Optimizing Medication Safety with Oral Antitumor Therapy: A Methodological Approach for the Real-World Implementation of the AMBORA Competence and Consultation Center
Abstract
:1. Introduction
2. Materials and Methods
2.1. Setting
2.2. Study Design and Patients
2.3. Implementation Process and Strategies
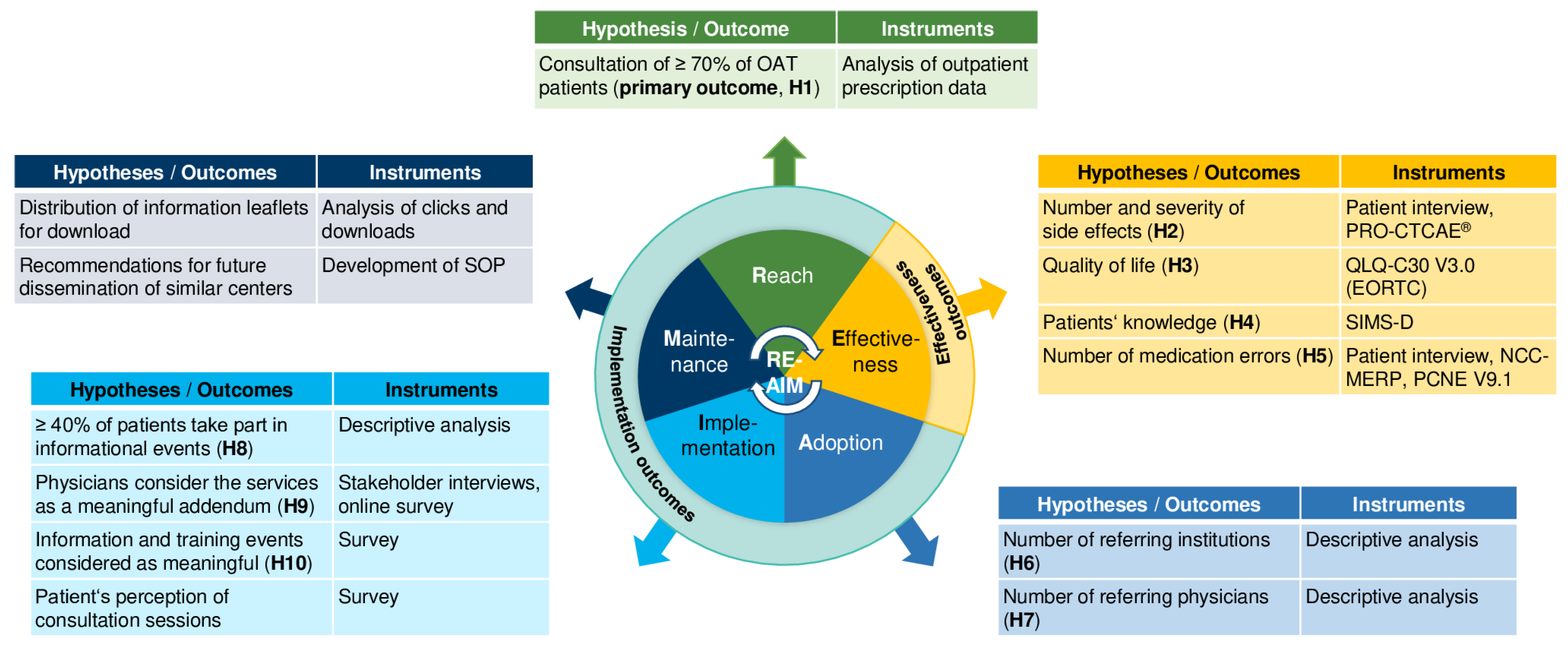
2.3.1. Dimension Reach
2.3.2. Dimension Effectiveness
2.3.3. Dimension Adoption
2.3.4. Dimension Implementation
2.3.5. Semi-Structured Stakeholder Interviews
2.3.6. Dimension Maintenance
2.4. Statistical Analysis
3. Results
3.1. Dimension Reach
3.2. Dimension Adoption
3.3. Dimension Implementation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bauer, M.S.; Kirchner, J. Implementation science: What is it and why should I care? Psychiatry Res. 2020, 283, 112376–112381. [Google Scholar] [CrossRef] [PubMed]
- Theobald, S.; Brandes, N.; Gyapong, M.; El-Saharty, S.; Proctor, E.; Diaz, T.; Wanji, S.; Elloker, S.; Raven, J.; Elsey, H.; et al. Implementation research: New imperatives and opportunities in global health. Lancet 2018, 392, 2214–2228. [Google Scholar] [CrossRef] [PubMed]
- Balas, E.A.; Boren, S.A. Managing clinical knowledge for health care improvement. Yearb. Med. Inform. 2000, 9, 65–70. [Google Scholar] [CrossRef] [Green Version]
- Grant, J.; Green, L.; Mason, B. Basic research and health: A reassessment of the scientific basis for the support of biomedical science. Res. Evaluat. 2003, 12, 217–224. [Google Scholar] [CrossRef]
- Morris, Z.S.; Wooding, S.; Grant, J. The answer is 17 years, what is the question: Understanding time lags in translational research. J. R. Soc. Med. 2011, 104, 510–520. [Google Scholar] [CrossRef] [PubMed]
- Mosteller, F. Innovation and evaluation. Science 1981, 211, 881–886. [Google Scholar] [CrossRef]
- Chalmers, I.; Glasziou, P. Avoidable waste in the production and reporting of research evidence. Lancet 2009, 374, 86–89. [Google Scholar] [CrossRef]
- Grimshaw, J.M.; Eccles, M.P.; Lavis, J.N.; Hill, S.J.; Squires, J.E. Knowledge translation of research findings. Implement Sci. 2012, 7, 50–66. [Google Scholar] [CrossRef] [Green Version]
