-10) and slightly detectable Th1 (IFN-ɣ, TNF-α, and IL-2) and Th17 (IL-17A) cytokines (Table 3).

Table 3.
Results from measurement of cytokine profile Th1/Th2/Th17 by BD CBA Th1/Th2/Th17 kit.
3. Discussion
The clinical and laboratory changes consistent with infection were weakly manifested, probably because of the patient’s comorbidity and deprived immune status. Clinical presentation was additionally concealed by the empirical antibiotic courses and later by the superposed fungal infection. The refusal for catheter removal interfered with the prompt identification and eradication of the infection.
Dialysis patients with central venous catheters are reported to have higher rates of mortality and complications, i.e., endocarditis, septic shock, and abscesses, compared to other vascular accesses: arterio-venous fistulas and vascular grafts. The same article pointed out that despite the many problems of catheters, their placement may be inevitable and, because of the profile of patients that begin hemodialysis, they are widely used, i.e., in older patients often with many comorbidities. The construction of an arterio-venous fistula and its maturation in these cases may be difficult [8].
The spectrum of the causative agents of hemodialysis-related infections is similar in cases of vascular access and catheter-related bacteremia. More than half of them are caused by Gram-positive bacteria, the most common of which is S. aureus, including methicillin-resistant S. aureus (MRSA). Coagulase-negative staphylococci (CoNS) are also common, predominantly S. epidermidis [9,10,11]. Approximately 25% of cases are caused by Gram-negative bacteria such as Escherichia coli (E. coli), Pseudomonas aeruginosa, Enterobacter spp., and Klebsiella spp. as well as Proteus spp. and fungi from the Candida genus [11,12].
With the increasing use of MALDI-TOF MS and 16S rRNA sequencing, bacteria previously not known to be associated with certain clinical syndromes have been newly identified. This particular patient population is also susceptible to opportunistic infections caused by rare pathogens such as Gordonia species. They are emerging pathogens in hemodialysis patients.
Little is known about the epidemiology of Gordonia spp. in general and its association with human diseases. The Gordonia genus has a complicated taxonomic history of several reclassifications. The picture is further complicated by the fact that identification is challenging and misidentification often occurs due to the close relation of other genera within the Mycobacteriales order, like Dietzia spp., Corynebacterium, Nocardia spp., Rhodococcus spp., and Tsukamurella spp., with Gordonia spp. Reports of human infections caused by Gordonia spp. are relatively rare when compared to other opportunistic pathogens of closely related taxonomic genera like Nocardia spp. and Rhodococcus spp. A bibliographic review indicates that the use of catheters for long-term intravenous access is a notable risk factor for bloodstream infections caused by Gordonia species. A recent report from France showed that Gordonia spp., including G. sputi, are indeed recovered from immunocompromised hosts like HIV-positive individuals, and individuals with malnutrition or long-term corticosteroid treatment, etc., but also when indwelling catheters of any type are present [13,14,15,16,17,18,19,20].
In our case, for the time between November 2021 and September 2022, the patient had multiple febrile episodes where conventional blood culture testing did not yield a definitive causative agent and the applied antimicrobial treatment had a temporary effect. The first possible causative agent in our patient, detected in September 2022, was identified by semi-automated biochemical testing (RapID CB Plus) as Corynebacterium striatum. The mentioned test does not include Gordonia spp. in its diagnostic spectrum. Because of this fact and due to the close relativity and overlapping of some morphological and biochemical characteristics of Gordonia spp. and its other neighboring genera, similar to the other authors, we cannot exclude the possibility for the first isolate to have been Gordonia sputi misidentified as Corynebacterium striatum. This goes to show that routine methods are insufficient and more complex and modern techniques are needed, e.g., proteomic analysis with mass-spectrometry or molecular genetic assays like polymerase chain reaction (PCR) or 16S ribosomal RNA sequencing. Lai et al. retrospectively re-evaluated 66 samples that were initially identified by conventional techniques as Rhodococcus spp. and found that when using the molecular method 15 of them were re-identified as Gordonia spp. [21].
