Blood Flow Restriction in Oncological Patients: Advantages and Safety Considerations
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Evaluation of Methodological Rigor
2.4. Selection and Data Extraction Process
2.5. Bias Risk
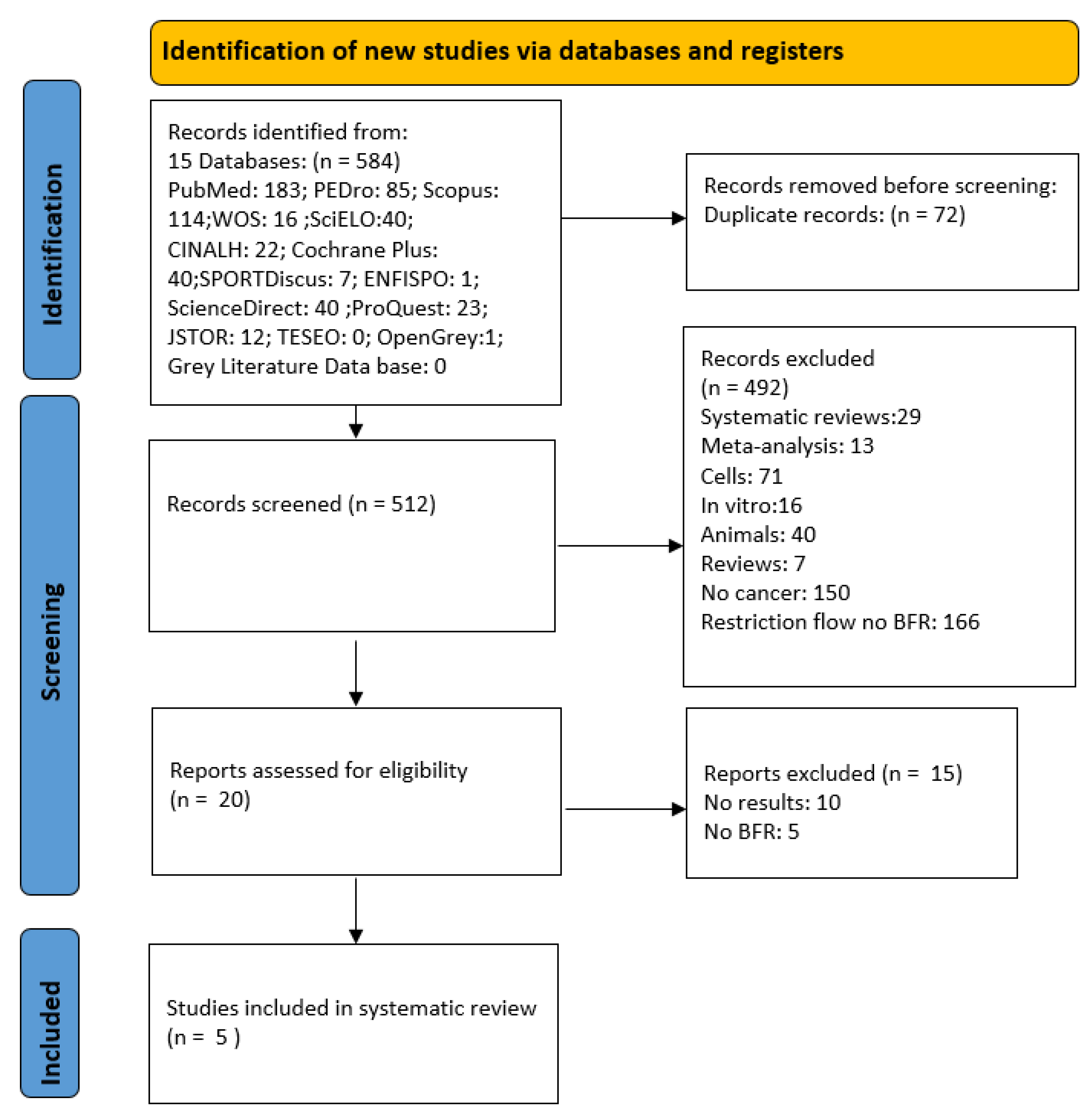
3. Results
3.1. Selection of Studies
3.2. Data Extraction
3.2.1. Characteristics of the Subjects
3.2.2. Characteristics of the Interventions
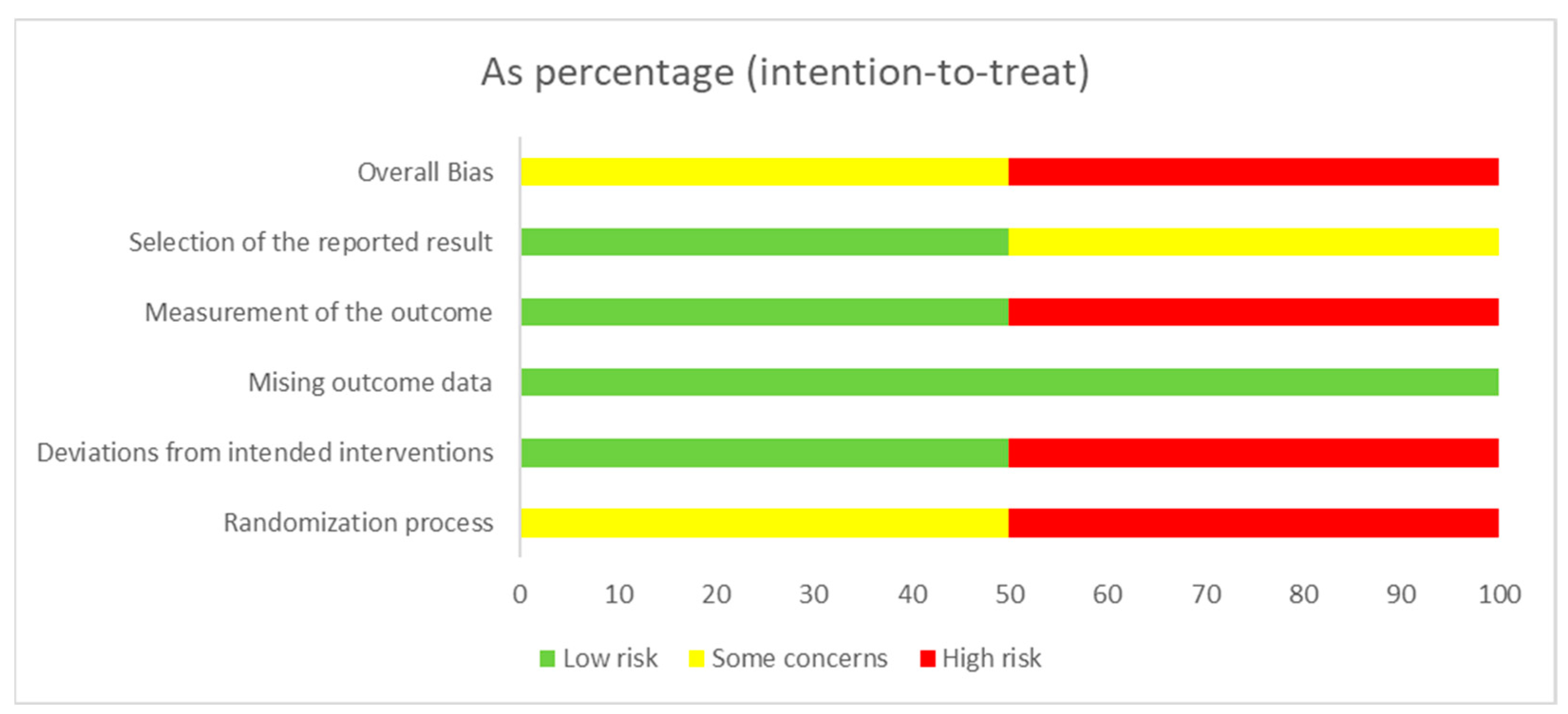
3.2.3. Methodological Quality Assessment and Risk of Bias
4. Discussion
4.1. The Potential of BFR in Cancer Treatment
4.2. Muscular Adaptation and Sarcopenia
4.3. BFR Exercise Interventions and Outcomes
4.4. Frequency and Variables Studied
4.5. Safety Considerations
4.6. Use the BFR in Prehabilitation
4.7. Limitations and Future Directions
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Miller, K.D.; Nogueira, L.; Devasia, T.; Mariotto, A.B.; Yabroff, K.R.; Jemal, A.; Kramer, J.; Siegel, R.L. Cancer Treatment and Survivorship Statistics, 2022. CA Cancer J. Clin. 2022, 72, 409–436. [Google Scholar] [CrossRef] [PubMed]
- Aydin, M.; Kose, E.; Odabas, I.; Bingul, B.M.; Demirci, D.; Aydin, Z. The Effect of Exercise on Life Quality and Depression Levels of Breast Cancer Patients. Asian Pac. J. Cancer Prev. 2021, 22, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Stout, N.L.; Baima, J.; Swisher, A.K.; Winters-Stone, K.M.; Welsh, J. A Systematic Review of Exercise Systematic Reviews in the Cancer Literature (2005–2017). PM R 2017, 9, S347–S384. [Google Scholar] [CrossRef] [PubMed]
- Pollán, M.; Casla-Barrio, S.; Alfaro, J.; Esteban, C.; Segui-Palmer, M.A.; Lucia, A.; Martín, M. Exercise and Cancer: A Position Statement from the Spanish Society of Medical Oncology. Clin. Transl. Oncol. 2020, 22, 1710–1729. [Google Scholar] [CrossRef]
