Sex-Based Differences in Clinical Profile and Complications among Individuals with Type 2 Diabetes Seen at a Private Tertiary Diabetes Care Centre in India
Abstract
:1. Introduction
2. Materials and Methods
3. Statistical Analysis
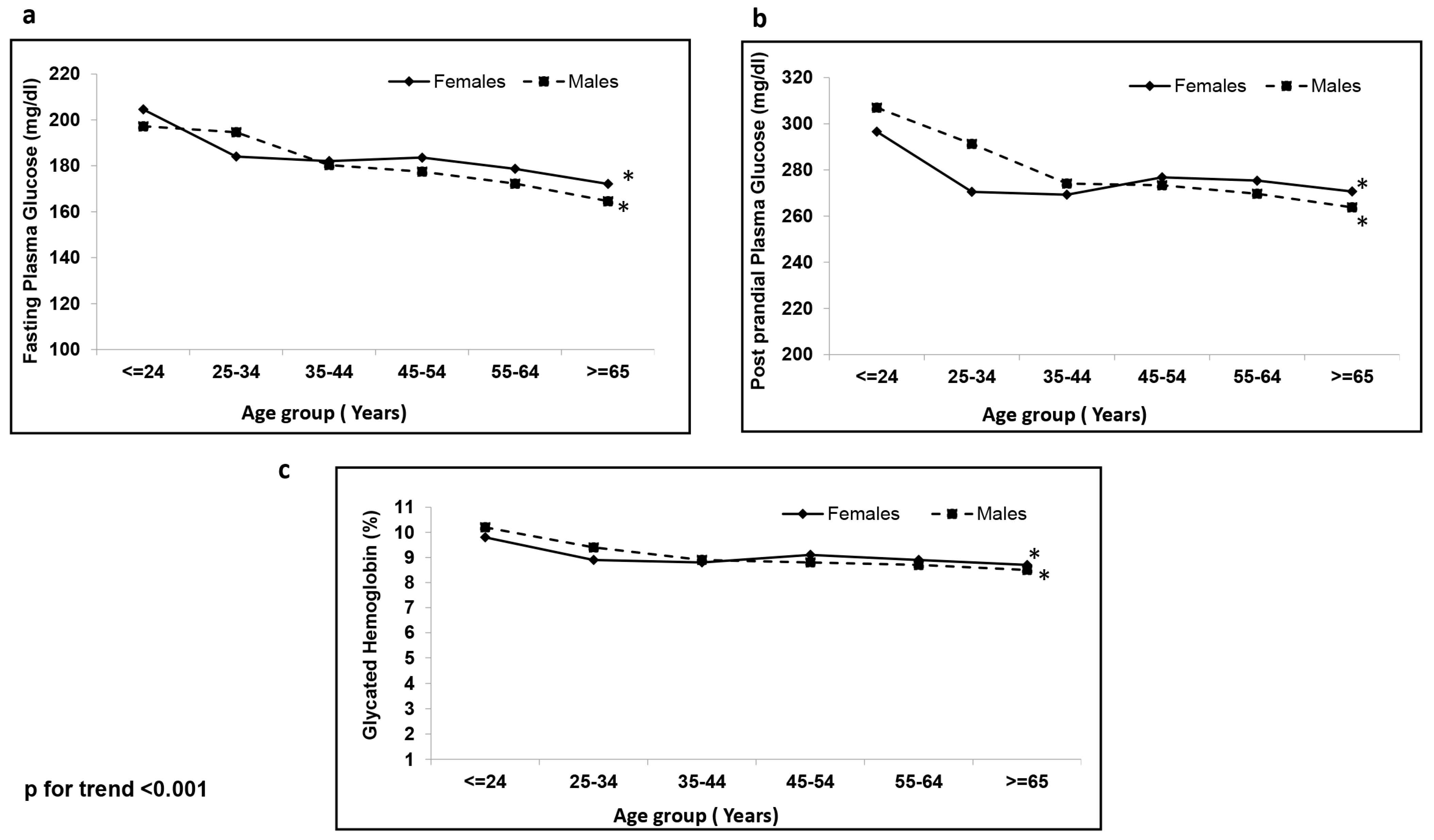
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mathur, P.; Leburu, S.; Kulothungan, V. Prevalence, Awareness, Treatment and Control of Diabetes in India From the Countrywide National NCD Monitoring Survey. Front. Public Health 2022, 10, 205. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.E.; Dorrance, K.A.; Oxenreiter, M.M.; Yan, K.R.; Close, K.L. The type 2 diabetes ‘modern preventable pandemic’ and replicable lessons from the COVID-19 crisis. Prev. Med. Rep. 2022, 25, 101636. [Google Scholar] [CrossRef] [PubMed]
- International Diabetes Federation. IDF Diabetes Atlas, 10th Edition. 2021. Available online: https://diabetesatlas.org/idfawp/resource-files/2021/07/IDF_Atlas_10th_Edition_2021.pdf (accessed on 20 May 2022).