Matrix-assisted laser desorption/ionization-time-of-flight (MALDI-TOF) mass spectrometry (MS)—MALDI-TOF MS—is a method for rapid and accurate identification that is becoming increasingly available in the clinical microbiology laboratory. It is a technique that is based on producing ionized particles from a bacterial–matrix mixture, which are then separated according to their mass-to-charge ratio. A unique spectrum is generated, which is in turn compared to a database of known and validated microorganisms. MALDI-TOF MS is capable of identifying a wide spectrum of microorganisms including Gram-positive and Gram-negative bacteria, mycobacteria, yeast, and molds. Often, the accuracy is comparable to molecular methods of identification such as 16S rRNA gene sequencing [22,23,24].
Thanks to the MALDI-TOF MS method, we managed to elucidate the etiology and initiate appropriate treatment. We consider MALDI-TOF MS a cheap, rapid, and reliable method for the accurate identification of G. sputi on a species level. Precise identification is also important in providing additional information about the association of G. sputi and extending our knowledge on the epidemiology of G. sputi and its role as an opportunistic pathogen.
Also, it is crucial to underline the significance of opportunistic isolates such as Gordonia sputi in immunosuppressed patients. For an adequate immune response towards infectious agents, a sufficient number of immune-competent cells are needed and, in our patient, the flowcytometry testing indisputably confirmed lymphopenia with suppression of major subsets of cells with the most remarkable decrease in the CD3+ T-cells count. This is an important factor supporting the invasiveness of infections. Such findings regarding the cellular immune status are also present in various studies [25,26].
Another reason for the impaired immune response is the dysregulation of cytokine production resulting in an imbalanced differentiation of Th lymphocytes to Th1 or Th2 cells. Each of the corresponding subpopulations secretes distinct cytokines—Th1 cytokines are IL-2, TNF-α, IFN-γ, etc., while IL-4, IL-6, IL-10, etc. belong to the group of Th2 cytokines [27]. Our patient’s immune status demonstrates an impairment of cell-mediated immunity, which is sustained by Th1 cells (slightly detectable levels of Th1 cytokines) with preserved humoral immunity marked by normal levels of total immunoglobulins and complement fractions C3 and C4 sustained by Th2 cells (increased levels of Th2 cytokines). It is known that IL-4 as well as IL-10 enhance Th2 and inhibit Th1 development [28,29]. According to some other authors, the levels of Th2 cytokines in hemodialyzed patients are increased [27] [30], which corresponds to the results in our patient. A study by Szabo et al. demonstrates that IL-4 inhibits the expression of the signal-transducing β2 subunit of the IL-12 receptor and thus the ability of the latter to induce a Th1 response [31]. Additionally, both IL-4 and IL-10 possess direct anti-inflammatory properties [32,33,34,35]. The hindrance of Th1 cytokines may result in complex defects of cell-mediated effector functions, including the phagocytic elimination of infectious agents, macrophage inflammatory cytokine production, and natural killer cell– and CD8+ T-cell–mediated cytotoxicity [36]. Moreover, chronic hepatitis C infection is associated with impaired function of helper/inducer CD4+ T cells and cytotoxic/suppressor CD8+ T cells and an overactive Th2 immune response [37,38,39,40,41,42] Thus, the comorbidities presented by chronic liver infection, long-term hemodialysis, and cancer in her adolescence correspond to the immune suppression in this patient.
Immune stimulation is an important therapeutic measure in such patients. Due to the intact levels of total immunoglobulins in this case, the administration of intravenous immunoglobulin (IVIG) was not taken into consideration. A therapeutic approach for this patient is Inosine pranobex (IP), commonly known as Isoprinosine, which is known to enhance T-cell lymphocyte proliferation and the activity of NK cells, leading to the restoration of the deficient responses in immunosuppressed patients with advantageous effects also on HCV infection [43].
4. Conclusions
The clinical presentation of catheter-associated bacteriemia in polymorbid hemodialysis patients may be vague because of a depressed immune system. Prophylaxis of infections by these patients is crucial because of the many life-threatening complications. These patients must be closely followed-up and even when mild symptoms are presented physicians should be encouraged to take blood cultures.