- Mishra, S.I.; Scherer, R.W.; Snyder, C.; Geigle, P.M.; Berlanstein, D.R.; Topaloglu, O. Exercise Interventions on Health-Related Quality of Life for People with Cancer during Active Treatment. Cochrane Database Syst. Rev. 2012, 2012, CD008465. [Google Scholar] [CrossRef] [PubMed]
- Avendaño, F.R.; Arenas, N.S.; Victoria, A.G.; Tello, N.C. Prescripción del ejercicio en el paciente oncológico. Revisión sistemáticaPrescription of exercise in cancer patients. Systematic review. Fisioterapia 2021, 43, 218–229. [Google Scholar] [CrossRef]
- Ligibel, J.A.; Denlinger, C.S. New NCCN Guidelines® for Survivorship Care. JNCCN J. Natl. Compr. Cancer Netw. 2013, 11, 640–644. [Google Scholar] [CrossRef]
- Winters-Stone, K.M.; Neil, S.E.; Campbell, K.L. Attention to Principles of Exercise Training: A Review of Exercise Studies for Survivors of Cancers Other than Breast. Br. J. Sports Med. 2014, 48, 987–995. [Google Scholar] [CrossRef]
- Wolin, K.Y.; Schwartz, A.L.; Matthews, C.E.; Courneya, K.S.; Schmitz, K.H. Implementing the Exercise Guidelines for Cancer Survivors. J. Support. Oncol. 2012, 10, 171–177. [Google Scholar] [CrossRef]
- Mustian, K.M.; Alfano, C.M.; Heckler, C.; Kleckner, A.S.; Kleckner, I.R.; Leach, C.R.; Mohr, D.; Palesh, O.G.; Peppone, L.J.; Piper, B.F.; et al. Comparison of Pharmaceutical, Psychological, and Exercise Treatments for Cancer-Related Fatigue: A Meta-Analysis. JAMA Oncol. 2017, 3, 961–968. [Google Scholar] [CrossRef]
- Schmitz, K.H.; Courneya, K.S.; Matthews, C.; Demark-Wahnefried, W.; Galvão, D.A.; Pinto, B.M.; Irwin, M.L.; Wolin, K.Y.; Segal, R.J.; Lucia, A.; et al. American College of Sports Medicine Roundtable on Exercise Guidelines for Cancer Survivors. Med. Sci. Sports Exerc. 2010, 42, 1409–1426. [Google Scholar] [CrossRef] [PubMed]
- Instituto Nacional del Cáncer Hoja Informativa Sobre La Actividad Física y El Cáncer. Available online: https://www.cancer.gov/espanol/cancer/causas-prevencion/riesgo/obesidad/actividad-fisica-hoja-informativa (accessed on 26 May 2023).
