The Effect of Kinesio Taping Combined with Virtual-Reality-Based Upper Extremity Training on Upper Extremity Function and Self-Esteem in Stroke Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Procedure
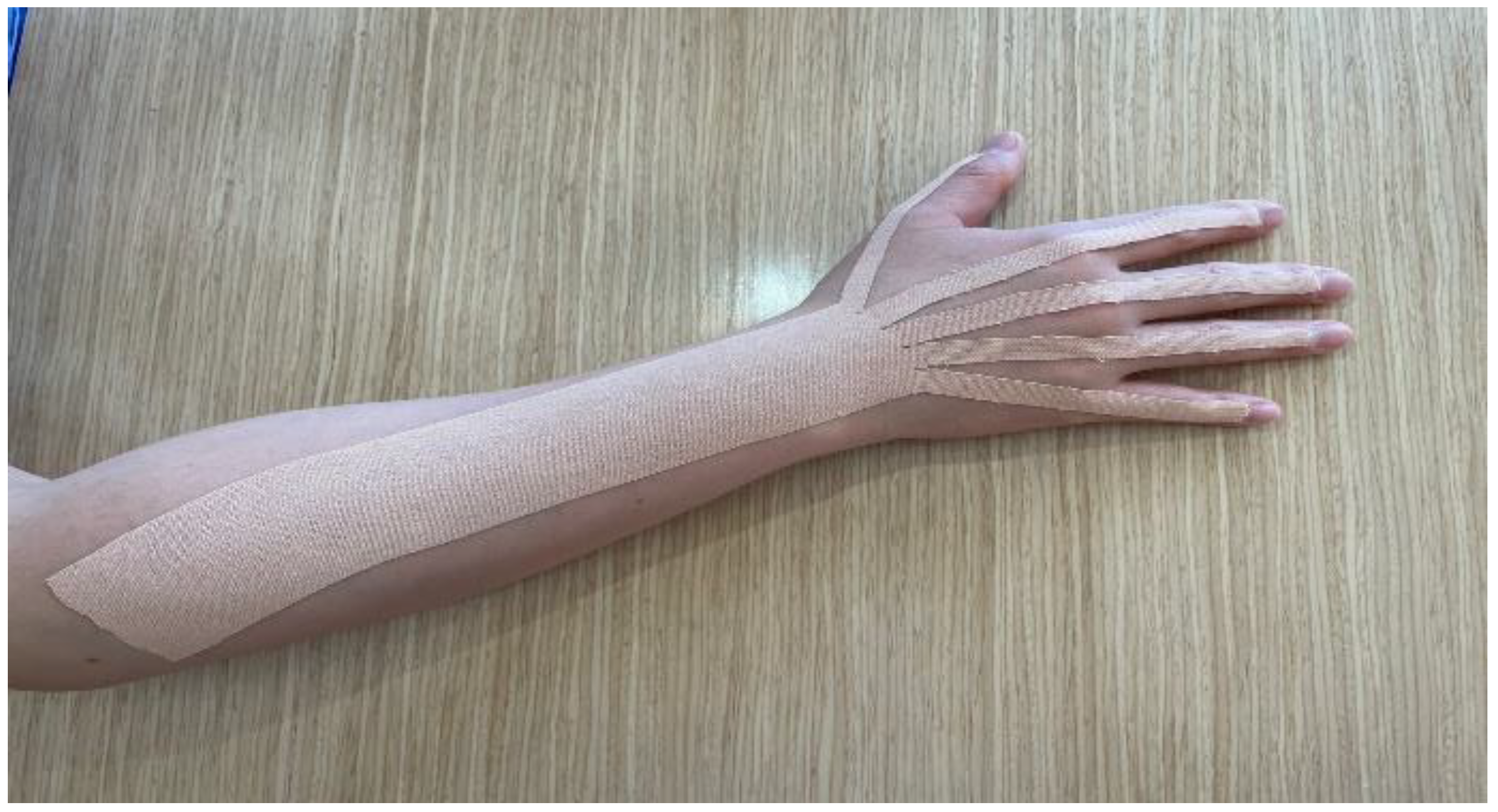
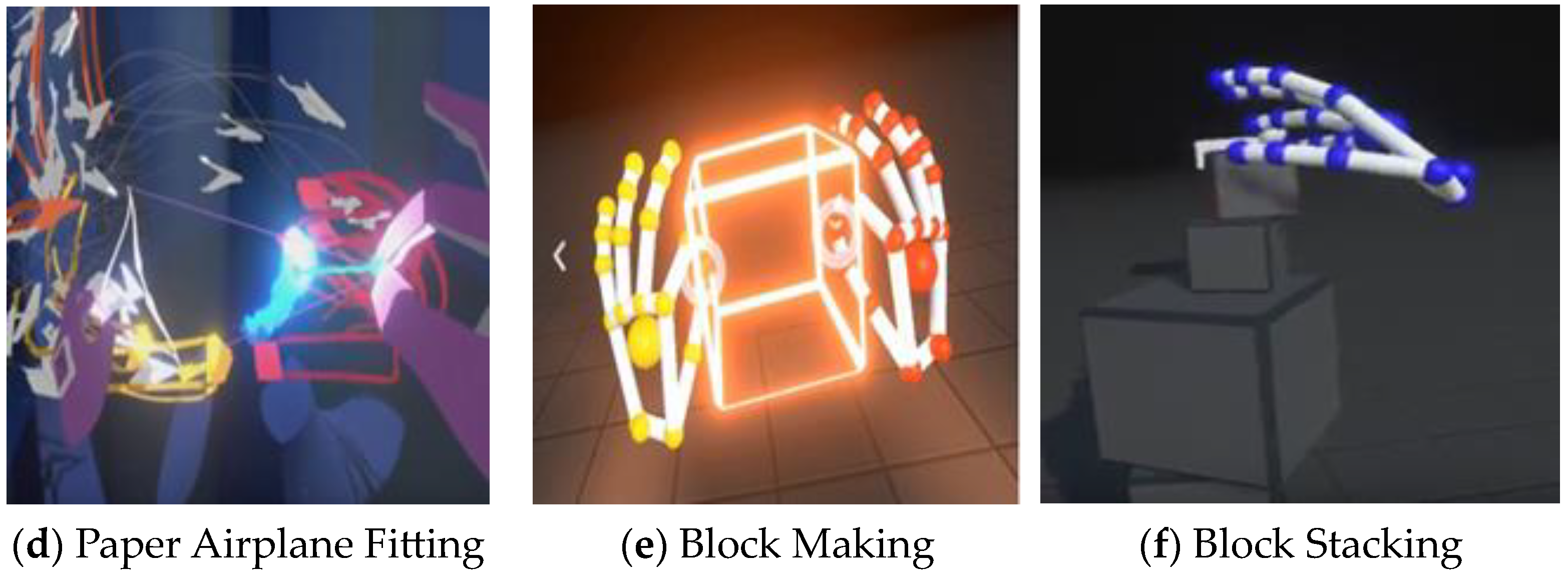
2.3. Intervention
2.4. Outcome Measures
2.4.1. Fugl–Meyer Assessment for the Upper Extremity (FMA-UE)
2.4.2. Wolf Motor Function Test (WMFT)
2.4.3. Motor Activity Log (MAL)
2.4.4. Self-Efficacy Scale (SEF)
2.5. Statistical Analysis
3. Results
3.1. Participants’ Characteristics
3.2. Comparison of Changes in Upper Extremity Function in Groups before and after Intervention
3.3. Comparison of Changes in Self-Efficacy within Groups before and after Intervention
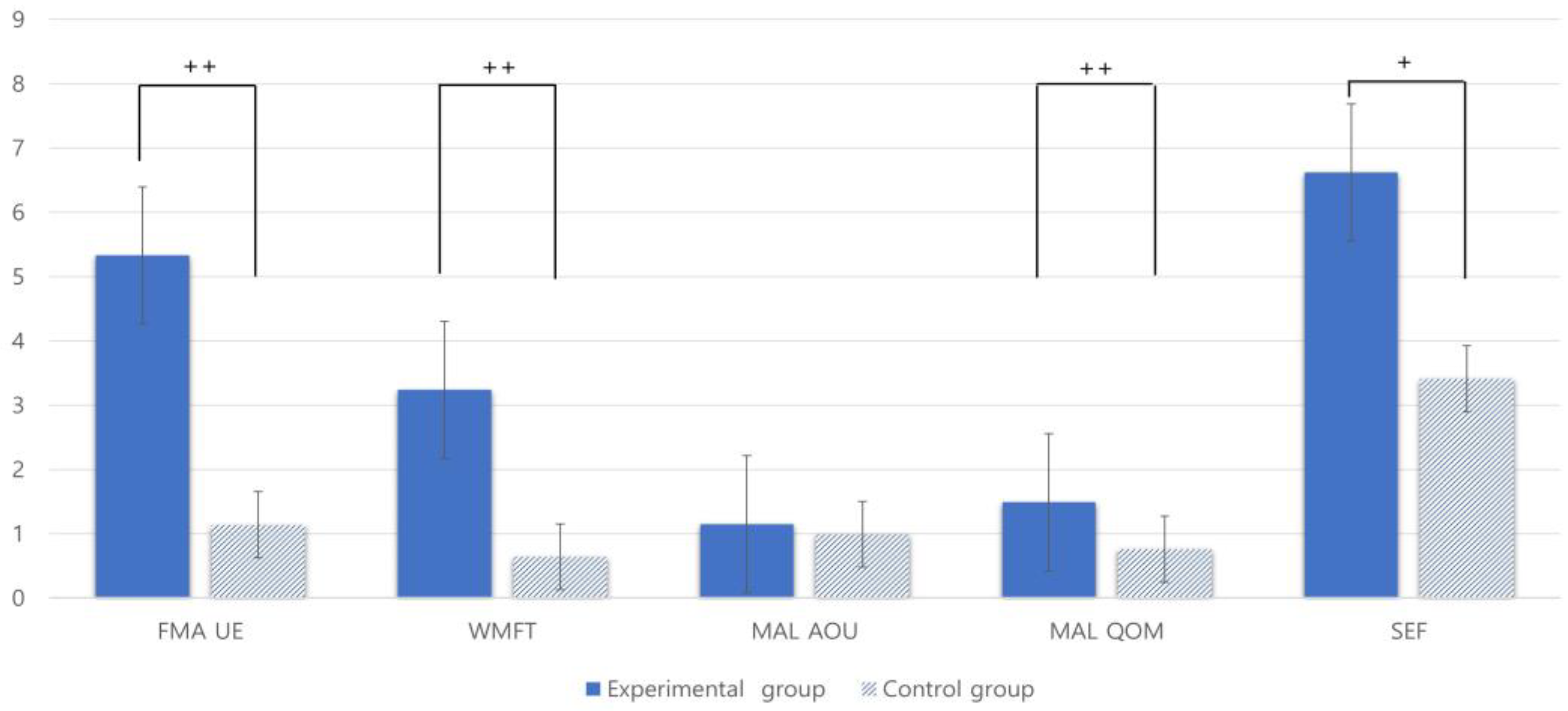
3.4. Comparison of Changes in Upper Extremity Function and Self-Efficacy between Groups before and after Intervention
3.5. Comparison of Changes in Upper Extremity Function and Self-Efficacy in Groups before and after Intervention
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Veldema, J.; Jansen, P. Aquatic therapy in stroke rehabilitation: Systematic review and meta-analysis. Acta Neurol. Scand. 2021, 143, 221–241. [Google Scholar] [CrossRef] [PubMed]
- Jang, D.E.; Shin, J.H. Self-Care Performance of Middle-Aged Stroke Patients in Korea. Clin. Nurs. Res. 2019, 28, 263–279. [Google Scholar] [CrossRef] [PubMed]
- Legg, L.A.; Lewis, S.R.; Schofield-Robinson, O.J.; Drummond, A.; Langhorne, P. Occupational therapy for adults with problems in activities of daily living after stroke. Cochrane Database Syst. Rev. 2017, 7, CD003585. [Google Scholar] [CrossRef] [PubMed]
- Chuluunbaatar, E.; Chou, Y.J.; Pu, C. Quality of life of stroke survivors and their informal caregivers: A prospective study. Disabil. Health J. 2016, 9, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Etoom, M.H.; Hawamdeh, Z.; Alwardat, M.G. Constraint-induced movement therapy as a rehabilitation intervention for upper extremity in stroke patients: Systematic