Self-Reported Medication Adherence Measured with Morisky Scales in Rare Disease Patients: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Information Sources and Search Strategy
2.3. Selection Process
2.4. Data Items
2.5. Risk of Bias Assessment
2.6. Outcome Measures
2.7. Synthesis Methods
3. Results
3.1. Study Selection
3.2. Study Characteristics
3.3. Risk of Study Bias
3.4. Results of Syntheses
Treatment Adherence
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Belzer, L.T.; Wright, S.M.; Goodwin, E.J.; Singh, M.N.; Carter, B.S. Psychosocial Considerations for the Child with Rare Disease: A Review with Recommendations and Calls to Action. Children 2022, 9, 933. [Google Scholar] [CrossRef] [PubMed]
- Collin-Histed, T.; Gershkowitz, J.; Stevens, B.; Timmins, G. The Patient Perspective on Rare Diseases. In Lysosomal Storage Disorders; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2022; pp. 299–307. ISBN 978-1-119-69731-2. [Google Scholar]
- Nguengang Wakap, S.; Lambert, D.M.; Olry, A.; Rodwell, C.; Gueydan, C.; Lanneau, V.; Murphy, D.; Le Cam, Y.; Rath, A. Estimating Cumulative Point Prevalence of Rare Diseases: Analysis of the Orphanet Database. Eur. J. Hum. Genet. 2020, 28, 165–173. [Google Scholar] [CrossRef] [PubMed]
- EURODIS. What Is a Rare Disease? Available online: https://www.eurordis.org/information-support/what-is-a-rare-disease/ (accessed on 19 December 2022).
- Hedley, V.; Hannah, M.; Charlotte, R.; Ségolène, A. Report on the State of the Art of Rare Disease Activities in Europe; European Commission: Brussels, Belgium, 2018. [Google Scholar]
- ICORD. Naoko Yamamoto (Disease Control Division, Ministry of Health, Labour and Welfare, Japan). Rare Disease Policies in Japan. In Proceedings of the 7th ICORD International Conference for Rare and Intractable Diseases and Orphan Drugs—C3: Connection and Collaboration, for Creation 2012, Tokyo, Japan, 4–6 February 2012. [Google Scholar]
- Richter, T.; Nestler-Parr, S.; Babela, R.; Khan, Z.M.; Tesoro, T.; Molsen, E.; Hughes, D.A. Rare Disease Terminology and Definitions—A Systematic Global Review: Report of the ISPOR Rare Disease Special Interest Group. Value Health 2015, 18, 906–914. [Google Scholar] [CrossRef] [PubMed]
- Haendel, M.; Vasilevsky, N.; Unni, D.; Bologa, C.; Harris, N.; Rehm, H.; Hamosh, A.; Baynam, G.; Groza, T.; McMurry, J.; et al. How Many Rare Diseases Are There? Nat. Rev. Drug Discov. 2020, 19, 77–78. [Google Scholar] [CrossRef] [PubMed]
- European Commission Rare Diseases. Available online: https://research-and-innovation.ec.europa.eu/research-area/health/rare-diseases_en (accessed on 5 December 2022).