- Conceição, M.S.; Ugrinowitsch, C. Exercise with Blood Flow Restriction: An Effective Alternative for the Non-Pharmaceutical Treatment for Muscle Wasting. J. Cachexia. Sarcopenia Muscle 2019, 10, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Pearson, S.J.; Hussain, S.R. A Review on the Mechanisms of Blood-Flow Restriction Resistance Training-Induced Muscle Hypertrophy. Sports Med. 2015, 45, 187–200. [Google Scholar] [CrossRef]
- Loenneke, J.P.; Wilson, J.M.; Marín, P.J.; Zourdos, M.C.; Bemben, M.G. Low Intensity Blood Flow Restriction Training: A Meta-Analysis. Eur. J. Appl. Physiol. 2012, 112, 1849–1859. [Google Scholar] [CrossRef] [PubMed]
- Wortman, R.J.; Brown, S.M.; Savage-Elliott, I.; Finley, Z.J.; Mulcahey, M.K. Blood Flow Restriction Training for Athletes: A Systematic Review. Am. J. Sports Med. 2021, 49, 1938–1944. [Google Scholar] [CrossRef] [PubMed]
- Hughes, L.; Paton, B.; Rosenblatt, B.; Gissane, C.; Patterson, S.D. Blood Flow Restriction Training in Clinical Musculoskeletal Rehabilitation: A Systematic Review and Meta-Analysis. Br. J. Sports Med. 2017, 51, 1003–1011. [Google Scholar] [CrossRef]
- Lorenz, D.S.; Bailey, L.; Wilk, K.E.; Mangine, R.E.; Head, P.; Grindstaff, T.L.; Morrison, S. Blood Flow Restriction Training. J. Athl. Train. 2021, 56, 937–944. [Google Scholar] [CrossRef]
- Thiebaud, R.; Loenneke, J.P.; Abe, T. COPD and Muscle Loss: Is Blood Flow Restriction a Potential Treatment? J. Trainology 2014, 3, 1–5. [Google Scholar] [CrossRef]
- Linero, C.; Choi, S.J. Effect of Blood Flow Restriction during Low-Intensity Resistance Training on Bone Markers and Physical Functions in Postmenopausal Women. J. Exerc. Sci. Fit. 2021, 19, 57–65. [Google Scholar] [CrossRef]
- Alves, T.C.; Santos, A.P.; Abdalla, P.P.; Venturini, A.C.R.; Angelotti, P.S.; Borges, F.G.; Reis, H.D.O.; Bollela, V.R.; Mota, J.; Machado, D.R.L. Resistance Training with Blood Flow Restriction: Impact on the Muscle Strength and Body Composition in People Living with HIV/AIDS. Eur. J. Sport Sci. 2020, 21, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Centner, C.; Wiegel, P.; Gollhofer, A.; König, D. Effects of Blood Flow Restriction Training on Muscular Strength and Hypertrophy in Older Individuals: A Systematic Review and Meta-Analysis. Sports Med. 2019, 49, 95–108. [Google Scholar] [CrossRef]
- De Souza, T.M.F.; Libardi, C.A.; Cavaglieri, C.R.; Gáspari, A.F.; Brunelli, D.T.; De Souza, G.V.; Ugrinowitsch, C.; Min Li, L.; Chacon-Mikahil, M.P.T. Concurrent Training with Blood Flow Restriction Does Not Decrease Inflammatory Markers. Int. J. Sports Med. 2018, 39, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Corrêa, H.L.; Neves, R.V.P.; Deus, L.A.; Souza, M.K.; Haro, A.S.; Costa, F.; Silva, V.L.; Santos, C.A.R.; Moraes, M.R.; Simões, H.G.; et al. Blood Flow Restriction Training Blunts Chronic Kidney Disease Progression in Humans. Med. Sci. Sports Exerc. 2021, 53, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Cahalin, L.P.; Formiga, M.F.; Owens, J.; Anderson, B.; Hughes, L. Beneficial Role of Blood Flow Restriction Exercise in Heart Disease and Heart Failure Using the Muscle Hypothesis of Chronic Heart Failure and a Growing Literature. Front. Physiol. 2022, 13, 924557. [Google Scholar] [CrossRef]
- Saatmann, N.; Zaharia, O.P.; Loenneke, J.P.; Roden, M.; Pesta, D.H. Effects of Blood Flow Restriction Exercise and Possible Applications in Type 2 Diabetes. Trends Endocrinol. Metab. 2021, 32, 106–117. [Google Scholar] [CrossRef]
- Lamberti, N.; Straudi, S.; Donadi, M.; Tanaka, H.; Basaglia, N.; Manfredini, F. Effectiveness of Blood Flow-Restricted Slow Walking on Mobility in Severe Multiple Sclerosis: A Pilot Randomized Trial. Scand. J. Med. Sci. Sports 2020, 30, 1999–2009. [Google Scholar] [CrossRef]
- Mun, D.-J.; Park, J.-C. Effect of Strength Training Combined with Blood Flow Restriction Exercise on Leg Muscle Thickness in Children with Cerebral Palsy. PNF Mov. 2021, 19, 441–449. [Google Scholar] [CrossRef]
- Skiba, G.H.; Andrade, S.F.; Rodacki, A.F. Effects of Functional Electro-Stimulation Combined with Blood Flow Restriction in Affected Muscles by Spinal Cord Injury. Neurol. Sci. 2022, 43, 603–613. [Google Scholar] [CrossRef]
- Minniti, M.C.; Statkevich, A.P.; Kelly, R.L.; Rigsby, V.P.; Exline, M.M.; Rhon, D.I.; Clewley, D. The Safety of Blood Flow Restriction Training as a Therapeutic Intervention for Patients With Musculoskeletal Disorders: A Systematic Review. Am. J. Sports Med. 2020, 48, 1773–1785. [Google Scholar] [CrossRef]