review and meta-analysis. Int. J. Rehabil. Res. 2016, 39, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Michaelsen, S.M.; Dannenbaum, R.; Levin, M.F. Task-specific training with trunk restraint on arm recovery in stroke: Randomized control trial. Stroke 2006, 37, 186–192. [Google Scholar] [CrossRef]
- Zhuang, J.Y.; Ding, L.; Shu, B.B.; Chen, D. Associated Mirror Therapy Enhances Motor Recovery of the Upper Extremity and Daily Function after Stroke: A Randomized Control Study. Neural Plast. 2021, 29, 263–269. [Google Scholar] [CrossRef]
- Stinear, C.M.; Lang, C.E.; Zeiler, S.; Byblow, W.D. Advances and challenges in stroke rehabilitation. Lancet Neurol. 2020, 19, 348–360. [Google Scholar] [CrossRef]
- Israely, S.; Leisman, G.; Carmeli, E. Improvement in arm and hand function after a stroke with task-oriented training. BMJ Case Rep. 2017, 17, 219–239. [Google Scholar] [CrossRef]
- Liepert, J. Evidence-based therapies for upper extremity dysfunction. Curr. Opin. Neurol. 2010, 23, 678–682. [Google Scholar] [CrossRef] [PubMed]
- Alt, M.M.; Björkdahl, A.; Forsberg-Wärleby, G.; Persson, C.U. Implementation of evidence-based assessment of upper extremity in stroke rehabilitation: From evidence to clinical practice. J. Rehabil. Med. 2021, 53, 1977–1986. [Google Scholar]
- Turolla, A.; Dam, M.; Ventura, L. Virtual reality for the rehabilitation of the upper limb motor function after stroke: A prospective controlled trial. Neuroeng. Rehabil. 2013, 10, 85. [Google Scholar] [CrossRef] [PubMed]
- Rizzolatti, G.; Fadiga, L.; Foqassi, L. Resonance behaviors and mirror neurons. Arch. Ital. Biol. 1999, 137, 85–100. [Google Scholar] [PubMed]
- Fabbri-Destro, M.; Rizzolatti, G. Mirror neurons and mirror systems in monkeys and humans. Physiology 2008, 23, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Parma, J.O.; de Assis Leão, S.E.S.; Sales, I.S.; Macedo, L.C. Mirror therapy in upper limb motor recovery and activities of daily living, and its neural correlates in stroke individuals: A systematic review and meta-analysis. Brain Res. Bull. 2021, 177, 217–238. [Google Scholar]