- Weingart, S.N.; Brown, E.; Bach, P.B.; Eng, K.; Johnson, S.A.; Kuzel, T.M.; Langbaum, T.S.; Leedy, R.D.; Muller, R.J.; Newcomer, L.N.; et al. NCCN Task force report: Oral chemotherapy. J. Natl. Compr. Canc. Netw. 2008, 6 (Suppl. 3), S1–S14. [Google Scholar] [CrossRef]
- Zerillo, J.A.; Goldenberg, B.A.; Kotecha, R.R.; Tewari, A.K.; Jacobson, J.O.; Krzyzanowska, M.K. Interventions to improve oral chemotherapy safety and quality: A systematic review. JAMA Oncol. 2018, 4, 105–117. [Google Scholar] [CrossRef]
- Schlichtig, K.; Dürr, P.; Dörje, F.; Fromm, M.F. New oral anti-cancer drugs and medication safety. Dtsch. Arztebl. Int. 2019, 116, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Weingart, S.N.; Zhang, L.; Sweeney, M.; Hassett, M. Chemotherapy medication errors. Lancet Oncol. 2018, 19, e191–e199. [Google Scholar] [CrossRef] [PubMed]
- Dürr, P.; Schlichtig, K.; Kelz, C.; Deutsch, B.; Maas, R.; Eckart, M.J.; Wilke, J.; Wagner, H.; Wolff, K.; Preuß, C.; et al. The randomized AMBORA trial: Impact of pharmacological/pharmaceutical care on medication safety and patient-reported outcomes during treatment with new oral anticancer agents. J. Clin. Oncol. 2021, 39, 1983–1994. [Google Scholar] [CrossRef] [PubMed]
- Schlichtig, K.; Dürr, P.; Dörje, F.; Fromm, M.F. Medication errors during treatment with new oral anticancer agents: Consequences for clinical practice based on the AMBORA study. Clin. Pharmacol. Ther. 2021, 110, 1075–1086. [Google Scholar] [CrossRef]
- Schlichtig, K.; Cuba, L.; Dürr, P.; Bellut, L.; Meidenbauer, N.; Kunath, F.; Goebell, P.J.; Mackensen, A.; Dörje, F.; Fromm, M.F.; et al. New oral antitumor drugs and medication safety in uro-oncology: Implications for clinical practice based on a subgroup analysis of the AMBORA trial. J. Clin. Med. 2022, 11, 4558–4571. [Google Scholar] [CrossRef]
- Dürr, P.; Schlichtig, K.; Krebs, S.; Schramm, A.; Schötz, L.; Fromm, M.F.; Dörje, F. Economic aspects in the care of patients with new oral anticancer drugs: Findings from the AMBORA trial. Z. Fur Evidenz Fortbild. Und Qual. Im Gesundh. 2022, 169, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Dürr, P.; Meier, F.; Schlichtig, K.; Schramm, A.; Schötz, L.; Fromm, M.F.; Dörje, F. Characteristics and cost of unscheduled hospitalizations in patients treated with new oral anticancer drugs in Germany: Evidence from the randomized AMBORA trial. J. Clin. Med. 2022, 11, 6392–6407. [Google Scholar] [CrossRef]