- Pin, F.; Couch, M.E.; Bonetto, A. Preservation of Muscle Mass as a Strategy to Reduce the Toxic Effects of Cancer Chemotherapy on Body Composition. Curr. Opin. Support. Palliat. Care 2018, 12, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Xiong, F.; Feng, J.; Mu, P.; Deng, Q.; Guo, H.; Zhang, W. Effects of Blood Flow Restriction Exercise on Muscular Endurance and Cardiopulmonary Endurance: A Systematic Review and Meta-Analysis. Res. Sq. 2022, preprint. [Google Scholar] [CrossRef]
- Kohlbrenner, D.; Aregger, C.; Osswald, M.; Sievi, N.A.; Clarenbach, C.F. Blood-Flow-Restricted Strength Training Combined With High-Load Strength and Endurance Training in Pulmonary Rehabilitation for COPD: A Case Report. Phys. Ther. 2021, 101, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Strasser, B.; Pesta, D. Resistance Training for Diabetes Prevention and Therapy: Experimental Findings and Molecular Mechanisms. BioMed Res. Int. 2013, 2013, 805217. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Altman, D.; Antes, G.; Atkins, D.; Barbour, V.; Barrowman, N.; Berlin, J.A.; et al. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, 873–880. [Google Scholar] [CrossRef]
- Hutton, B.; Catalá-López, F.; Moher, D. La Extensión de La Declaración PRISMA Para Revisiones Sistemáticas Que Incorporan Metaanálisis En Red: PRISMA-NMA. Med. Clin. 2016, 147, 262–266. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Vandenbroucke, J.P.; von Elm, E.; Altman, D.G.; Gøtzsche, P.C.; Mulrow, C.D.; Pocock, S.J.; Poole, C.; Schlesselman, J.J.; Egger, M.; Blettner, M.; et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and Elaboration. Int. J. Surg. 2014, 12, 1500–1524. [Google Scholar] [CrossRef]
- Green, S. JPT Higgins Cochrane Handbook for Systematic Reviews of Interventions; Version 5.0.2; The Cochrane Collaboration: Melbourne, Australia, 2009. [Google Scholar]
- Wooten, S.V.; Fleming, R.Y.D.; Wolf, J.S.; Stray-Gundersen, S.; Bartholomew, J.B.; Mendoza, D.; Stanforth, P.R.; Stanforth, D.; Hernandez, L.M.; Tanaka, H. Prehabilitation Program Composed of Blood Flow Restriction Training and Sports Nutrition Improves Physical Functions in Abdominal Cancer Patients Awaiting Surgery. Eur. J. Surg. Oncol. 2021, 47, 2952–2958. [Google Scholar] [CrossRef]
- Wooten, S.V.; Wolf, J.S.; Mendoza, D.; Bartholomew, J.B.; Stanforth, P.R.; Stanforth, D.; Tanaka, H.; Fleming, R.Y.D. The Impact of a Multimodal Sport Science-Based Prehabilitation Program on Clinical Outcomes in Abdominal Cancer Patients: A Cohort Study. Am. Surg. 2022, 88, 2302–2308. [Google Scholar] [CrossRef]
- Wang, T.; Stanforth, P.R.; Fleming, R.Y.D.; Wolf, J.S., Jr.; Stanforth, D.; Tanaka, H. A Mobile App With Multimodality Prehabilitation Programs for Patients Awaiting Elective Surgery: Development and Usability Study. JMIR Perioper. Med. 2021, 4, e32575. [Google Scholar] [CrossRef] [PubMed]
- Adimi, S.; Azarbayjani, M.A.; Naderi, N.; Alizadehasl, A. Comparative Study of the Effect of Aerobic Training Mode and Blood Flow Restriction on Quality of Life in Cardiotoxic Women after Chemotherapy for Breast Cancer: A Double-Blind Randomized Clinical Trial. J. Cardiovasc. Nurs. 2020, 9, 176–185. [Google Scholar]
- Adimi, S.; Azarbayjani, M.A.; Naderi, N.; Alizadehasl, A. Effects of 12 Weeks of Aerobic Exercise (High-Intensity Interval Training or Moderate-Intensity Continuous Training) with and without Blood Flow Restriction on Anthropometric Indices in Women with Cardiotoxicity after Breast Cancer Treatment. Iran. J. Breast Dis. 2022, 15, 18–25. [Google Scholar] [CrossRef]
- Sanderson, S.; Tatt, I.D.; Higgins, J.P.T. Tools for Assessing Quality and Susceptibility to Bias in Observational Studies in Epidemiology: A Systematic Review and Annotated Bibliography. Int. J. Epidemiol. 2007, 36, 666–676. [Google Scholar] [CrossRef] [PubMed]
- Messaggi-Sartor, M.; Marco, E.; Martínez-Téllez, E.; Rodriguez-Fuster, A.; Palomares, C.; Chiarella, S.; Muniesa, J.M.; Orozco-Levi, M.; Barreiro, E.; Güell, M.R. Combined Aerobic Exercise and High-Intensity Respiratory Muscle Training in Patients Surgically Treated for Non-Small Cell Lung Cancer: A Pilot Randomized Clinical Trial. Eur. J. Phys. Rehabil. Med. 2019, 55, 113–122. [Google Scholar] [CrossRef]
- Samuel, S.R.; Maiya, A.G.; Fernandes, D.J.; Guddattu, V.; Saxena, P.P.; Kurian, J.R.; Lin, P.J.; Mustian, K.M. Effectiveness of Exercise-Based Rehabilitation on Functional Capacity and Quality of Life in Head and Neck Cancer Patients Receiving Chemo-Radiotherapy. Support. Care Cancer 2019, 27, 3913–3920. [Google Scholar] [CrossRef]
- Dieli-Conwright, C.M.; Courneya, K.S.; Demark-Wahnefried, W.; Sami, N.; Lee, K.; Sweeney, F.C.; Stewart, C.; Buchanan, T.A.; Spicer, D.; Tripathy, D.; et al. Aerobic and Resistance Exercise Improves Physical Fitness, Bone Health, and Quality of Life in Overweight and Obese Breast Cancer Survivors: A Randomized Controlled Trial. Breast Cancer Res. 2018, 20, 124. [Google Scholar] [CrossRef]