- Sucar, L.E.; Orihuela-Espina, F.; Velazquez, R.L.; Reinkensmeyer, D.J.; Leder, R.; Hernandez-Franco, J. Gesture therapy: An upper limb virtual reality-based motor rehabilitation platform. IEEE. Trans. Neural Syst. Rehabil. Eng. 2014, 22, 634–643. [Google Scholar] [CrossRef]
- Ikbali, A.S.; Mirzayev, I.; Umit, Y.O.; Cosar Saracgil, S.N. Virtual Reality in Upper Extremity Rehabilitation of Stroke Patients: A Randomized Controlled Trial. J. Stroke Cerebrovasc. Dis. 2018, 27, 3473–3478. [Google Scholar] [CrossRef]
- Namdari, S.; Alosh, H.; Baldwin, K.; Mehta, S.; Keenan, M.A. Shoulder tenotomies to improve passive motion and relieve pain in patients with spastic hemiplegia after upper motor neuron injury. J. Shoulder Elbow Surg. 2011, 20, 802–806. [Google Scholar] [CrossRef]
- Kim, H.; Lee, B. The effects of Kinesio tape on isokinetic muscular function of horseracing jockeys. J. Phys. Ther. Sci. 2013, 25, 1273–1277. [Google Scholar] [CrossRef]
- Gramatikova, M.; Nikolova, E.; Mitova, S. Nature, application and effect of kinesio-taping. J. Act. Phys. Educ. Sport 2014, 4, 115–119. [Google Scholar]
- Zhang, S.; Fu, W.; Pan, J.; Wang, L.; Rui, X.; Liu, Y. Acute effects of Kinesio taping on muscle strength and fatigue in the forearm of tennis players. J. Sci. Med. Sport 2015, 6, 1214–1220. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.C.; Chen, P.C.; Tso, H.H.; Yang, Y.C.; Ho, T.L.; Leong, C.P. Effects of kinesio taping on hemiplegic hand in patients with upper limb post-stroke spasticity: A randomized controlled pilot study. Eur. J. Phys. Rehabil. Med. 2019, 55, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Alireza, A.; Amed Aly Ibrahim, A.; Thorsten Alexander, K. Developing a VR training environment for fingers rehabilitation. EuroHaptics 2022, 13, 22–25. [Google Scholar]
- Kim, K.J.; Gang, M.Y. Effect of Taping and Virtual Reality Combined Exercise on Static and Dynamic Balance With Functional Ankle Instability. Phys. Ther. Korea 2020, 27, 292–297. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.G. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef]