- Bauer, M.S.; Damschroder, L.; Hagedorn, H.; Smith, J.; Kilbourne, A.M. An introduction to implementation science for the non-specialist. BMC Psychol. 2015, 3, 32–43. [Google Scholar] [CrossRef] [Green Version]
- Eccles, M.P.; Mittman, B.S. Welcome to implementation science. Implement. Sci. 2006, 1, 13. [Google Scholar] [CrossRef] [Green Version]
- Curran, G.M.; Bauer, M.; Mittman, B.; Pyne, J.M.; Stetler, C. Effectiveness-implementation hybrid designs: Combining elements of clinical effectiveness and implementation research to enhance public health impact. Med. Care 2012, 50, 217–226. [Google Scholar] [CrossRef] [Green Version]
- Glasgow, R.E.; Harden, S.M.; Gaglio, B.; Rabin, B.; Smith, M.L.; Porter, G.C.; Ory, M.G.; Estabrooks, P.A. RE-AIM planning and evaluation framework: Adapting to new science and practice with a 20-year review. Front. Public Health 2019, 7, 64–72. [Google Scholar] [CrossRef] [Green Version]
- Glasgow, R.E.; Vogt, T.M.; Boles, S.M. Evaluating the public health impact of health promotion interventions: The RE-AIM framework. Am. J. Public Health 1999, 89, 1322–1327. [Google Scholar] [CrossRef] [Green Version]
- Pinnock, H.; Barwick, M.; Carpenter, C.R.; Eldridge, S.; Grandes, G.; Griffiths, C.J.; Rycroft-Malone, J.; Meissner, P.; Murray, E.; Patel, A.; et al. Standards for Reporting Implementation Studies (StaRI): Explanation and elaboration document. BMJ Open 2017, 7, e013318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Snyder, R.A.; Abarca, J.; Meza, J.L.; Rothschild, J.M.; Rizos, A.; Bates, D.W. Reliability evaluation of the adapted national coordinating council medication error reporting and prevention (NCC MERP) index. Pharmacoepidemiol. Drug Saf. 2007, 16, 1006–1013. [Google Scholar] [CrossRef]
- Pharmaceutical Care Network Europe Association. Classification for Drug Related Problems, V9.1. Available online: https://www.pcne.org/upload/files/230_PCNE_classification_V8-02.pdf (accessed on 7 February 2023).
- National Cancer Institute: Patient Reported Outcomes Version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE®) Measurement System, Item Library Version 1.0, German. Available online: https://healthcaredelivery.cancer.gov/pro-ctcae/ (accessed on 7 February 2023).