- International Institute for Population Sciences (IIPS) and ICF. National Family Health Survey (NFHS-5), 2019–2021: India. Mumbai: IIPS. 2021. Available online: http://rchiips.org/nfhs/NFHS-5_FCTS/India.pdf (accessed on 20 May 2022).
- Anjana, R.M.; Deepa, M.; Pradeepa, R.; Mahanta, J.; Narain, K.; Das, H.K.; Adhikari, P.; Rao, P.V.; Saboo, B.; Kumar, A.; et al. Prevalence of diabetes and prediabetes in 15 states of India: Results from the ICMR–INDIAB population-based cross-sectional study. Lancet Diabetes Endocrinol. 2017, 5, 585–596. [Google Scholar] [CrossRef]
- Kautzky-Willer, A.; Harreiter, J.; Pacini, G. Sex and Gender Differences in Risk, Pathophysiology and Complications of Type 2 Diabetes Mellitus. Endocr. Rev. 2016, 37, 278–316. [Google Scholar] [CrossRef] [Green Version]
- Muilwijk, M.; Bolijn, R.; Galenkamp, H.; Stronks, K.; van Charante, E.M.; van Valkengoed, I.G. The association between gender-related characteristics and type 2 diabetes risk in a multi-ethnic population: The HELIUS study. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 142–150. [Google Scholar] [CrossRef]
- Hammarström, A.; Annandale, E. A conceptual muddle: An empirical analysis of the use of ‘sex’ and ‘gender’ in ‘gender-specific medicine’ journals. PLoS ONE 2012, 7, e34193. [Google Scholar] [CrossRef] [Green Version]
- Schober, E.; Holl, R.W.; Grabert, M.; Thon, A.; Rami, B.; Kapellen, T.; Seewi, O.; Reinehr, T.; Rami-Merhar, B. Diabetes mellitus type 2 in childhood and adolescence in Germany and parts of Austria. Eur. J. Pediatr. 2005, 164, 705–707. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Prasad, H.C. Changing epidemiology of metabolic syndrome and type 2 diabetes in Chinese youth. Curr. Diab. Rep. 2014, 14, 447. [Google Scholar] [CrossRef]
- Mayer-Davis, E.J.; Dabelea, D.; Lawrence, J.M. Incidence Trends of Type 1 and Type 2 Diabetes among Youths, 2002–2012. N. Engl. J. Med. 2017, 377, 301. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Ni, J.; Wu, Y.; Zhang, H.; Liu, J.; Tu, J.; Cui, J.; Ning, X.; Wang, J. Sex Differences in the Prevalence, Awareness, Treatment, and Control of Diabetes Mellitus Among Adults Aged 45 Years and Older in Rural Areas of Northern China: A Cross-Sectional, Population-Based Study. Front. Endocrinol. 2019, 10, 147. [Google Scholar] [CrossRef] [Green Version]
- Sattar, N. Gender aspects in type 2 diabetes mellitus and cardiometabolic risk. Best Pract. Res. Clin. Endocrinol. Metab. 2013, 27, 501–507. [Google Scholar] [CrossRef]
- Choi, Y.J.; Kim, H.C.; Kim, H.M.; Park, S.W.; Kim, J.; Kim, D.J. Prevalence and management of diabetes in Korean adults: Korea National Health and Nutrition Examination Surveys 1998–2005. Diabetes Care 2009, 32, 2016–2020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, Y.; Cai, R.; Sun, J.; Dong, X.; Huang, R.; Tian, S.; Wang, S. Diabetes mellitus as a risk factor for incident chronic kidney disease and end-stage renal disease in women compared with men: A systematic review and meta-analysis. Endocrine 2017, 55, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Ohkuma, T.; Peters, S.A.E.; Woodward, M. Sex differences in the association between diabetes and cancer: A systematic review and meta-analysis of 121 cohorts including 20 million individuals and one million events. Diabetologia 2018, 61, 2140–2154. [Google Scholar] [CrossRef] [Green Version]