- Kripalani, S.; Yao, X.; Haynes, R.B. Interventions to Enhance Medication Adherence in Chronic Medical Conditions: A Systematic Review. Arch. Intern. Med. 2007, 167, 540–550. [Google Scholar] [CrossRef]
- Patel, V.; Flisher, A.J.; Hetrick, S.; McGorry, P. Mental Health of Young People: A Global Public-Health Challenge. Lancet 2007, 369, 1302–1313. [Google Scholar] [CrossRef]
- Pereira, E.D.B.; de Matos Cavalcante, A.G. Prescription Is Not Enough: The Importance of Adherence to Pharmacological Treatment of COPD. J. Bras. Pneumol. Publicacao Of. Soc. Bras. Pneumol. E Tisilogia 2022, 48, e20220058. [Google Scholar] [CrossRef]
- Mongkhon, P.; Ashcroft, D.M.; Scholfield, C.N.; Kongkaew, C. Hospital Admissions Associated with Medication Non-Adherence: A Systematic Review of Prospective Observational Studies. BMJ Qual. Saf. 2018, 27, 902–914. [Google Scholar] [CrossRef]
- Jacquelet, E.; Poujois, A.; Pheulpin, M.-C.; Demain, A.; Tinant, N.; Gastellier, N.; Woimant, F. Adherence to Treatment, a Challenge Even in Treatable Metabolic Rare Diseases: A Cross Sectional Study of Wilson’s Disease. J. Inherit. Metab. Dis. 2021, 44, 1481–1488. [Google Scholar] [CrossRef]
- Masełbas, W.; Członkowska, A.; Litwin, T.; Niewada, M. Persistence with Treatment for Wilson Disease: A Retrospective Study. BMC Neurol. 2019, 19, 278. [Google Scholar] [CrossRef]
- Rajiah, K.; Sivarasa, S.; Maharajan, M.K. Impact of Pharmacists’ Interventions and Patients’ Decision on Health Outcomes in Terms of Medication Adherence and Quality Use of Medicines among Patients Attending Community Pharmacies: A Systematic Review. Int. J. Environ. Res. Public. Health 2021, 18, 4392. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Szmelter, A. Global Pharmaceutical Industry: Characteristics and Trends. Available online: https://www.igi-global.com/chapter/global-pharmaceutical-industry/www.igi-global.com/chapter/global-pharmaceutical-industry/216205 (accessed on 5 December 2022).
- Torreya Global Pharma Industry Study. Torreya The Future of the Global Pharmaceutical Industry. 2017. Available online: https://torreya.com/publications/torreya_global_pharma_industry_study_october2017.pdf (accessed on 28 May 2023).
- Orphanet. The Portal for Rare Diseases and Orphan Drugs. Available online: http://www.orpha.net/consor/www/cgi-bin/index.php?lng=ES (accessed on 5 December 2022).
- Hoy, D.; Brooks, P.; Woolf, A.; Blyth, F.; March, L.; Bain, C.; Baker, P.; Smith, E.; Buchbinder, R. Assessing Risk of Bias in Prevalence Studies: Modification of an Existing Tool and Evidence of Interrater Agreement. J. Clin. Epidemiol. 2012, 65, 934–939. [Google Scholar] [CrossRef]