- Minnella, E.M.; Awasthi, R.; Loiselle, S.E.; Agnihotram, R.V.; Ferri, L.E.; Carli, F. Effect of Exercise and Nutrition Prehabilitation on Functional Capacity in Esophagogastric Cancer Surgery: A Randomized Clinical Trial. JAMA Surg. 2018, 153, 1081–1089. [Google Scholar] [CrossRef]
- Pérez Regalado, S.; León, J.; Feriche, B. Therapeutic Approach for Digestive System Cancers and Potential Implications of Exercise under Hypoxia Condition: What Little Is Known? A Narrative Review. J. Cancer Res. Clin. Oncol. 2022, 148, 1107–1121. [Google Scholar] [CrossRef]
- Ashcraft, K.A.; Warner, A.B.; Jones, L.W.; Dewhirst, M.W. Exercise as Adjunct Therapy in Cancer. Semin. Radiat. Oncol. 2019, 29, 16–24. [Google Scholar] [CrossRef]
- Arvelo, F.; Cotte, C. Hipoxia En La Malignidad Del Cáncer. Revisión. Investig. Clin. 2009, 50, 529–546. [Google Scholar]
- Xu, Y.; Kuai, R.; Chu, Y.M.; Zhou, L.; Zhang, H.Q.; Li, J. Hypoxia Facilitates the Proliferation of Colorectal Cancer Cells by Inducing Cancer-Associated Fibroblast-Derived IL6. Neoplasma 2021, 68, 1015–1022. [Google Scholar] [CrossRef] [PubMed]
- Loenneke, J.P.; Wilson, G.J.; Wilson, J.M. A Mechanistic Approach to Blood Flow Occlusion. Int. J. Sports Med. 2010, 31, 1–4. [Google Scholar] [CrossRef]
- Zhang, X.M.; Dou, Q.L.; Zeng, Y.; Yang, Y.; Cheng, A.S.K.; Zhang, W.W. Sarcopenia as a Predictor of Mortality in Women with Breast Cancer: A Meta-Analysis and Systematic Review. BMC Cancer 2020, 20, 172. [Google Scholar] [CrossRef] [PubMed]
- Bahamondes-Ávila, C.; Ponce-Fuentes, F.; Chahin-Inostroza, N.; Bracho-Milic, F.; Navarrete-Hidalgo, C. Entrenamiento de Fuerza Con Restricción Parcial Del Flujo Sanguíneo En Adultos Mayores Con Sarcopenia. SciELO Public. Health 2021, 46, e1105. [Google Scholar]
- van Waart, H.; Stuiver, M.M.; van Harten, W.H.; Geleijn, E.; de Maaker-Berkhof, M.; Schrama, J.; Geenen, M.M.; Meerum Terwogt, J.M.; van den Heiligenberg, S.M.; Hellendoorn-van Vreeswijk, J.A.J.H.; et al. Recruitment to and Pilot Results of the PACES Randomized Trial of Physical Exercise during Adjuvant Chemotherapy for Colon Cancer. Int. J. Colorectal Dis. 2018, 33, 29–40. [Google Scholar] [CrossRef]
- McMillan, E.M.; Newhouse, I.J. Exercise Is an Effective Treatment Modality for Reducing Cancer-Related Fatigue and Improving Physical Capacity in Cancer Patients and Survivors: A Meta-Analysis. Appl. Physiol. Nutr. Metab. 2011, 36, 892–903. [Google Scholar] [CrossRef]
- Stout, N.L.; Binkley, J.M.; Schmitz, K.H.; Andrews, K.; Hayes, S.C.; Campbell, K.L.; McNeely, M.L.; Soballe, P.W.; Berger, A.M.; Cheville, A.L.; et al. A Prospective Surveillance Model for Rehabilitation for Women with Breast Cancer. Cancer 2012, 118, 2191–2200. [Google Scholar] [CrossRef]
- Cataldi, S.; Greco, G.; Mauro, M.; Fischetti, F. Effect of Exercise on Cancer-Related Fatigue: A Systematic Review. J. Hum. Sport Exerc. 2021, 16, 476–492. [Google Scholar] [CrossRef]
- Clark, B.C.; Manini, T.M.; Hoffman, R.L.; Williams, P.S.; Guiler, M.K.; Knutson, M.J.; McGlynn, M.L.; Kushnick, M.R. Relative Safety of 4 Weeks of Blood Flow-Restricted Resistance Exercise in Young, Healthy Adults. Scand. J. Med. Sci. Sports 2011, 21, 653–662. [Google Scholar] [CrossRef]
- Campbell, K.L.; Winters-Stone, K.; Wiskemann, J.; May, A.M.; Schwartz, A.L.; Courneya, K.S.; Zucker, D.; Matthews, C.; Ligibel, J.; Gerber, L.; et al. Exercise Guidelines for Cancer Survivors: Consensus Statement from International Multidisciplinary Roundtable. Med. Sci. Sports Exerc. 2019, 51, 2375. [Google Scholar] [CrossRef] [PubMed]
- Toohey, K.; Pumpa, K.; McKune, A.; Cooke, J.; Semple, S. High-Intensity Exercise Interventions in Cancer Survivors: A Systematic Review Exploring the Impact on Health Outcomes. J. Cancer Res. Clin. Oncol. 2018, 144, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Loenneke, J.P.; Kim, D.; Fahs, C.A.; Thiebaud, R.S.; Abe, T.; Larson, R.D.; Bemben, D.A.; Bemben, M.G. The Influence of Exercise Load with and without Different Levels of Blood Flow Restriction on Acute Changes in Muscle Thickness and Lactate. Clin. Physiol. Funct. Imaging 2017, 37, 734–740. [Google Scholar] [CrossRef]
- Romero-Arenas, S.; Martínez-Pascual, M.; Alcaraz, P.E. Impact of Resistance Circuit Training on Neuromuscular, Cardiorespiratory and Body Composition Adaptations in the Elderly. Aging Dis. 2013, 4, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Perera, E.; Zhu, X.M.; Horner, N.S.; Bedi, A.; Ayeni, O.R.; Khan, M. Effects of Blood Flow Restriction Therapy for Muscular Strength, Hypertrophy, and Endurance in Healthy and Special Populations: A Systematic Review and Meta-Analysis. Clin. J. Sport Med. 2022, 32, 531–545. [Google Scholar] [CrossRef] [PubMed]