- Wang, M.; Pei, Z.-W.; Xiong, B.-D.; Meng, X.-M.; Chen, X.-L.; Liao, W.-J. Use of Kinesio taping in lower-extremity rehabilitation of post-stroke patients: A systematic review and meta-analysis. Complement. Ther. Clin. Pract. 2019, 35, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Irene, C.P.; Noelia, Z.A.; Desirée, M.C.; Rafael, L.V. Leap Motion Controller Video Game-Based Therapy for Upper Extremity Motor Recovery in Patients with Central Nervous System Diseases. A Systematic Review with Meta-Analysis. Sensors 2021, 21, 2065. [Google Scholar]
- Gladstone, D.J.; Danells, C.J.; Black, S.E. The Fugl-Meyer assessment of motor recovery after stroke: A critical review of its measurement properties. Neurorehabil. Neural Repair 2002, 16, 232–240. [Google Scholar] [CrossRef]
- Rech, K.D.; Salazar, A.P.; Marchese, R.R.; Schifino, G.; Cimolin, V.; Pagnussat, A.S. Fugl-Meyer Assessment Scores Are Related With Kinematic Measures in People with Chronic Hemiparesis after Stroke. J. Stroke Cerebrovasc. Dis. 2020, 29, 463–477. [Google Scholar] [CrossRef]
- Wolf, S.L.; Thompson, P.A.; Morris, D.M. The EXCITE trial: Attributes of the Wolf Motor Function Test in patients with subacute stroke. Neurorehabil. Neural Repair 2005, 19, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Cavuoto, L.A.; Subryan, H.; Stafford, M.; Yang, Z.; Bhattacharjya, S.; Xu, W.; Langan, J. Understanding User Requirements for the Design of a Home-Based Stroke Rehabilitation System. Proc. Hum. Factors Ergon. Soc. Annu. Meet. 2018, 62, 1037–1041. [Google Scholar] [CrossRef] [PubMed]
- Magwood, G.S.; Nichols, M.; Jenkins, C.; Logan, A.; Qanungo, S.; Zigbuo-Wenzler, E.; Ellis, C., Jr. Community-Based Interventions for Stroke Provided by Nurses and Community Health Workers: A Review of the Literature. J. Neurosci. Nurs. 2020, 52, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Hiragami, S.; Inoue, Y.; Harada, K. Minimal clinically important difference for the Fugl-Meyer assessment of the upper extremity in convalescent stroke patients with moderate to severe hemiparesis. J. Phys. Ther. Sci. 2019, 31, 917–921. [Google Scholar] [CrossRef] [PubMed]