Given the current rise in immunocompromised hosts as well as the prominent increase in venous catheter use, it is crucial to precisely identify Gordonia spp. on a species level. This, in turn, will not only help us better understand the epidemiology of G. sputi infections but also aid in improving strategies and optimizing the treatment guidelines. Increased awareness among clinicians, including clinical microbiologists, would be beneficial to high-risk populations and public health in general. This case illustrates that some rapid commercially available microbiological identification systems may provide inaccurate results, and the precise identification to the species level can be achieved by assays that are more complex but still accessible for most laboratories like MALDI-TOF mass spectrometry. We consider MALDI-TOF mass spectrometry a reliable alternative to molecular methods that can provide rapid, cheap, and accurate identification of Gordonia spp. on a species level.
We may also conclude that the treatment of immunocompromised comorbid hemodialysis patients should always include a consideration of the constant risk of opportunistic infections. Their management should involve protective measures against the latter, prophylaxis of fungal infections, and appropriate immune stimulation.
Author Contributions
B.V. and A.A. were responsible for the conceptualization. B.V. and G.T. were responsible for patient treatment and follow-up. B.V. mainly wrote the manuscript but all the authors contributed to writing, reviewing, and editing the manuscript. A.B. and M.M. contributed to the immunological analyses and interpretations. Microbiological evaluation and interpretations were performed by A.A. and Y.K. In addition, M.M. was also responsible for supervision and final correction. All authors have read and agreed to the published version of the manuscript.
Funding
The study was funded by the National University Complex for Biomedical and Translational Research (NUCBTR), with participation in BBMRI-ERIC—Stage 2, third-year Agreements D01-395/18 December 2020 and D01-278 of 14 December 2022.
Institutional Review Board Statement
Ethical review and approval were waived for this study due to the fact that no additional investigations were performed to the patient for research purposes. We analyze and describe retrospective the findings. All tests were made for diagnose and treatment purposes. We have applied patient informed consent only.
Informed Consent Statement
Written informed consent was obtained from the patient involved in the study. All procedures performed in the presented study were in accordance with the ethical standards of the institution and with the 1964 Helsinki Declaration and its later amendments.
Data Availability Statement
The raw clinical and laboratory data associated with the current study are available from the corresponding author, without undue reservation on a reasonable request.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Stenvinkel, P.; Ketteler, M.; Johnson, R.J.; Lindholm, B.; Pecoits-Filho, R.; Riella, M.; Heimbürger, O.; Cederholm, T.; Girndt, M. IL-10, IL-6, and TNF-α: Central factors in the altered cytokine network of uremia—The good, the bad, and the ugly. Kidney Int. 2005, 67, 1216–1233. [Google Scholar] [CrossRef]
- Mailloux, L.U.; Bellucci, A.G.; Wilkes, B.M.; Napolitano, B.; Mossey, R.T.; Lesser, M.; Bluestone, P.A. Mortality in dialysis patients: Analysis of the causes of death. Am. J. Kidney Dis. 1991, 18, 326–335. [Google Scholar] [CrossRef]
- Annual Data Report|USRDS. Available online: https://usrds-adr.niddk.nih.gov/2022/end-stage-renal-disease/6-mortality (accessed on 8 July 2023).