- Chatterjee, S.; Peters, S.A.E.; Woodward, M.; Arango, S.M.; Batty, G.D.; Beckett, N.; Beiser, A.; Borenstein, A.R.; Crane, P.K.; Haan, M.N.; et al. Type 2 Diabetes as a Risk Factor for Dementia in Women Compared with Men: A Pooled Analysis of 2.3 Million People Comprising More Than 100,000 Cases of Dementia. Diabetes Care 2016, 39, 300–307. [Google Scholar] [CrossRef] [Green Version]
- Anjana, R.M.; Unnikrishnan, R.; Deepa, M.; Venkatesan, U.; Pradeepa, R.; Joshi, S.; Saboo, B.; Das, A.K.; Bajaj, S.; Bhansali, A.; et al. Achievement of guideline recommended diabetes treatment targets and health habits in people with self-reported diabetes in India (ICMR-INDIAB-13): A national cross-sectional study. Lancet Diabetes Endocrinol. 2022, 10, 430–441. [Google Scholar] [CrossRef] [PubMed]
- Snehalatha, C.; Ramachandran, A.; Mohan, V.; Viswanathan, M. Pancreatic beta cell response in insulin treated NIDDM patients limitations of a random C-peptide measurement. Diabete Metab. 1987, 13, 27–30. [Google Scholar]
- Amutha, A.; Datta, M.; Unnikrishnan, I.R.; Anjana, R.M.; Rema, M.; Narayan, K.M.V.; Mohan, V. Clinical profile of diabetes in the young seen between 1992 and 2009 at a specialist diabetes centre in south India. Prim. Care Diabetes 2011, 5, 223–229. [Google Scholar] [CrossRef]
- Pradeepa, R.; Prabu, A.V.; Jebarani, S.; Subhashini, S.; Mohan, V. Use of a Large Diabetes Electronic Medical Record System in India: Clinical and Research Applications. J. Diabetes Sci. Technol. 2011, 5, 543–552. [Google Scholar] [CrossRef]
- Early Treatment Diabetic Retinopathy Study Research Group. Early Treatment Diabetic Retinopathy Study Design and Baseline Patient Characteristics: ETDRS Report Number 7. Ophthalmology 1991, 98, 741–756. [Google Scholar] [CrossRef]
- Unnikrishnan, R.I.; Rema, M.; Pradeepa, R.; Deepa, M.; Shanthirani, C.S.; Deepa, R.; Mohan, V. Prevalence and risk factors of diabetic nephropathy in an urban South Indian population: The Chennai Urban Rural Epidemiology Study (CURES 45). Diabetes Care 2007, 30, 2019–2024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pradeepa, R.; Rema, M.; Vignesh, J.; Deepa, M.; Deepa, R.; Mohan, V. Prevalence and risk factors for diabetic neuropathy in an urban south Indian population: The Chennai Urban Rural Epidemiology Study (CURES-55). Diabet. Med. 2008, 25, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Pradeepa, R.; Chella, S.; Surendar, J.; Indulekha, K.; Anjana, R.M.; Mohan, V. Prevalence of peripheral vascular disease and its association with carotid intima-media thickness and arterial stiffness in type 2 diabetes: The Chennai Urban Rural Epidemiology Study (CURES 111). Diabetes Vasc. Dis. Res. 2014, 11, 190–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rose, G.A.; Blackburn, H.; Gillum, R.F.; Prineas, R.J. Cardiovascular Survey Methods. In Minnesota Code for Resting Electrocardiograms, 2nd ed.; Springer: London, UK, 1982; pp. 124–143. [Google Scholar]
- Sherwani, S.I.; Khan, H.A.; Ekhzaimy, A.; Masood, A.; Sakharkar, M.K. Significance of HbA1c Test in Diagnosis and Prognosis of Diabetic Patients. Biomark. Insights 2016, 11, BMI-S38440. [Google Scholar] [CrossRef] [PubMed]
- Misra, R.; Lager, J. Ethnic and gender differences in psychosocial factors, glycemic control, and quality of life among adult type 2 diabetic patients. J. Diabetes Its Complicat. 2009, 23, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Arnetz, L.; Ekberg, N.R.; Alvarsson, M. Sex differences in type 2 diabetes: Focus on disease course and outcomes. Diabetes Metab. Syndr. Obes. Targets Ther. 2014, 7, 409–420. [Google Scholar] [CrossRef] [Green Version]