- Norouzi-Gheidari, N.; Hernandez, A.; Archambault, P.S.; Higgins, J.; Poissant, L.; Kairy, D. Feasibility, Safety and Efficacy of a Virtual Reality Exergame System to Supplement Upper Extremity Rehabilitation Post-Stroke: A Pilot Randomized Clinical Trial and Proof of Principle. Int. J. Environ. Res. Public Health 2019, 17, 113. [Google Scholar] [CrossRef]
- Wee, S.K.; Hughes, A.M.; Warner, M.; Burridge, J.H. Trunk restraint to promote upper extremity recovery in stroke patients: A systematic review and meta-analysis. Neurorehabil. Neural Repair 2014, 28, 660–677. [Google Scholar] [CrossRef] [PubMed]
- Jassi, F.J.; Del Antonio, T.T.; Azevedo, B.O.; Moraes, R.; George, S.Z.; Chaves, T.C. Star-Shape Kinesio Taping Is Not Better Than a Minimal Intervention or Sham Kinesio Taping for Pain Intensity and Postural Control in Chronic Low Back Pain: A Randomized Controlled Trial. Arch. Phys. Med. Rehabil. 2021, 102, 1352–1360.e3. [Google Scholar] [CrossRef]
- Song, G.B.; Park, E.C. Effect of virtual reality games on stroke patients’ balance, gait, depression, and interpersonal relationships. J. Phys. Ther. Sci. 2015, 27, 2057–2060. [Google Scholar] [CrossRef] [PubMed]



| Characteristics | Experimental Group (n = 21) | Control Group (n = 22) | X2/t | p |
|---|---|---|---|---|
| Age (year), mean ± SD | 59.00 ± 12.66 | 63.09 ± 9.66 | −1.186 | 0.243 |
| Gender (male/female) | 11/10 | 13/9 | 0.433 | 0.667 |
| Type of stroke (Hemorrhage/Infarction) | 11/10 | 15/7 | −1.688 | 0.100 |
| Side of stroke (Right/Left) | 12/9 | 13/9 | −0.741 | 0.463 |
| Time since onset of stroke (months), mean ± SD 1 | 3.81 ± 1.66 | 3.86 ± 1.28 | −0.119 | 0.906 |
| Experimental Group | Control Group | Between Groups p-Values | |||||
|---|---|---|---|---|---|---|---|