- Campbell, K.L.; Zadravec, K.; Bland, K.A.; Chesley, E.; Wolf, F.; Janelsins, M.C. The Effect of Exercise on Cancer-Related Cognitive Impairment and Applications for Physical Therapy: Systematic Review of Randomized Controlled Trials. Phys. Ther. 2020, 100, 523–542. [Google Scholar] [CrossRef]
- Argilés, J.M.; Busquets, S.; Stemmler, B.; López-Soriano, F.J. Cancer Cachexia: Understanding the Molecular Basis. Nat. Rev. Cancer 2014, 14, 754–762. [Google Scholar] [CrossRef]
- van de Worp, W.R.P.H.; Schols, A.M.W.J.; Theys, J.; van Helvoort, A.; Langen, R.C.J. Nutritional Interventions in Cancer Cachexia: Evidence and Perspectives From Experimental Models. Front. Nutr. 2020, 7, 601329. [Google Scholar] [CrossRef]
- Hylden, C.; Burns, T.; Stinner, D.; Owens, J. Blood Flow Restriction Rehabilitation for Extremity Weakness: A Case Series. J. Spec. Oper. Med. 2015, 15, 50–56. [Google Scholar] [CrossRef]
- Colapietro, M.; Portnoff, B.; Miller, S.J.; Sebastianelli, W.; Vairo, G.L. Effects of Blood Flow Restriction Training on Clinical Outcomes for Patients With ACL Reconstruction: A Systematic Review. Sports Health 2023, 15, 260–273. [Google Scholar] [CrossRef]
- Giles, L.; Webster, K.E.; Mcclelland, J.; Cook, J.L. Quadriceps Strengthening with and without Blood Flow Restriction in the Treatment of Patellofemoral Pain: A Double-Blind Randomised Trial. Br. J. Sports Med. 2017, 51, 1688–1694. [Google Scholar] [CrossRef] [PubMed]
- The American Cancer Society Managing Cancer-Related Side Effects: Managing Fatigue and Weakness. Available online: https://www.cancer.org/treatment/treatments-and-side-effects/physical-side-effects/fatigue.html (accessed on 30 March 2023).
- Bower, J.E. Cancer-Related Fatigue—Mechanisms, Risk Factors, and Treatments. Nat. Rev. Clin. Oncol. 2014, 11, 597–609. [Google Scholar] [CrossRef] [PubMed]
- Cognetti, D.J.; Sheean, A.J.; Owens, J.G. Blood Flow Restriction Therapy and Its Use for Rehabilitation and Return to Sport: Physiology, Application, and Guidelines for Implementation. Arthrosc. Sports Med. Rehabil. 2022, 4, e71–e76. [Google Scholar] [CrossRef]
- Didier, K.D.; Ederer, A.K.; Reiter, L.K.; Brown, M.; Hardy, R.; Caldwell, J.; Black, C.; Bemben, M.G.; Ade, C.J. Altered Blood Flow Response to Small Muscle Mass Exercise in Cancer Survivors Treated with Adjuvant Therapy. J. Am. Heart Assoc. 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Takarada, Y.; Sato, Y.; Ishii, N. Effects of Resistance Exercise Combined with Vascular Occlusion on Muscle Function in Athletes. Eur. J. Appl. Physiol. 2002, 86, 308–314. [Google Scholar] [CrossRef]
- Cheema, B.; Gaul, C.A.; Lane, K.; Fiatarone Singh, M.A. Progressive Resistance Training in Breast Cancer: A Systematic Review of Clinical Trials. Breast Cancer Res. Treat. 2008, 109, 9–26. [Google Scholar] [CrossRef]
- Aleixo, G.F.P.; Shachar, S.S.; Nyrop, K.A.; Muss, H.B.; Malpica, L.; Williams, G.R. Myosteatosis and Prognosis in Cancer: Systematic Review and Meta-Analysis. Crit. Rev. Oncol. Hematol. 2020, 145, 102839. [Google Scholar] [CrossRef]
- Lan, C.; Liu, Y.; Wang, Y. Effects of Different Exercise Programs on Cardiorespiratory Fitness and Body Composition in College Students. J. Exerc. Sci. Fit. 2022, 20, 62–69. [Google Scholar] [CrossRef]
- Kim, D.; Kuk, D.; Park, H. Effects of Low Intensity Combined Exercise Training with Blood Flow Restriction on Body Composition and Cardiovascular Responses in Elderly Females. J. Korea Acad. Coop. Soc. 2019, 20, 362–370. [Google Scholar] [CrossRef]
- Nakajima, T.; Kurano, M.; Iida, H.; Takano, H.; Oonuma, H.; Morita, T.; Meguro, K.; Sato, Y.; Nagata, T.; KAATSU Training Group. Use and Safety of KAATSU Training: Results of a National Survey. Int. J. KAATSU Train. Res. 2006, 2, 5–13. [Google Scholar] [CrossRef]
- Barbalho, M.; Rocha, A.C.; Seus, T.L.; Raiol, R.; Del Vecchio, F.B.; Coswig, V.S. Addition of Blood Flow Restriction to Passive Mobilization Reduces the Rate of Muscle Wasting in Elderly Patients in the Intensive Care Unit: A within-Patient Randomized Trial. Clin. Rehabil. 2019, 33, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Husmann, F.; Mittlmeier, T.; Bruhn, S.; Zschorlich, V.; Behrens, M. Impact of Blood Flow Restriction Exercise on Muscle Fatigue Development and Recovery. Med. Sci. Sports Exerc. 2018, 50, 436–446. [Google Scholar] [CrossRef] [PubMed]