- Duarte, F.G.; da Silva Moreira, S.; Maria da Conceição, C.A.; de Souza Teles, C.A.; Andrade, C.S.; Reingold, A.; Moreira, E.D., Jr. Sex differences and correlates of poor glycaemic control in type 2 diabetes: A cross-sectional study in Brazil and Venezuela. BMJ Open 2019, 9, e023401. [Google Scholar] [CrossRef] [Green Version]
- Mauvais-Jarvis, F. Gender differences in glucose homeostasis and diabetes. Physiol. Behav. 2018, 187, 20–23. [Google Scholar] [CrossRef]
- Pond, N.; Sturock, N.; Jeffcoate, W. Age related changes in glycosylated haemoglobin in patients with IDDM. Diabet. Med. 1996, 13, 510–513. [Google Scholar] [CrossRef]
- Raum, E.; Krämer, H.U.; Rüter, G.; Rothenbacher, D.; Rosemann, T.; Szecsenyi, J.; Brenner, H. Medication non-adherence and poor glycaemic control in patients with type 2 diabetes mellitus. Diabetes Res. Clin. Pract. 2012, 97, 377–384. [Google Scholar] [CrossRef]
- Sonmez, A.; Yumuk, V.; Haymana, C.; Demirci, I.; Barcin, C.; Kıyıcı, S.; Güldiken, S.; Örük, G.; Saydam, B.O.; Baldane, S.; et al. Impact of Obesity on the Metabolic Control of Type 2 Diabetes: Results of the Turkish Nationwide Survey of Glycemic and Other Metabolic Parameters of Patients with Diabetes Mellitus (TEMD Obesity Study). Obes. Facts 2019, 12, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.B. Overweight and Obesity in Women: Health Risks and Consequences. J. Women’s Health 2003, 12, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Luhar, S.; Timæus, I.M.; Jones, R.; Cunningham, S.; Patel, S.A.; Kinra, S.; Clarke, L.; Houben, R. Forecasting the prevalence of overweight and obesity in India to 2040. PLoS ONE 2020, 15, e0229438. [Google Scholar] [CrossRef] [PubMed]
- Wiklund, P. The role of physical activity and exercise in obesity and weight management: Time for critical appraisal. J. Sport Health Sci. 2016, 5, 151–154. [Google Scholar] [CrossRef] [Green Version]
- Nantel, J.; Mathieu, M.-E.; Prince, F. Physical Activity and Obesity: Biomechanical and Physiological Key Concepts. J. Obes. 2010, 2011, 650230. [Google Scholar] [CrossRef]
- The Lancet Public Health. Time to tackle the physical activity gender gap. Lancet Public Health 2019, 4, e360. [Google Scholar] [CrossRef] [Green Version]
- Forouhi, N.G. Nutrition and Type 2 Diabetes: Computational Optimization Modeling to Expand the Evidence Base for South Asians. Diabetes Care 2022, 45, 2811–2813. [Google Scholar] [CrossRef]
- Ley, S.H.; Hamdy, O.; Mohan, V.; Hu, F.B. Prevention and management of type 2 diabetes: Dietary components and nutritional strategies. Lancet 2014, 383, 1999–2007. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.-L.; Wang, Q.; Hong, Y.; Ojo, O.; Jiang, Q.; Hou, Y.-Y.; Huang, Y.-H.; Wang, X.-H. The Effect of Low-Carbohydrate Diet on Glycemic Control in Patients with Type 2 Diabetes Mellitus. Nutrients 2018, 10, 661. [Google Scholar] [CrossRef] [Green Version]
- Scientific Advisory Commission on Nutrition. Lower Carbohydrate Diets for Adults with Type 2 Diabetes. 2021; London, Scientific Advisory Commission on Nutrition. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1014673/SACN_report_on_lower_carbohydrate_diets_for_type_2_diabetes.pdf (accessed on 12 May 2023).