- Morisky, D.E.; Green, L.W.; Levine, D.M. Concurrent and Predictive Validity of a Self-Reported Measure of Medication Adherence. Med. Care 1986, 24, 67–74. [Google Scholar] [CrossRef]
- Morisky, D.E.; Ang, A.; Krousel-Wood, M.; Ward, H.J. Predictive Validity of a Medication Adherence Measure in an Outpatient Setting. J. Clin. Hypertens. 2008, 10, 348–354. [Google Scholar] [CrossRef]
- Nyaga, V.N.; Arbyn, M.; Aerts, M. Metaprop: A Stata Command to Perform Meta-Analysis of Binomial Data. Arch. Public Health 2014, 72, 39. [Google Scholar] [CrossRef]
- Lin, L.; Chu, H. Meta-Analysis of Proportions Using Generalized Linear Mixed Models. Epidemiol. Camb. Mass 2020, 31, 713–717. [Google Scholar] [CrossRef]
- Newcombe, R.G. Two-Sided Confidence Intervals for the Single Proportion: Comparison of Seven Methods. Stat. Med. 1998, 17, 857–872. [Google Scholar] [CrossRef]
- Barendregt, J.J.; Doi, S.A.; Lee, Y.Y.; Norman, R.E.; Vos, T. Meta-Analysis of Prevalence. J. Epidemiol. Community Health 2013, 67, 974–978. [Google Scholar] [CrossRef]
- Lin, L.; Xu, C. Arcsine-Based Transformations for Meta-Analysis of Proportions: Pros, Cons, and Alternatives. Health Sci. Rep. 2020, 3, e178. [Google Scholar] [CrossRef]
- Badawy, S.M.; Thompson, A.A.; Lai, J.-S.; Penedo, F.J.; Rychlik, K.; Liem, R.I. Health-Related Quality of Life and Adherence to Hydroxyurea in Adolescents and Young Adults with Sickle Cell Disease. Pediatr. Blood Cancer 2017, 64, e26369. [Google Scholar] [CrossRef] [PubMed]
- Badawy, S.M.; Thompson, A.A.; Penedo, F.J.; Lai, J.-S.; Rychlik, K.; Liem, R.I. Barriers to Hydroxyurea Adherence and Health-Related Quality of Life in Adolescents and Young Adults with Sickle Cell Disease. Eur. J. Haematol. 2017, 98, 608–614. [Google Scholar] [CrossRef] [PubMed]
- Galadanci, N.A.; Umar Abdullahi, S.; Vance, L.D.; Musa Tabari, A.; Ali, S.; Belonwu, R.; Salihu, A.; Amal Galadanci, A.; Wudil Jibir, B.; Bello-Manga, H.; et al. Feasibility Trial for Primary Stroke Prevention in Children with Sickle Cell Anemia in Nigeria (SPIN Trial). Am. J. Hematol. 2017, 92, 780–788. [Google Scholar] [CrossRef] [PubMed]
- Pernell, B.M.; DeBaun, M.R.; Becker, K.; Rodeghier, M.; Bryant, V.; Cronin, R.M. Improving Medication Adherence with Two-Way Short Message Service Reminders in Sickle Cell Disease and Asthma. A Feasibility Randomized Controlled Trial. Appl. Clin. Inform. 2017, 8, 541–559. [Google Scholar] [CrossRef]