- Da Cunha Nascimento, D.; Petriz, B.; Da Cunha Oliveira, S.; Vieira, D.C.L.; Funghetto, S.S.; Silva, A.O.; Prestes, J. Effects of Blood Flow Restriction Exercise on Hemostasis: A Systematic Review of Randomized and Non-Randomized Trials. Int. J. Gen. Med. 2019, 12, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Martin, P.M.; Bart, R.M.; Ashley, R.L.; Velasco, T.; Wise, S.R. An Overview of Blood Flow Restriction Physiology and Clinical Considerations. Curr. Sports Med. Rep. 2022, 21, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Ferlito, J.V.; Pecce, S.A.P.; Oselame, L.; De Marchi, T. The Blood Flow Restriction Training Effect in Knee Osteoarthritis People: A Systematic Review and Meta-Analysis. Clin. Rehabil. 2020, 34, 1378–1390. [Google Scholar] [CrossRef]
- Murray, J.; Bennett, H.; Boyle, T.; Williams, M.; Davison, K. Approaches to Determining Occlusion Pressure for Blood Flow Restricted Exercise Training: Systematic Review. J. Sports Sci. 2021, 39, 663–672. [Google Scholar] [CrossRef]
- Clarkson, M.J.; May, A.K.; Warmington, S.A. Is There Rationale for the Cuff Pressures Prescribed for Blood Flow Restriction Exercise? A Systematic Review. Scand. J. Med. Sci. Sports 2020, 30, 1318–1336. [Google Scholar] [CrossRef]
- Michael, C.M.; Lehrer, E.J.; Schmitz, K.H.; Zaorsky, N.G. Prehabilitation Exercise Therapy for Cancer: A Systematic Review and Meta-Analysis. Cancer Med. 2021, 10, 4195–4205. [Google Scholar] [CrossRef]
- Carli, F.; Charlebois, P.; Stein, B.; Feldman, L.; Zavorsky, G.; Kim, D.J.; Scott, S.; Mayo, N.E. Randomized Clinical Trial of Prehabilitation in Colorectal Surgery. Br. J. Surg. 2010, 97, 1187–1197. [Google Scholar] [CrossRef]
- Gillis, C.; Li, C.; Lee, L.; Awasthi, R.; Augustin, B.; Gamsa, A.; Liberman, A.S.; Stein, B.; Charlebois, P.; Feldman, L.S.; et al. Prehabilitation versus RehabilitationA Randomized Control Trial in Patients Undergoing Colorectal Resection for Cancer. Anesthesiology 2014, 121, 937–947. [Google Scholar] [CrossRef]
- Li, C.; Carli, F.; Lee, L.; Charlebois, P.; Stein, B.; Liberman, A.S.; Kaneva, P.; Augustin, B.; Wongyingsinn, M.; Gamsa, A.; et al. Impact of a Trimodal Prehabilitation Program on Functional Recovery after Colorectal Cancer Surgery: A Pilot Study. Surg. Endosc. 2013, 27, 1072–1082. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
| Databases | Search Strategy |
|---|---|
| PubMed | (cancer* OR neoplasm* OR malignan* OR carcinoma OR tumor OR tumor OR oncolog*) AND (blood flow restriction therapy OR Limb occlusion pressure OR blood flow restriction OR Blood flow restriction exercise OR BFR exercise OR KAATSU training OR limb occlusion OR blood flow restrict* OR vascular restrict* OR KAATSU) |
| PEDro | blood flow restriction |
| Scopus | (cancer* OR neoplasm* OR malignan* OR carcinoma OR tumor OR tumor OR oncolog*) AND (Limb occlusion pressure OR “blood flow restriction” OR “Blood flow restriction exercise” OR “Blood flow restriction therapy” OR “BFR exercise” OR “KAATSU training” OR “limb occlusion” OR “blood flow restrict*” OR KAATSU) |
| WOS | (cancer* OR neoplasm* OR malignan* OR carcinoma OR tumor OR tumor OR oncolog*) AND (Limb occlusion pressure OR blood flow restriction OR Blood flow restriction exercise OR BFR exercise OR KAATSU training OR limb occlusion OR blood flow restrict* OR vascular restrict* OR KAATSU) |
| SciELO | “blood flow restriction” AND oncolog* OR cancer OR neoplasm In all indexes |
| CINAHL | (cancer* OR neoplasm* OR malignan* OR carcinoma OR tumor OR tumor OR oncolog*) AND (Limb occlusion pressure OR blood flow restriction OR Blood flow restriction exercise OR BFR exercise OR KAATSU training OR limb occlusion OR blood flow restrict* OR vascular restrict* OR KAATSU) |
| Cochrane Plus | blood flow restriction AND cancer in title, abstract, keywords |
| SPORTDiscus | (cancer* OR neoplasm* OR malignan* OR carcinoma OR tumor OR tumor OR oncolog*) AND (Limb occlusion pressure OR “blood flow restriction” OR “Blood flow restriction exercise” OR “BFR exercise” OR “KAATSU training” OR “limb occlusion” OR “blood flow restrict*” OR KAATSU) |
| ENFISPO | (Limb occlusion pressure OR blood flow restriction OR Blood flow restriction exercise OR BFR exercise OR KAATSU training OR limb occlusion OR blood flow restrict* OR vascular restrict* OR KAATSU) |
| ScienceDirect | “Blood Flow Restriction Therapy” |
| ProQuestResearch Library | abstract (blood flow and exercise) AND abstract (Cancer) |
| Journal Storage | “blood flow restriction” |
| TESEO | “blood flow restriction” |
| OpenGrey | “blood flow restriction” |
| Grey Literature Database | “blood flow restriction” |
| Author/Country | Type of Cancer | Stage of the Disease | Type of Treatment |
|---|---|---|---|
| Adimi et al. (2022) [45] Iran | Breast cancer with cardiotoxity | Not mentioned | chemotherapy |