- Ajala, O.; English, P.; Pinkney, J. Systematic review and meta-analysis of different dietary approaches to the management of type 2 diabetes. Am. J. Clin. Nutr. 2013, 97, 505–516. [Google Scholar] [CrossRef] [Green Version]
- Wheeler, M.L.; Dunbar, S.A.; Jaacks, L.M.; Karmally, W.; Mayer-Davis, E.J.; Wylie-Rosett, J.; Yancy, W.S. Response to Comment on: Wheeler et al. Macronutrients, Food Groups, and Eating Patterns in the Management of Diabetes: A Systematic Review of the Literature, 2010. Diabetes Care 2012, 35, 434–445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anjana, R.M.; Srinivasan, S.; Sudha, V.; Joshi, S.R.; Saboo, B.; Tandon, N.; Das, A.K.; Jabbar, P.K.; Madhu, S.V.; Gupta, A.; et al. Macronutrient Recommendations for Remission and Prevention of Diabetes in Asian Indians Based on a Data-Driven Optimization Model: The ICMR-INDIAB National Study. Diabetes Care 2022, 45, 2883–2891. [Google Scholar] [CrossRef] [PubMed]
- Edwards, E.; Sackett, S.C. Psychosocial Variables Related to Why Women are Less Active than Men and Related Health Implications. Clin. Med. Insights Women’s Health 2016, 9, 47–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Afaya, R.A.; Bam, V.; Azongo, T.B.; Afaya, A. Knowledge of chronic complications of diabetes among persons living with type 2 diabetes mellitus in northern Ghana. PLoS ONE 2020, 15, e0241424. [Google Scholar] [CrossRef]
- Deshpande, A.D.; Harris-Hayes, M.; Schootman, M. Epidemiology of Diabetes and Diabetes-Related Complications. Phys. Ther. 2008, 88, 1254–1264. [Google Scholar] [CrossRef] [Green Version]
- Roman, G.; Pantea Stoian, A. Cardiovascular Risk/Disease in Type 2 Diabetes Mellitus. In Type 2 Diabetes—From Pathophysiology to Cyber Systems; IntechOpen: London, UK, 2021. [Google Scholar]
- Madonna, R.; Balistreri, C.R.; De Rosa, S.; Muscoli, S.; Selvaggio, S.; Selvaggio, G.; Ferdinandy, P.; De Caterina, R. Impact of Sex Differences and Diabetes on Coronary Atherosclerosis and Ischemic Heart Disease. J. Clin. Med. 2019, 8, 98. [Google Scholar] [CrossRef] [Green Version]
- Malmborg, M.; Schmiegelow, M.D.S.; Nørgaard, C.H.; Munch, A.; Gerds, T.; Schou, M.; Kistorp, C.; Torp-Pedersen, C.; Hlatky, M.A.; Gislason, G. Does type 2 diabetes confer higher relative rates of cardiovascular events in women compared with men? Eur. Heart J. 2020, 41, 1346–1353. [Google Scholar] [CrossRef] [Green Version]
- Ballotari, P.; Venturelli, F.; Greci, M.; Rossi, P.G.; Manicardi, V. Sex Differences in the Effect of Type 2 Diabetes on Major Cardiovascular Diseases: Results from a Population-Based Study in Italy. Int. J. Endocrinol. 2017, 2017, 6039356. [Google Scholar] [CrossRef] [Green Version]