- Siga, M.M.; Ducher, M.; Florens, N.; Roth, H.; Mahloul, N.; Fouque, D.; Fauvel, J.P. Prediction of all-cause mortality in haemodialysis patients using a Bayesian network. Nephrol. Dial. Transplant. 2020, 35, 1420–1425. [Google Scholar] [CrossRef]
- Tsukamura, M. Proposal of a New Genus, Gordona, for Slightly Acid-fast Organisms Occurring in Sputa of Patients with Pulmonary Disease and in Soil. J. Gen. Microbiol. 1971, 68, 15–26. [Google Scholar] [CrossRef]
- Arenskötter, M.; Bröker, D.; Steinbüchel, A. Biology of the Metabolically Diverse Genus Gordonia. Appl. Environ. Microbiol. 2004, 70, 3195. [Google Scholar] [CrossRef]
- Linos, A.; Berekaa, M.M.; Steinbüchel, A.; Kim, K.K.; Sproer, C.; Kroppenstedt, R.M. Gordonia westfalica sp. nov., a novel rubber-degrading actinomycete. Int. J. Syst. Evol. Microbiol. 2002, 52 Pt 4, 1133–1139. [Google Scholar]
- Böhlke, M.; Uliano, G.; Barcellos, F.C. Hemodialysis Catheter-related Infection: Prophylaxis, Diagnosis and Treatment. J. Vasc. Access 2015, 16, 347–355. [Google Scholar] [CrossRef]
- Danese, M.D.; Griffiths, R.I.; Dylan, M.; Yu, H.T.; Dubois, R.; Nissenson, A.R. Mortality differences among organisms causing septicemia in hemodialysis patients. Hemodial. Int. 2006, 10, 56–62. [Google Scholar] [CrossRef]
- Loo, L.W.; Liew, Y.X.; Choong, H.L.; Tan, A.L.; Chlebicki, P. Microbiology and audit of vascular access-associated bloodstream infections in multi-ethnic Asian hemodialysis patients in a tertiary hospital. Infect. Dis. 2015, 47, 225–230. [Google Scholar] [CrossRef]
- D’Amato-Palumbo, S.; Kaplan, A.A.; Feinn, R.S.; Lalla, R.V. Retrospective study of microorganisms associated with vascular access infections in hemodialysis patients. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2013, 115, 56–61. [Google Scholar] [CrossRef]
- Aslam, S.; Vaida, F.; Ritter, M.; Mehta, R.L. Systematic review and meta-analysis on management of hemodialysis catheter-related bacteremia. J. Am. Soc. Nephrol. 2014, 25, 2927–2941. [Google Scholar] [CrossRef] [PubMed]
- Sng, L.H.; Koh, T.H.; Toney, S.R.; Floyd, M.; Butler, W.R.; Tan, B.H. Bacteremia Caused by Gordonia bronchialis in a Patient with Sequestrated Lung. J. Clin. Microbiol. 2004, 42, 2870. [Google Scholar] [CrossRef] [PubMed]
- Kempf, V.A.J.; Schmalzing, M.; Yassin, A.F.; Schaal, K.P.; Baumeister, D.; Arenskötter, M.; Steinbüchel, A.; Autenrieth, I.B. Gordonia polyisoprenivorans septicemia in a bone marrow transplant patient. Eur. J. Clin. Microbiol. Infect. Dis. 2004, 23, 226–228. [Google Scholar] [CrossRef] [PubMed]
- Pham, A.S.; Dé, I.; Rolston, K.V.; Tarrand, J.J.; Han, X.Y. Catheter-related bacteremia caused by the nocardioform actinomycete Gordonia terrae. Clin. Infect. Dis. 2003, 36, 524–527. [Google Scholar] [CrossRef]
- Buchman, A.L.; McNeil, M.M.; Brown, J.M.; Lasker, B.A.; Anient, M.E. Central venous catheter sepsis caused by unusual Gordona (Rhodococcus species: Identification with a digoxigenin-labeled rDNA probe. Clin. Infect. Dis. 1992, 15, 694–697. [Google Scholar] [CrossRef] [PubMed]
- Lesens, O.; Hansmann, Y.; Riegel, P.; Heller, R.; Benaissa-Djellouli, M.; Martinot, M.; Petit, H.; Christmann, D. Bacteremia and endocarditis caused by a Gordonia species in a patient with a central venous catheter. Emerg. Infect. Dis. 2000, 6, 382–385. [Google Scholar] [CrossRef]