- Viola, A.S.; Drachtman, R.; Kaveney, A.; Sridharan, A.; Savage, B.; Delnevo, C.D.; Coups, E.J.; Porter, J.S.; Devine, K.A. Feasibility of Medical Student Mentors to Improve Transition in Sickle Cell Disease. J. Pediatr. Psychol. 2021, 46, 650–661. [Google Scholar] [CrossRef]
- Fogarty, H.; Gaul, A.; Syed, S.; Aleksejenko, N.; Geoghegan, R.; Conroy, H.; Crampton, E.; Ngwenya, N.; Tuohy, E.; McMahon, C. Adherence to Hydroxyurea, Health-Related Quality of Life Domains and Attitudes towards a Smartphone App among Irish Adolescents and Young Adults with Sickle Cell Disease. Ir. J. Med. Sci. 2022, 191, 809–816. [Google Scholar] [CrossRef]
- Ivarsson, B.; Hesselstrand, R.; Rådegran, G.; Kjellström, B. Adherence and Medication Belief in Patients with Pulmonary Arterial Hypertension or Chronic Thromboembolic Pulmonary Hypertension: A Nationwide Population-Based Cohort Survey. Clin. Respir. J. 2018, 12, 2029–2035. [Google Scholar] [CrossRef]
- Dzemaili, S.; Tiemensma, J.; Quinton, R.; Pitteloud, N.; Morin, D.; Dwyer, A.A. Beyond Hormone Replacement: Quality of Life in Women with Congenital Hypogonadotropic Hypogonadism. Endocr. Connect. 2017, 6, 404–412. [Google Scholar] [CrossRef]
- Vitturi, B.K.; Pellegrinelli, A.; Valerio, B.C.O. Medication Adherence in Patients with Myasthenia Gravis in Brazil: A Cross-Sectional Study. Acta Neurol. Belg. 2020, 120, 83–89. [Google Scholar] [CrossRef]
- Viswanathan, K.; Swaminathan, N.; Viswanathan, R.; Lakkaraja, M. Caregiver’s Health Locus of Control and Medication Adherence in Sickle Cell Disease. J. Natl. Med. Assoc. 2015, 107, 51–55. [Google Scholar] [CrossRef]
- Thuret, I.; Hacini, M.; Pégourié-Bandelier, B.; Gardembas-Pain, M.; Bisot-Locard, S.; Merlat-Guitard, A.; Bachir, D. Socio-Psychological Impact of Infused Iron Chelation Therapy with Deferoxamine in Metropolitan France: ISOSFER Study Results. Hematol. Amst. Neth. 2009, 14, 315–322. [Google Scholar] [CrossRef]
- Treadwell, M.J.; Law, A.W.; Sung, J.; Hackney-Stephens, E.; Quirolo, K.; Murray, E.; Glendenning, G.A.; Vichinsky, E. Barriers to Adherence of Deferoxamine Usage in Sickle Cell Disease. Pediatr. Blood Cancer 2005, 44, 500–507. [Google Scholar] [CrossRef]
- Raji, S.O.; Lawani, A.O.; James, B.O. Prevalence and Correlates of Major Depression among Nigerian Adults with Sickle Cell Disease. Int. J. Psychiatry Med. 2016, 51, 456–466. [Google Scholar] [CrossRef]
- Lamiani, G.; Strada, I.; Mancuso, M.E.; Coppola, A.; Vegni, E.; Moja, E.A. Pro-Adherence Study Group Factors Influencing Illness Representations and Perceived Adherence in Haemophilic Patients: A Pilot Study. Haemophilia 2015, 21, 598–604. [Google Scholar] [CrossRef]