- de Ritter, R.; de Jong, M.; Vos, R.C.; van der Kallen, C.J.H.; Sep, S.J.S.; Woodward, M.; Stehouwer, C.D.A.; Bots, M.L.; Peters, S.A.E. Sex differences in the risk of vascular disease associated with diabetes. Biol Sex Differ. 2020, 11, 1. [Google Scholar] [CrossRef] [Green Version]
- Vashist, P.; Senjam, S.; Gupta, V.; Manna, S.; Gupta, N.; Shamanna, B.; Bhardwaj, A.; Kumar, A.; Gupta, P. Prevalence of diabetic retinopahty in India: Results from the National Survey 2015–2519. Indian J. Ophthalmol. 2021, 69, 3087. [Google Scholar] [CrossRef]
- Qian, J.; Haq, Z.; Yang, D.; Stewart, J.M. Male sex increases the risk of diabetic retinopathy in an urban safety-net hospital population without impacting the relationship between axial length and retinopathy. Sci. Rep. 2022, 12, 9780. [Google Scholar] [CrossRef] [PubMed]
- Tomic, D.; Shaw, J.E.; Magliano, D.J. The burden and risks of emerging complications of diabetes mellitus. Nat. Rev. Endocrinol. 2022, 18, 525–539. [Google Scholar] [CrossRef] [PubMed]
- Ramteke, P.; Deb, A.; Shepal, V.; Bhat, M.K. Hyperglycemia Associated Metabolic and Molecular Alterations in Cancer Risk, Progression, Treatment, and Mortality. Cancers 2019, 11, 1402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Habib, S.L.; Rojna, M. Diabetes and risk of cancer. ISRN Oncol. 2013, 2013, 583786. [Google Scholar] [CrossRef] [Green Version]
- De Pergola, G.; Silvestris, F. Obesity as a Major Risk Factor for Cancer. J. Obes. 2013, 2013, 291546. [Google Scholar] [CrossRef] [Green Version]
- Taylor, P.N.; Albrecht, D.; Scholz, A.; Gutierrez-Buey, G.; Lazarus, J.H.; Dayan, C.M.; Okosieme, O.E. Global epidemiology of hyperthyroidism and hypothyroidism. Nat. Rev. Endocrinol. 2018, 14, 301–316. [Google Scholar] [CrossRef]
- Meng, Z.; Liu, M.; Zhang, Q.; Liu, L.; Song, K.; Tan, J.; Jia, Q.; Zhang, G.; Wang, R.; He, Y.; et al. Gender and Age Impacts on the Association Between Thyroid Function and Metabolic Syndrome in Chinese. Medicine 2015, 94, e2193. [Google Scholar] [CrossRef]
- Unnikrishnan, A.; Kalra, S.; Sahay, R.; Bantwal, G.; John, M.; Tewari, N. Prevalence of hypothyroidism in adults: An epidemiological study in eight cities of India. Indian J. Endocrinol. Metab. 2013, 17, 647–652. [Google Scholar] [CrossRef]
- Cleveland Clinic. Available online: https://my.clevelandclinic.org/health/diseases/21608-perimenopause (accessed on 15 June 2022).