| Wooten et al. (2022) [42] United States | Abdominal cancer: Pheochromocytoma, Colon with/without hepatic metastasis, Esophageal, Gall bladder, Jejunum, Pancreas, Stomach, Rectal with/without hepatic metastasis, Retroperitoneal, Small intestine with/without hepatic metastasis, Cecum, Leiomyosarcoma, Liver | CG: 2.7 ± 1.6 EC: 3.3 ± 1.0 | Underwent elective cancer-related surgery and usual preoperative care |
| Wooten et al. (2021) [41] United States | Abdominal cancer: Pheochromocytoma/Adrenocortical, Colon or/with Hepatic Metastasis, Esophageal, Gall Bladder 1 Jejunum, Pancreas, Rectal or/with Hepatic Metastasis, Retroperitoneal, Small Intestine or/with Hepatic Metastasis |
| Surgery with different complexity: simple, intermediate or complex. |
| Wang et al. (2021) [43] United States | Abdominal cancer | Not mentioned | surgery |
| Adimi et al. (2020) [44] Iran | Breast cancer with cardiotoxicity | Not mentioned |
|
| Author/Type of Cancer | Treatment | Type of BFR | Variables/Assessment Tools | Results |
|---|---|---|---|---|
| Adimi et al. (2022) [45] Breast cancer | n = 20 G1: n = 5 HITT G2: n = 5 MIT G3: n = 5 HITT+BFR G4: n = 5 MIT+BFR Treadmill 3 days/week 12 weeks | Not mentioned |
|
|
| Wooten et al. (2022) [42] Abdominal cancer | n = 92 BFR group: n = 21 Nutrition supplement + home-based exercise of low-intensity upper and lower body (followed video 45 min) + BFR resistance exercises or 15 min of walking with leg BFR bands (alternated every other day) 5–6 days/week 4 weeks CG: n = 71 no prehabilitation (prior surgery) |
|
|
|
| Wooten et al. (2021) [41] Abdominal cancer | n = 24 BFR + nutrition supplement Body weight and light resistance exercises+ BFR 3 sets (20–30 repetitions/1-min rest between sets) or 15 min of walking with leg BFR bands (alternated every other day) 5–6 days/week 4 weeks (prior surgery) |
|
|
Time to complete the test reduced significantly Baseline: 14.6 s, End of prehabilitation: 9.8 s p-value: 0.03.
Standard Error: ±0.72 s ES: Not provided p: <0.01
4-weeks: 10.8 points p-value: 0.01
|
| Wang et al. (2021) [43] Abdominal cancer | n = 8 BFR exercise and sport nutrition supplement intervention (pro-gram integrated into a mobile app) 4 weeks | Band placement refers to the process of setting up and inflating the BFR (Blood Flow Restriction) bands on users’ arms or legs. This instructional video, created by B Strong, LLC, provides guidance on how to properly set up and use the bands for BFR training. |
program, ease of use of the app, and information load))
|
|
| Adimi et al. (2020) [44] Breast cancer | n = 20 G1: n = 5 HITT G2: n = 5 MIT G3: n = 5 HITT+BFR G4: n = 5 MIT+BFR Treadmill 3 days/week 12 weeks |
|
|
|
| Analyzed Portion | Object | Wooten et al. (2021) [41] | Wooten et al. (2021) [42] | Wang et al. (2021) [43] |
|---|---|---|---|---|
| Title and abstract | 1 | × | × | |
| I: background/rationale | 2 | × | × | × |
| I: objectives | 3 | × | × | × |
| M: study design | 4 | |||
| M: setting | 5 | × | × | |
| M: participants | 6 | × | × | × |
| M: variables | 7 | × | × | × |
| M: data sources/measures | 8 | × | × | × |
| M: biases | 9 | × | ||
| M: study size | 10 | |||
| M: quantitative variables | 11 | × | × | |
| M: statistical methods | 12 | × | × | |
| R: participants | 13 | × | × | × |
| R: descriptive data | 14 | × | × | × |
| R: outcome data | 15 | × | × | × |
| R: main results | 16 | × | × | × |
| R: other analyses | 17 | |||
| D: key results | 18 | × | × | × |
| D: limitations | 19 | × | × | × |
| D: interpretation | 20 | × | × | × |
| D: generalizability | 21 | × | × | |
| D: Other information: funding | 22 | × | × |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vinolo-Gil, M.J.; García-Campanario, I.; Estebanez-Pérez, M.-J.; Pastora-Bernal, J.-M.; Rodríguez-Huguet, M.; Martín-Vega, F.J. Blood Flow Restriction in Oncological Patients: Advantages and Safety Considerations. Healthcare 2023, 11, 2062. https://doi.org/10.3390/healthcare11142062
Vinolo-Gil MJ, García-Campanario I, Estebanez-Pérez M-J, Pastora-Bernal J-M, Rodríguez-Huguet M, Martín-Vega FJ. Blood Flow Restriction in Oncological Patients: Advantages and Safety Considerations. Healthcare. 2023; 11(14):2062. https://doi.org/10.3390/healthcare11142062
Chicago/Turabian StyleVinolo-Gil, Maria Jesus, Ismael García-Campanario, María-José Estebanez-Pérez, José-Manuel Pastora-Bernal, Manuel Rodríguez-Huguet, and Francisco Javier Martín-Vega. 2023. "Blood Flow Restriction in Oncological Patients: Advantages and Safety Considerations" Healthcare 11, no. 14: 2062. https://doi.org/10.3390/healthcare11142062
APA StyleVinolo-Gil, M. J., García-Campanario, I., Estebanez-Pérez, M.-J., Pastora-Bernal, J.-M., Rodríguez-Huguet, M., & Martín-Vega, F. J. (2023). Blood Flow Restriction in Oncological Patients: Advantages and Safety Considerations. Healthcare, 11(14), 2062. https://doi.org/10.3390/healthcare11142062