- Knudsen, K.B.; Pressler, T.; Mortensen, L.H.; Jarden, M.; Skov, M.; Quittner, A.L.; Katzenstein, T.; Boisen, K.A. Associations between Adherence, Depressive Symptoms and Health-Related Quality of Life in Young Adults with Cystic Fibrosis. SpringerPlus 2016, 5, 1216. [Google Scholar] [CrossRef]
- Karaca, C.; Dincer, M.T.; Ozcan, S.G.; Sarac, B.; Ahmadzada, S.; Alagoz, S.; Bakir, A.; Kiykim, E.; Trabulus, S.; Seyahi, N. The Impact of the COVID-19 Pandemic on Fabry Disease Patients: An Examination of Mood Status, Therapy Adherence, and COVID-19 Infection. Orphanet J. Rare Dis. 2022, 17, 338. [Google Scholar] [CrossRef]
- Badawy, S.M.; Thompson, A.A.; Holl, J.L.; Penedo, F.J.; Liem, R.I. Healthcare Utilization and Hydroxyurea Adherence in Youth with Sickle Cell Disease. Pediatr. Hematol. Oncol. 2018, 35, 297–308. [Google Scholar] [CrossRef]
- Bacci, E.D.; Coyne, K.S.; Poon, J.-L.; Harris, L.; Boscoe, A.N. Understanding Side Effects of Therapy for Myasthenia Gravis and Their Impact on Daily Life. BMC Neurol. 2019, 19, 335. [Google Scholar] [CrossRef]
- Introna, A.; D’Errico, E.; Modugno, B.; Scarafino, A.; Fraddosio, A.; Distaso, E.; Tempesta, I.; Mastronardi, A.; Simone, I.L. Adherence to Riluzole in Patients with Amyotrophic Lateral Sclerosis: An Observational Study. Neuropsychiatr. Dis. Treat. 2018, 14, 193–203. [Google Scholar] [CrossRef]
- Grady, D.; Weiss, M.; Hernandez-Sanchez, J.; Pepke-Zaba, J. Medication and Patient Factors Associated with Adherence to Pulmonary Hypertension Targeted Therapies. Pulm. Circ. 2018, 8, 2045893217743616. [Google Scholar] [CrossRef]
- Galadanci, N.A.; Abdullahi, S.U.; Tabari, M.A.; Abubakar, S.; Belonwu, R.; Salihu, A.; Neville, K.; Kirkham, F.; Inusa, B.; Shyr, Y.; et al. Primary Stroke Prevention in Nigerian Children with Sickle Cell Disease (SPIN): Challenges of Conducting a Feasibility Trial. Pediatr. Blood Cancer 2015, 62, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, A.A.; Tiemensma, J.; Quinton, R.; Pitteloud, N.; Morin, D. Adherence to Treatment in Men with Hypogonadotrophic Hypogonadism. Clin. Endocrinol. 2017, 86, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Aşiret, G.D.; Kapucu, S.; Kaymaz, T.T.; Kurt, C.E.B. Psychosocial Adjustment and Adherence to Medication in Patients with Myasthenia Gravis. Gazi Med. J. 2021, 32, 3. [Google Scholar]
- Idiaquez, J.F.; Gonzalez, S.; Lasso-Penafiel, J.; Barnett, C. Pharmacological treatment compliance and a description of its associated factors in patients with myasthenia gravis. Rev. Neurol. 2018, 66, 15–20. [Google Scholar]