- Chedraui, P.; Pérez-López, F.R. Metabolic syndrome during female midlife: What are the risks? Climacteric 2019, 22, 127–132. [Google Scholar] [CrossRef]
- Slopien, R.; Wender-Ozegowska, E.; Rogowicz-Frontczak, A.; Meczekalski, B.; Zozulinska-Ziolkiewicz, D.; Jaremek, J.D.; Cano, A.; Chedraui, P.; Goulis, D.G.; Lopes, P.; et al. Menopause and diabetes: EMAS clinical guide. Maturitas 2018, 117, 6–10. [Google Scholar] [CrossRef] [Green Version]
- Muka, T.; Asllanaj, E.; Avazverdi, N.; Jaspers, L.; Stringa, N.; Milic, J.; Ligthart, S.; Ikram, M.A.; Laven, J.S.E.; Kavousi, M.; et al. Age at natural menopause and risk of type 2 diabetes: A prospective cohort study. Diabetologia 2017, 60, 1951–1960. [Google Scholar] [CrossRef] [Green Version]
- Penno, G.; Solini, A.; Bonora, E.; Fondelli, C.; Orsi, E.; Zerbini, G.; Trevisan, R.; Vedovato, M.; Gruden, G.; Laviola, L.; et al. Gender differences in cardiovascular disease risk factors, treatments and complications in patients with type 2 diabetes: The RIACE Italian multicentre study. J. Intern. Med. 2013, 274, 176–191. [Google Scholar] [CrossRef] [PubMed]
- Krämer, H.U.; Rüter, G.; Schöttker, B.; Rothenbacher, D.; Rosemann, T.; Szecsenyi, J.; Brenner, H.; Raum, E. Gender differences in healthcare utilization of patients with diabetes. Am. J. Manag. Care 2012, 18, 362–369. [Google Scholar] [PubMed]
- Rossi, M.C.; Cristofaro, M.R.; Gentile, S.; Lucisano, G.; Manicardi, V.; Mulas, M.F.; Napoli, A.; Nicolucci, A.; Pellegrini, F.; Suraci, C.; et al. Sex disparities in the quality of diabetes care: Biological and cultural factors may play a different role for different outcomes: A cross-sectional observational study from the AMD annals initiative. Diabetes Care 2013, 36, 3162–3168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krämer, H.U.; Raum, E.; Rüter, G.; Schöttker, B.; Rothenbacher, D.; Rosemann, T.; Szecsenyi, J.; Brenner, H. Gender disparities in diabetes and coronary heart disease medication among patients with type 2 diabetes: Results from the DIANA study. Cardiovasc. Diabetol. 2012, 11, 88. [Google Scholar] [CrossRef] [Green Version]

| Males (n = 36,490) | Females (n = 36,490) | |
|---|---|---|
| Age (years) | 53 ± 10 | 53 ± 10 |
| Age at onset of diabetes (years) | 45 ± 9 | 46 ± 10 * |
| Height (cms) | 167.2 ± 6.5 | 153.4 ± 6 * |
| Weight (kgs) | 73.5 ± 12.9 | 66.5 ± 12.4 * |
| Body mass index (kg/m2) | 26.2 ± 4.1 | 28.2 ± 4.9 * |
| BMI Categories n (%) | ||
| <18.5 | 400 (1.1) | 264 (0.7) * |
| 18.5–24.9 | 14,534 (39.8) | 9393 (25.7) * |
| 25.0–29.9 | 15,853 (43.4) | 15,390 (42.2) * |
| ≥30 | 5703 (15.6) | 11,443 (31.4) * |
| Systolic blood pressure (mmHg) | 130 ± 16 | 130 ± 17 |
| Diastolic blood pressure (mmHg) | 79 ± 8.4 | 78.5 ± 8.1 |
| Duration of diabetes (years) | 7.7 ± 6.8 | 6.8 ± 6.3 * |
| Occupation n (%) | ||
| Home-maker | 0 | 4801 (13.2) |
| Private employee | 14,375 (39.7) | 27,601 (76.1) * |
| Government employee | 3019 (8.3) | 1654 (4.6) * |
| Self-employed/Business | 13,329 (36.8) | 484 (1.3) * |
| Retired | 5417 (14.9) | 1662 (4.6) * |
| Un-employed/Student | 105 (0.3) | 66 (0.2) ** |
| Physical activity | ||
| None | 17,518 (48.0) | 21,436 (58.7) * |
| <150 min/week | 9892 (27.1) | 10,357 (28.4) * |
| ≥150 min/week | 9080 (24.9) | 4697 (12.9) * |