| Before Treatment | After Treatment | p-Value | Before Treatment | After Treatment | p-Value | ||
| FMA-UE | 22.00 (5.12) | 27.33 (5.25) | <0.000 ** | 22.41 (5.73) | 23.55 (6.03) | <0.000 ** | 0.034 † |
| WMFT | 18.48 (4.78) | 21.71 (5.02) | <0.000 ** | 17.41 (8.23) | 18.05 (5.43) | <0.036 * | 0.027 † |
| MAL-AOU | 0.94 (0.37) | 2.09 (0.78) | <0.000 ** | 1.15 (0.30) | 2.15 (0.56) | <0.000 ** | 0.782 |
| MAL-QOM | 0.91 (0.32) | 2.41 (0.51) | <0.000 ** | 1.13 (0.34) | 1.90 (0.60) | <0.000 ** | 0.015 † |
| SEF | 15.33 (3.74) | 21.95 (4.21) | <0.000 ** | 15.64 (4.53) | 19.05 (4.82) | <0.000 ** | 0.041 † |
| Experimental Group | Control Group | p-Value | |
|---|---|---|---|
| FMA-UE | 5.33 (4.32) | 1.14 (1.16) | <0.000 †† |
| WMFT | 3.24 (2.82) | 0.64 (1.32) | <0.001 †† |
| MAL-AOU | 1.15 (0.65) | 0.99 (0.59) | <0.417 |
| MAL-QOM | 1.49 (0.48) | 0.76 (0.73) | <0.000 †† |
| SEF | 6.62 (4.85) | 3.41 (2.88) | <0.011 † |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.-W.; Ma, S.-R.; Choi, J.-B. The Effect of Kinesio Taping Combined with Virtual-Reality-Based Upper Extremity Training on Upper Extremity Function and Self-Esteem in Stroke Patients. Healthcare 2023, 11, 1813. https://doi.org/10.3390/healthcare11131813
Yang S-W, Ma S-R, Choi J-B. The Effect of Kinesio Taping Combined with Virtual-Reality-Based Upper Extremity Training on Upper Extremity Function and Self-Esteem in Stroke Patients. Healthcare. 2023; 11(13):1813. https://doi.org/10.3390/healthcare11131813
Chicago/Turabian StyleYang, Seo-Won, Sung-Ryong Ma, and Jong-Bae Choi. 2023. "The Effect of Kinesio Taping Combined with Virtual-Reality-Based Upper Extremity Training on Upper Extremity Function and Self-Esteem in Stroke Patients" Healthcare 11, no. 13: 1813. https://doi.org/10.3390/healthcare11131813
APA StyleYang, S.-W., Ma, S.-R., & Choi, J.-B. (2023). The Effect of Kinesio Taping Combined with Virtual-Reality-Based Upper Extremity Training on Upper Extremity Function and Self-Esteem in Stroke Patients. Healthcare, 11(13), 1813. https://doi.org/10.3390/healthcare11131813