- Gimenez-Lozano, C.; Páramo-Rodríguez, L.; Cavero-Carbonell, C.; Corpas-Burgos, F.; López-Maside, A.; Guardiola-Vilarroig, S.; Zurriaga, O. Rare Diseases: Needs and Impact for Patients and Families: A Cross-Sectional Study in the Valencian Region, Spain. Int. J. Environ. Res. Public. Health 2022, 19, 10366. [Google Scholar] [CrossRef]
- Gorini, F.; Santoro, M.; Pierini, A.; Mezzasalma, L.; Baldacci, S.; Bargagli, E.; Boncristiano, A.; Brunetto, M.R.; Cameli, P.; Cappelli, F.; et al. Orphan Drug Use in Patients with Rare Diseases: A Population-Based Cohort Study. Front. Pharmacol. 2022, 13, 869842. [Google Scholar] [CrossRef]
- Adebiyi, M.G.; Manalo, J.M.; Xia, Y. Metabolomic and Molecular Insights into Sickle Cell Disease and Innovative Therapies. Blood Adv. 2019, 3, 1347–1355. [Google Scholar] [CrossRef]
- Loiselle, K.; Lee, J.L.; Szulczewski, L.; Drake, S.; Crosby, L.E.; Pai, A.L.H. Systematic and Meta-Analytic Review: Medication Adherence Among Pediatric Patients with Sickle Cell Disease. J. Pediatr. Psychol. 2016, 41, 406–418. [Google Scholar] [CrossRef]
- Walsh, K.E.; Cutrona, S.L.; Kavanagh, P.L.; Crosby, L.E.; Malone, C.; Lobner, K.; Bundy, D.G. Medication Adherence Among Pediatric Patients with Sickle Cell Disease: A Systematic Review. Pediatrics 2014, 134, 1175–1183. [Google Scholar] [CrossRef]
- Dean, A.J.; Walters, J.; Hall, A. A Systematic Review of Interventions to Enhance Medication Adherence in Children and Adolescents with Chronic Illness. Arch. Dis. Child. 2010, 95, 717–723. [Google Scholar] [CrossRef]
- Suresh, A.B.; Asuncion, R.M.D. Myasthenia Gravis; StatPearls Publishing: Tampa, FL, USA, 2022. [Google Scholar]
- Guptill, J.T.; Soni, M.; Meriggioli, M.N. Current Treatment, Emerging Translational Therapies, and New Therapeutic Targets for Autoimmune Myasthenia Gravis. Neurotherapeutics 2016, 13, 118–131. [Google Scholar] [CrossRef]
- Alhaidar, M.K.; Abumurad, S.; Soliven, B.; Rezania, K. Current Treatment of Myasthenia Gravis. J. Clin. Med. 2022, 11, 1597. [Google Scholar] [CrossRef]
- Gao, Y.; Yu, B.; Mao, J.; Wang, X.; Nie, M.; Wu, X. Assisted Reproductive Techniques with Congenital Hypogonadotropic Hypogonadism Patients: A Systematic Review and Meta-Analysis. BMC Endocr. Disord. 2018, 18, 85. [Google Scholar] [CrossRef]
- Klinger, J.R.; Elliott, C.G.; Levine, D.J.; Bossone, E.; Duvall, L.; Fagan, K.; Frantsve-Hawley, J.; Kawut, S.M.; Ryan, J.J.; Rosenzweig, E.B.; et al. Therapy for Pulmonary Arterial Hypertension in Adults: Update of the CHEST Guideline and Expert Panel Report. Chest 2019, 155, 565–586. [Google Scholar] [CrossRef]