- Barthel, A.; Ursenbach, A.; Kaeuffer, C.; Koebel, C.; Gravet, A.; De Briel, D.; Dubois, J.; Haerrel, E.; Rougier, E.; Gerber, V. Characteristics and Treatment of Gordonia spp. Bacteremia, France. Emerg. Infect. Dis. 2023, 29, 1025–1028. [Google Scholar] [CrossRef]
- Drzyzga, O. The strengths and weaknesses of Gordonia: A review of an emerging genus with increasing biotechnological potential. Crit. Rev. Microbiol. 2012, 38, 300–316. [Google Scholar] [CrossRef]
- Verma, P.; Brown, J.M.; Nunez, V.H.; Morey, R.E.; Steigerwalt, A.G.; Pellegrini, G.J.; Kessler, H.A. Native valve endocarditis due to Gordonia polyisoprenivorans: Case report and review of literature of bloodstream infections caused by Gordonia species. J. Clin. Microbiol. 2006, 44, 1905–1908. [Google Scholar] [CrossRef]
- Lai, C.C.; Wang, C.Y.; Liu, C.Y.; Tan, C.K.; Lin, S.H.; Liao, C.H.; Chou, C.H.; Huang, Y.T.; Lin, H.I.; Hsueh, P.R. Infections caused by Gordonia species at a medical centre in Taiwan, 1997 to 2008. Clin. Microbiol. Infect. 2010, 16, 1448–1453. [Google Scholar] [CrossRef]
- Singhal, N.; Kumar, M.; Kanaujia, P.K.; Virdi, J.S. MALDI-TOF mass spectrometry: An emerging technology for microbial identification and diagnosis. Front. Microbiol. 2015, 6, 791. [Google Scholar] [CrossRef] [PubMed]
- Rychert, J. Commentary: Benefits and Limitations of MALDI-TOF Mass Spectrometry for the Identification of Microorganisms. J. Infect. 2019, 2, 1–5. [Google Scholar] [CrossRef]
- Grenga, L.; Pible, O.; Armengaud, J. Pathogen proteotyping: A rapidly developing application of mass spectrometry to address clinical concerns. Clin. Mass Spectrom. 2019, 14 Pt A, 9–17. [Google Scholar] [CrossRef]
- Kato, S.; Chmielewski, M.; Honda, H.; Pecoits-Filho, R.; Matsuo, S.; Yuzawa, Y.; Tranaeus, A.; Stenvinkel, P.; Lindholm, B. Aspects of immune dysfunction in end-stage renal disease. Clin. J. Am. Soc. Nephrol. 2008, 3, 1526–1533. [Google Scholar] [CrossRef]
- Deenitchina, S.S.; Ando, T.; Okuda, S.; Kinukawa, N.; Hirakata, H.; Nagashima, A.; Fujishima, M. Cellular Immunity in Hemodialysis Patients: A Quantitative Analysis of Immune Cell Subsets by Flow Cytometry. Am. J. Nephrol. 1995, 15, 57–65. [Google Scholar] [CrossRef]
- Clunk, J.M.; Lin, C.Y.; Curtis, J.J. Polarization of t-helper lymphocytes toward the Th2 phenotype in uremic patients. Am. J. Kidney Dis. 2001, 38, 286–295. [Google Scholar]
- Marchant, A.; Bruyns, C.; Vandenabeele, P.; Ducarme, M.; Gérard, C.; Delvaux, A.; De Groote, D.; Abramowicz, D.; Velu, T.; Goldman, M. Interleukin-10 controls interferon-γ and tumor necrosis factor production during experimental endotoxemia. Eur. J. Immunol. 1994, 24, 1167–1171. [Google Scholar] [CrossRef]
- Abbas, A.K.; Lichtman, A.H.; Pillai, S. Celullar and Molecular Immunology. In Celullar and Molecular Immunology, 10th ed.; Saunder Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Kimmel, P.L.; Phillips, T.M.; Simmens, S.J.; Peterson, R.A.; Weihs, K.L.; Alleyne, S.; Cruz, I.; Yanovski, J.A.; Veis, J.H. Immunologic function and survival in hemodialysis patients. Kidney Int. 1998, 54, 236. [Google Scholar] [CrossRef]