- Aaronson, N.K.; Ahmedzai, S.; Bergman, B.; Bullinger, M.; Cull, A.; Duez, N.J.; Filiberti, A.; Flechtner, H.; Fleishman, S.B.; de Haes, J.C.; et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J. Natl. Cancer Inst. 1993, 85, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Mahler, C.; Jank, S.; Hermann, K.; Horne, R.; Ludt, S.; Haefeli, W.E.; Szecsenyi, J. Psychometric properties of a German version of the “Satisfaction with Information about Medicines Scale” (SIMS-D). Value Health 2009, 12, 1176–1179. [Google Scholar] [CrossRef] [Green Version]
- Waltz, T.J.; Powell, B.J.; Matthieu, M.M.; Damschroder, L.J.; Chinman, M.J.; Smith, J.L.; Proctor, E.K.; Kirchner, J.E. Use of concept mapping to characterize relationships among implementation strategies and assess their feasibility and importance: Results from the Expert Recommendations for Implementing Change (ERIC) study. Implement. Sci. 2015, 10, 109–116. [Google Scholar] [CrossRef] [Green Version]
- Wirtz, M.A.; Bitzer, E.M.; Albert, U.S.; Ansmann, L.; Bögel, M.; Ernstmann, N.; Hollederer, A.; Hower, K.I.; Nowak, M.; Vollmar, H.C. DNVF-Memorandum III—Methods for health services research, part 4—Concept and methods for organizational health services research. Chapter 3—Methodological approaches for the evaluation and implementation of complex interventions in healthcare organizations. Gesundheitswesen 2019, 81, e82–e91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Damschroder, L.J.; Aron, D.C.; Keith, R.E.; Kirsh, S.R.; Alexander, J.A.; Lowery, J.C. Fostering implementation of health services research findings into practice: A consolidated framework for advancing implementation science. Implement Sci. 2009, 4, 50–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Regauer, V.; Seckler, E.; Campbell, C.; Phillips, A.; Rotter, T.; Bauer, P.; Müller, M. German translation and pre-testing of Consolidated Framework for Implementation Research (CFIR) and Expert Recommendations for Implementing Change (ERIC). Implement Sci. Commun. 2021, 2, 120–127. [Google Scholar] [CrossRef]
- Kirk, M.A.; Kelley, C.; Yankey, N.; Birken, S.A.; Abadie, B.; Damschroder, L. A systematic review of the use of the Consolidated Framework for Implementation Research. Implement Sci. 2016, 11, 72–84. [Google Scholar] [CrossRef] [Green Version]
- Comprehensive Cancer Center (CCC) Erlangen-EMN: Orale Tumortherapie (AMBORA). Available online: https://www.ccc.uk-erlangen.de/beratung/orale-tumortherapie-ambora/ (accessed on 7 February 2023).
- Blinder, V.S.; Garrett-Mayer, E.; Jacobsen, P.B.; Kozlik, M.M.; Markham, M.J.; Siegel, R.D.; Kamal, A.H.; Crist, S.T.S.; Rosenthal, J.; Chiang, A.C. Oral chemotherapy metric performance in Quality Oncology Practice Initiative practices: Updated trends and analysis. J. Natl. Compr. Canc. Netw. 2022, 20, 1099–1106.e1092. [Google Scholar] [CrossRef] [PubMed]
- Holle, L.M.; Segal, E.M.; Jeffers, K.D. The expanding role of the oncology pharmacist. Pharmacy 2020, 8, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Vulaj, V.; Hough, S.; Bedard, L.; Farris, K.; Mackler, E. Oncology pharmacist opportunities: Closing the gap in quality care. J. Oncol. Pract. 2018, 14, e403–e411. [Google Scholar] [CrossRef] [PubMed]
- Minogue, V.; Matvienko-Sikar, K.; Hayes, C.; Morrissey, M.; Gorman, G.; Terres, A. The usability and applicability of knowledge translation theories, models, and frameworks for research in the context of a national health service. Health Res. Policy Syst. 2021, 19, 105–118. [Google Scholar] [CrossRef]
- Nilsen, P. Making sense of implementation theories, models and frameworks. Implement Sci. 2015, 10, 53–65. [Google Scholar] [CrossRef] [Green Version]