- Macdonald, M.; Martin-Misener, R.; Helwig, M.; Smith, L.J.; Godfrey, C.M.; Curran, J.; Murphy, A. Experiences of Adults with Cystic Fibrosis in Adhering to Medication Regimens: A Qualitative Systematic Review. JBI Database Syst. Rev. Implement. Rep. 2016, 14, 258–285. [Google Scholar] [CrossRef]
- Appenzeller-Herzog, C.; Mathes, T.; Heeres, M.L.S.; Weiss, K.H.; Houwen, R.H.J.; Ewald, H. Comparative Effectiveness of Common Therapies for Wilson Disease: A Systematic Review and Meta-Analysis of Controlled Studies. Liver Int. 2019, 39, 2136–2152. [Google Scholar] [CrossRef]
- Kleinsinger, F. The Unmet Challenge of Medication Nonadherence. Perm. J. 2018, 22, 18–033. [Google Scholar] [CrossRef]

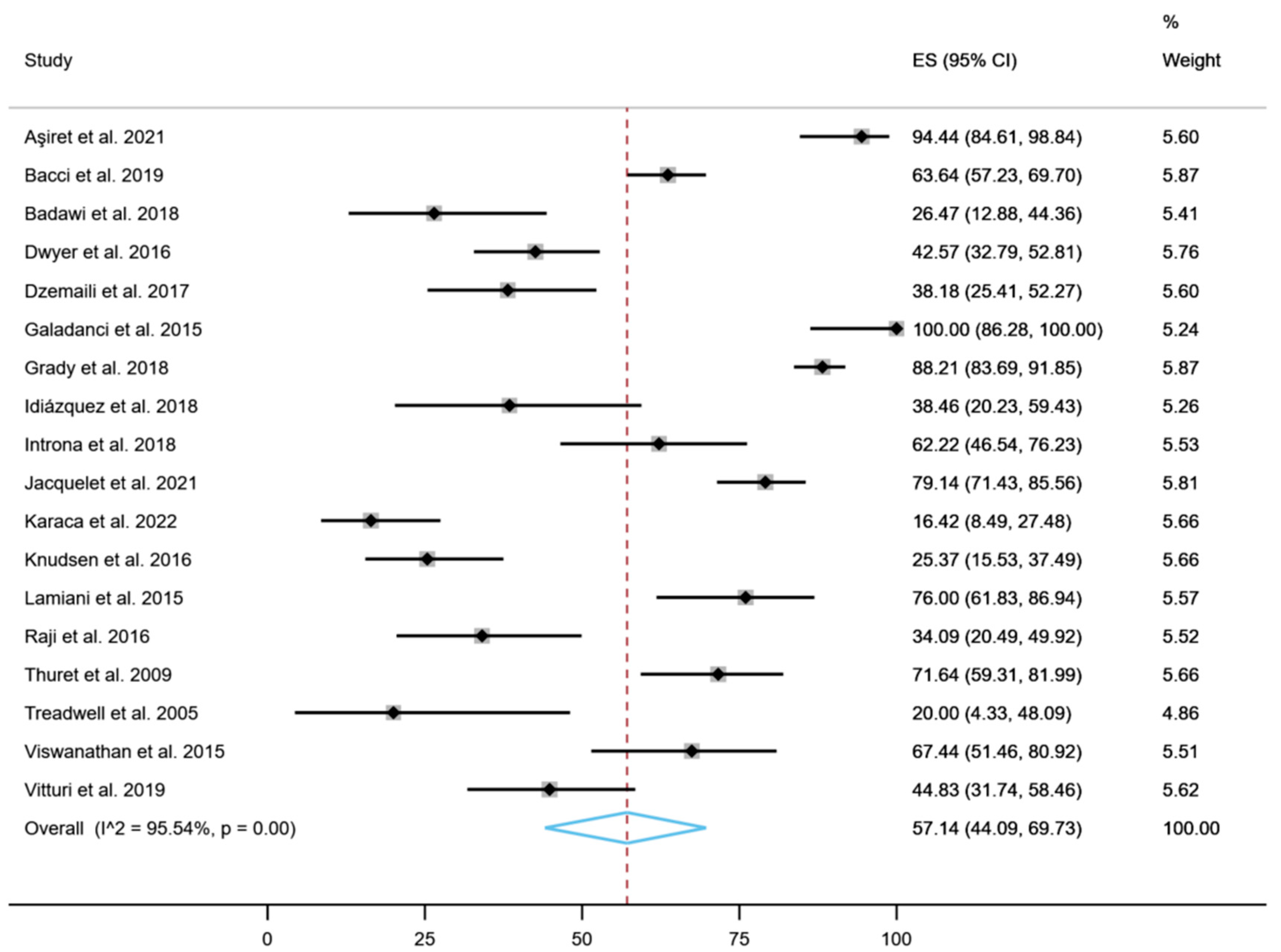


| Reference | Year | Country | Study Design | Total (n) | Women (%) | Age (Mean) | Rare Disease | Tool | Therapy | Adherence (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Aşiret et al. [51] | 2021 | Turkey | Cross-sectional | 54 | 64.8 | 44.1 | Myasthenia gravis | MMAS-4 | Cholinesterase inhibitors, Glucocorticoids (methyl prednisolone, prednisone vb.), Ciclosporin | 94.5 |
| Bacci et al. [46] | 2019 | USA | Cross-sectional | 242 | 64.0 | 58.4 | Myasthenia gravis | MMAS-8 | Intravenous immunoglobulin | 63.7 |
| Badawi et al. [45] | 2018 | USA | Cross-sectional | 34 | 41.0 | 14.0 | Sickle cell disease | MMAS-8 | Hydroxyurea | 26.0 |
| Dwyer et al. [50] | 2016 | Online | Cross-sectional | 101 | 0 | 37.0 | Congenital hypogonadotropic hypogonadism | MMAS-8 | Testosterone replacement therapy or fertility-inducing treatment via exogenous | 42.6 |
| Dzemaili et al. [36] | 2017 | Social media sites | Cross-sectional | 55 | 100 | 20.7 | Congenital hypogonadotropic hypogonadism | MMAS-8 | Hormone replacement | 38.2 |
| Galadanci et al. [49] | 2015 | Nigeria | Randomized clinical trial | 25 | 52.0 | 6.8 | Sickel cell disease | MMAS-8 | Hydroxyurea | 100 |
| Grady et al. [48] | 2018 | United Kingdom | Cross-sectional | 263 | 70.6 | 61.6 | Pulmonary arterial hypertension | MMAS-8 | Ambrisentan, bosentan, sildenafil, tadalafil, iloprost, epoprostenol, ERA + PDE5i, Iloprost (nebulized) + PDE5i, IV/SC Prostanoid + ERA, IV/SC Prostanoid + PDE5i, Trial drug + ERA +/− PDE5i | 88.2 |
| Idiázquez et al. [52] | 2018 | Chile | Cross-sectional | 26 | 57.7 | 55.5 | Myasthenia gravis | MMAS-4 | Cholinesterase inhibitors or immunosuppressors | 38.5 |
| Introna et al. [47] | 2018 | Italy | Cross-sectional | 45 | 40.0 | 63.8 | Amyotrophic lateral sclerosis | MMAS-8 | Riluzole tablet or oral suspension of riluzole | 62.2 |