| Smoking n (%) | 11,718 (32.1) | 348 (1.0) * |
| Alcohol n (%) | 14,399 (39.5) | 64 (0.2) * |
| Non-Vegetarians n (%) | 28,862 (79.7) | 26,115 (72.1) * |
| Dietary intake | ||
| Total calories (Kcals/day) | 1367 ± 425 | 1211 ± 349 * |
| Carbohydrate (gms /day) | 224 ± 72 | 200 ± 60 * |
| Protein (gms/ day) | 48 ± 14 | 43 ± 12 * |
| Fat (gms/day) | 32 ± 19 | 27 ± 16 * |
| Management n (%) | ||
| Oral hypoglycemic agents | 31,332 (85.9) | 31,114 (85.3) ** |
| Insulin | 235 (0.6) | 189 (0.5) ** |
| Oral hypoglycemic agents + insulin | 4923 (13.5) | 5187 (14.2) ** |
| Other comorbidities n (%) | ||
| Cancers | 216 (0.6) | 476 (1.3) * |
| Asthma/ chronic obstructive pulmonary disease | 180 (0.5) | 122 (0.3) ** |
| Hypothyroidism | 1292 (3.5) | 4549 (12.5) * |
| Biochemical Parameters | Males (n = 36,490) | Females (n = 36,490) |
|---|---|---|
| Fasting plasma glucose (mg/dL) | 176 ± 68 | 180 ± 73 * |
| Post prandial plasma glucose (mg/dL) | 273 ± 94 | 274 ± 97 ** |
| HbA1c (%) | 8.8 ± 2 | 8.9 ± 2 * |
| Serum cholesterol (mg/dL) | 177 ± 44 | 187 ± 45 * |
| Serum triglycerides (mg/dL) | 179 ± 133 | 167 ± 105 * |
| Serum HDL cholesterol (mg/dL) | 39 ± 9 | 44 ± 10 * |
| Serum LDL cholesterol (mg/dL) | 102 ± 38 | 109 ± 38 * |
| Total cholesterol/HDL cholesterol ratio | 4.7 ± 1.3 | 4.4 ± 1.2 * |
| Blood urea (mg/dL) | 25 ± 11 | 23 ± 10 * |
| Serum creatinine (mg/dL) | 0.9 ± 0.4 | 0.7 ± 0.6 * |
| Complications | Adjusted Odds Ratio * (95% CI) |
|---|---|
| Taking females as reference = 1 | |
| Microvascular | |
| Retinopathy (yes) | 1.59 (1.52–1.66), p < 0.001 |
| Nephropathy (yes) | 1.03 (0.99–1.08), p = 0.169 |
| Neuropathy (yes) | 1.35 (1.30–1.40), p < 0.001 |
| Macrovascular | |
| Cardiovascular disease(yes) | 1.75 (1.64–1.87), p < 0.001 |
| Peripheral vascular disease (yes) | 1.16 (1.08–1.26), p < 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pradeepa, R.; Shreya, L.; Anjana, R.M.; Jebarani, S.; Venkatesan, U.; Kamal Raj, N.; Swami, O.C.; Mohan, V. Sex-Based Differences in Clinical Profile and Complications among Individuals with Type 2 Diabetes Seen at a Private Tertiary Diabetes Care Centre in India. Healthcare 2023, 11, 1634. https://doi.org/10.3390/healthcare11111634
Pradeepa R, Shreya L, Anjana RM, Jebarani S, Venkatesan U, Kamal Raj N, Swami OC, Mohan V. Sex-Based Differences in Clinical Profile and Complications among Individuals with Type 2 Diabetes Seen at a Private Tertiary Diabetes Care Centre in India. Healthcare. 2023; 11(11):1634. https://doi.org/10.3390/healthcare11111634
Chicago/Turabian StylePradeepa, Rajendra, Lal Shreya, Ranjit Mohan Anjana, Saravanan Jebarani, Ulagamathesan Venkatesan, Nithyanantham Kamal Raj, Onkar C. Swami, and Viswanathan Mohan. 2023. "Sex-Based Differences in Clinical Profile and Complications among Individuals with Type 2 Diabetes Seen at a Private Tertiary Diabetes Care Centre in India" Healthcare 11, no. 11: 1634. https://doi.org/10.3390/healthcare11111634
APA StylePradeepa, R., Shreya, L., Anjana, R. M., Jebarani, S., Venkatesan, U., Kamal Raj, N., Swami, O. C., & Mohan, V. (2023). Sex-Based Differences in Clinical Profile and Complications among Individuals with Type 2 Diabetes Seen at a Private Tertiary Diabetes Care Centre in India. Healthcare, 11(11), 1634. https://doi.org/10.3390/healthcare11111634