- Bu, S.; Smith, A.; Janssen, A.; Donnelly, C.; Dadich, A.; Mackenzie, L.J.; Smith, A.L.; Young, A.L.; Wu, V.S.; Smith, S.J.; et al. Optimising implementation of telehealth in oncology: A systematic review examining barriers and enablers using the RE-AIM planning and evaluation framework. Crit. Rev. Oncol. Hematol. 2022, 180, 103869–103890. [Google Scholar] [CrossRef]
- Williams, L.B.; Shelton, B.J.; Gomez, M.L.; Al-Mrayat, Y.D.; Studts, J.L. Using implementation science to disseminate a lung cancer screening education intervention through community health workers. J. Community Health 2021, 46, 165–173. [Google Scholar] [CrossRef]
- Purdy, G.M.; Sobierajski, F.M.; Dolgoy, N.D.; McNeely, M.L. Evaluating implementation and pragmatism of cancer-specific exercise programs: A scoping review. J. Cancer Surviv. 2022, 16, 374–387. [Google Scholar] [CrossRef]
- Holtrop, J.S.; Estabrooks, P.A.; Gaglio, B.; Harden, S.M.; Kessler, R.S.; King, D.K.; Kwan, B.M.; Ory, M.G.; Rabin, B.A.; Shelton, R.C.; et al. Understanding and applying the RE-AIM framework: Clarifications and resources. J. Clin. Transl. Sci. 2021, 5, e126. [Google Scholar] [CrossRef]
- King, D.K.; Shoup, J.A.; Raebel, M.A.; Anderson, C.B.; Wagner, N.M.; Ritzwoller, D.P.; Bender, B.G. Planning for implementation success using RE-AIM and CFIR frameworks: A qualitative study. Front. Public Health 2020, 8, 59–72. [Google Scholar] [CrossRef] [Green Version]
- Birken, S.A.; Powell, B.J.; Presseau, J.; Kirk, M.A.; Lorencatto, F.; Gould, N.J.; Shea, C.M.; Weiner, B.J.; Francis, J.J.; Yu, Y.; et al. Combined use of the Consolidated Framework for Implementation Research (CFIR) and the Theoretical Domains Framework (TDF): A systematic review. Implement Sci. 2017, 12, 2–15. [Google Scholar] [CrossRef] [Green Version]
- Muluneh, B. Enhancing the design and evaluation of care delivery for patients on oral anticancer agents. J. Natl. Compr. Canc. Netw. 2022, 20, 1185–1187. [Google Scholar] [CrossRef] [PubMed]
- Verot, E.; Falandry, C.; Régnier Denois, V.; Feutrier, C.; Chapoton, B.; Okala, J.; Pupier, S.; Rousset, V.; Bridet, F.; Ravot, C.; et al. Conditions for the implementation of a patient education program dedicated to cancer patients treated by oral anticancer therapy. Patient Prefer. Adherence 2020, 14, 2263–2277. [Google Scholar] [CrossRef] [PubMed]
- Kinnaer, L.M.; Van de Vyver, M.; Kenis, I.; Decoene, E.; Foulon, V.; Van Hecke, A. A qualitative evaluation of the process of creating and implementing an interprofessional care pathway for patients treated with oral anticancer drugs. Eur. J. Oncol. Nurs. 2022, 61, 102218. [Google Scholar] [CrossRef] [PubMed]
- Farris, K.B.; Cadwallader, T.; Farley, J.; Gatwood, K.; Mackler, E.; Gatwood, J. Implementation of a model integrating primary and oncology pharmacists’ care for patients taking oral anticancer agents (OAA). Explor. Res. Clin. Soc. Pharm. 2022, 7, 100163–100170. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cuba, L.; Schlichtig, K.; Dürr, P.; Inwald, E.C.; Fromm, M.F.; Dörje, F. Optimizing Medication Safety with Oral Antitumor Therapy: A Methodological Approach for the Real-World Implementation of the AMBORA Competence and Consultation Center. Healthcare 2023, 11, 1640. https://doi.org/10.3390/healthcare11111640
Cuba L, Schlichtig K, Dürr P, Inwald EC, Fromm MF, Dörje F. Optimizing Medication Safety with Oral Antitumor Therapy: A Methodological Approach for the Real-World Implementation of the AMBORA Competence and Consultation Center. Healthcare. 2023; 11(11):1640. https://doi.org/10.3390/healthcare11111640
Chicago/Turabian StyleCuba, Lisa, Katja Schlichtig, Pauline Dürr, Elisabeth C. Inwald, Martin F. Fromm, and Frank Dörje. 2023. "Optimizing Medication Safety with Oral Antitumor Therapy: A Methodological Approach for the Real-World Implementation of the AMBORA Competence and Consultation Center" Healthcare 11, no. 11: 1640. https://doi.org/10.3390/healthcare11111640