| Jacquelet et al. [14] | 2021 | France | Cross-sectional | 139 | 50.4 | 39.0 | Wilson’s disease | MMAS-8 | D-Penicillamine, Trientine 2HCl, Zinc acetate, Zinc sulfate and Zinc sulfate | 79.1 |
| Karaca et al. [44] | 2022 | Turkey | Cross-sectional | 67 | 52.2 | 37.0 | Fabry disease | MMAS-4 | Enzyme replacement therapy | 16.4 |
| Knudsen et al. [43] | 2016 | Denmark | Cross-sectional | 67 | 59.0 | 24.1 | Cystic fibrosis | MMAS-8 | NR | 25.8 |
| Lamiani et al. [42] | 2015 | Italy | Cross-sectional | 50 | 0 | 39.7 | Hemophilia A | MMAS-4 | On-demand and prophylaxis | 76.0 |
| Raji et al. [41] | 2016 | Nigeria | Cross-sectional | 205 | 86.3 | 25.4 | Sickle cell disease | MMAS-8 | Hydroxyurea | 34.1 |
| Thuret et al. [39] | 2009 | France | Cross-sectional | 70 | 54.0 | 44.6 | Sickle cell disease myelodysplastic syndromes and β-thalassemia | MMAS-4 | DFO, deferiprone, deferiprone + DFO or deferasirox | 72.0 |
| Treadwell et al. [40] | 2005 | USA | Cross-sectional | 15 | 53.5 | 12.1 | Sickle cell disease | MMAS-4 | Chelation therapy | 20.0 |
| Viswanathan et al. [38] | 2015 | USA | Cross-sectional | 43 | 48.8 | 5.7 | Sickle cell disease | MMAS-8 | Hydroxyurea and Penicillin | 69.0 |
| Vitturi et al. [37] | 2020 | Brazil | Cross-sectional | 58 | 81.0 | 46.3 | Myasthenia gravis | MMAS-8 | NR | 44.8 |
| Variable | Coefficient | Lower Limit Confidence Interval | Upper Limit Confidence Interval | p-Value |
|---|---|---|---|---|
| Age mean | 0.32 | −0.44 | 1.08 | 0.403 |
| Year of publication | −0.34 | −3.78 | 3.10 | 0.845 |
| Quality score | −5.42 | −19.78 | 8.94 | 0.459 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Muñoz, A.M.; Victoria-Montesinos, D.; Cerdá, B.; Ballester, P.; de Velasco, E.M.; Zafrilla, P. Self-Reported Medication Adherence Measured with Morisky Scales in Rare Disease Patients: A Systematic Review and Meta-Analysis. Healthcare 2023, 11, 1609. https://doi.org/10.3390/healthcare11111609
García-Muñoz AM, Victoria-Montesinos D, Cerdá B, Ballester P, de Velasco EM, Zafrilla P. Self-Reported Medication Adherence Measured with Morisky Scales in Rare Disease Patients: A Systematic Review and Meta-Analysis. Healthcare. 2023; 11(11):1609. https://doi.org/10.3390/healthcare11111609
Chicago/Turabian StyleGarcía-Muñoz, Ana María, Desirée Victoria-Montesinos, Begoña Cerdá, Pura Ballester, Eloisa María de Velasco, and Pilar Zafrilla. 2023. "Self-Reported Medication Adherence Measured with Morisky Scales in Rare Disease Patients: A Systematic Review and Meta-Analysis" Healthcare 11, no. 11: 1609. https://doi.org/10.3390/healthcare11111609