- Szabo, S.J.; Dighe, A.S.; Gubler, U.; Murphy, K.M. Regulation of the Interleukin (IL)-12R β2 Subunit Expression in Developing T Helper 1 (Th1) and Th2 Cells. J. Exp. Med. 1997, 185, 817. [Google Scholar] [CrossRef]
- te Velde, A.; Huijbens, R.; Heije, K.; de Vries, J.; Figdor, C. Interleukin-4 (IL-4) Inhibits Secretion of IL-1β, Tumor Necrosis Factor a, and IL-6 by Human Monocytes. Blood 1990, 76, 1392–1397. [Google Scholar] [CrossRef]
- Abrams, J.; Figdor, C.G.; De Waal Malefyt, R.; Bennett, B.; De Vries, J.E. Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: An autoregulatory role of IL-10 produced by monocytes. J. Exp. Med. 1991, 174, 1209. [Google Scholar]
- Fiorentino, D.F.; Zlotnik, A.; Mosmann, T.R.; Howard, M.; O’Garra, A. IL-10 inhibits cytokine production by activated macrophages. J. Immunol. 1991, 147, 3815–3822. [Google Scholar] [CrossRef] [PubMed]
- Poe, J.C.; Wagner, D.H.; Miller, R.W.; Stout, R.D.; Suttles, J. IL-4 and IL-10 modulation of CD40-mediated signaling of monocyte IL-1beta synthesis and rescue from apoptosis. J. Immunol. 1997, 159, 846–852. [Google Scholar] [CrossRef] [PubMed]
- Boehm, U.; Klamp, T.; Groot, M.; Howard, J.C. Cellular responses to interferon-gamma. Annu. Rev. Immunol. 1997, 15, 749–795. [Google Scholar] [CrossRef]
- Franceschini, D.; Paroli, M.; Francavilla, V.; Videtta, M.; Morrone, S.; Labbadia, G.; Cerino, A.; Mondelli, M.U.; Barnaba, V. PD-L1 negatively regulates CD4+CD25+Foxp3+ Tregs by limiting STAT-5 phosphorylation in patients chronically infected with HCV. J. Clin. Investig. 2009, 119, 551. [Google Scholar] [CrossRef]
- Boettler, T.; Spangenberg, H.C.; Neumann-Haefelin, C.; Panther, E.; Urbani, S.; Ferrari, C.; Blum, H.E.; von Weizsäcker, F.; Thimme, R. T Cells with a CD4+CD25+ Regulatory Phenotype Suppress In Vitro Proliferation of Virus-Specific CD8+ T Cells during Chronic Hepatitis C Virus Infection. J. Virol. 2005, 79, 7860. [Google Scholar] [CrossRef]
- Cabrera, R.; Tu, Z.; Xu, Y.; Firpi, R.J.; Rosen, H.R.; Liu, C.; Nelson, D.R. An immunomodulatory role for CD4(+)CD25(+) regulatory T lymphocytes in hepatitis C virus infection. Hepatology 2004, 40, 1062–1071. [Google Scholar] [CrossRef]
- Rushbrook, S.M.; Ward, S.M.; Unitt, E.; Vowler, S.L.; Lucas, M.; Klenerman, P.; Alexander, G.J.M. Regulatory T Cells Suppress In Vitro Proliferation of Virus-Specific CD8+ T Cells during Persistent Hepatitis C Virus Infection. J. Virol. 2005, 79, 7852. [Google Scholar] [CrossRef]
- Semmo, N.; Day, C.L.; Ward, S.M.; Lucas, M.; Harcourt, G.; Loughry, A.; Klenerman, P. Preferential loss of IL-2-secreting CD4+ T helper cells in chronic HCV infection. Hepatology 2005, 41, 1019–1028. [Google Scholar] [CrossRef]
- Prezzi, C.; Casciaro, M.A.; Francavilla, V.; Schiaffella, E.; Finocchi, L.; Chircu, L.V.; Bruno, G.; Sette, A.; Abrignani, S.; Barnaba, V. Virus-specific CD8 + T cells with type 1 or type 2 cytokine profile are related to different disease activity in chronic hepatitis C virus infection. Eur. J. Immunol. 2001, 31, 894–906. [Google Scholar] [CrossRef]
- Sliva, J.; Pantzartzi, C.N.; Votava, M. Inosine Pranobex: A Key Player in the Game Against a Wide Range of Viral Infections and Non-Infectious Diseases. Adv. Ther. 2019, 36, 1878. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).