Advanced Techniques for Bone Restoration and Immediate Loading after Implant Failure: A Case Report
Abstract
1. Introduction
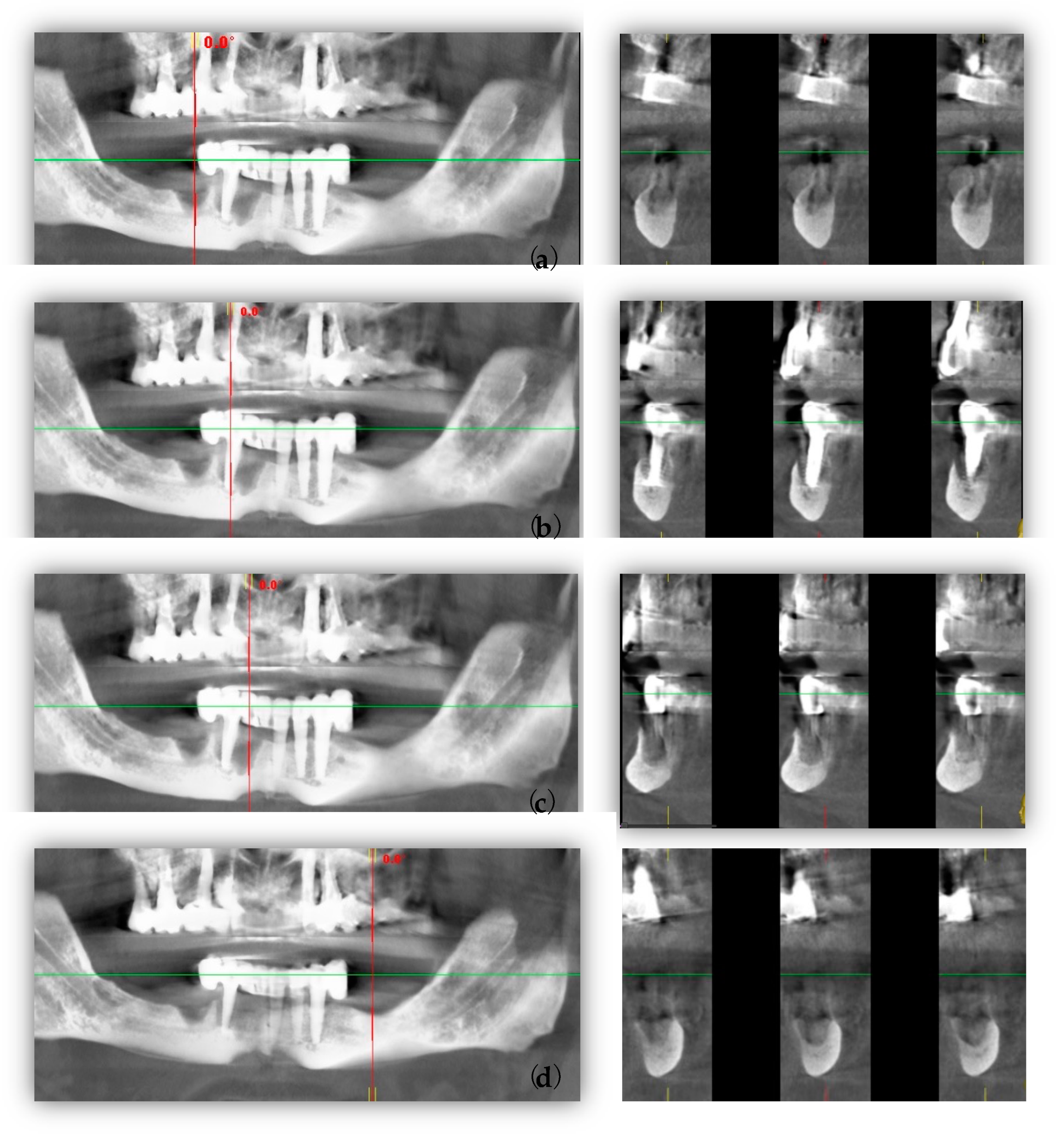
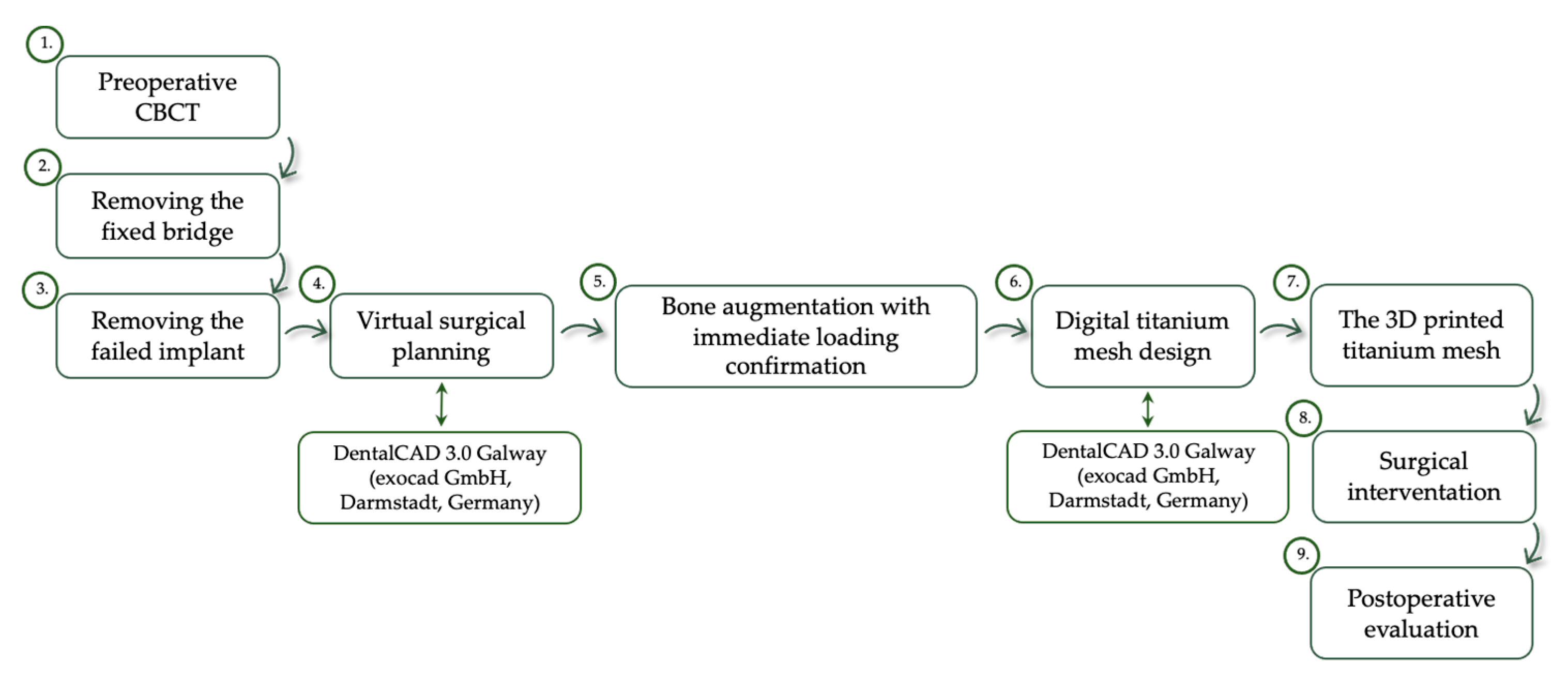
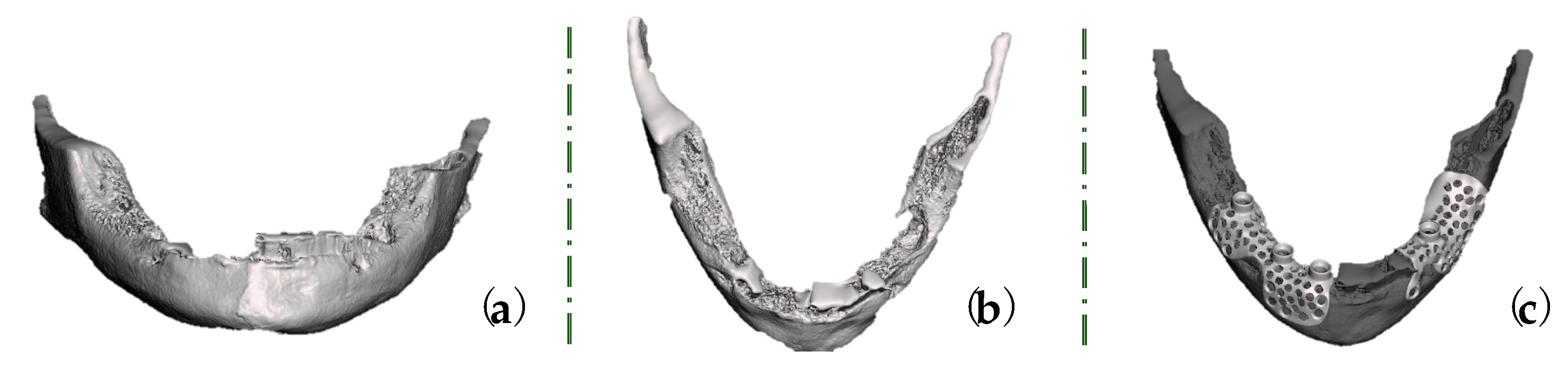
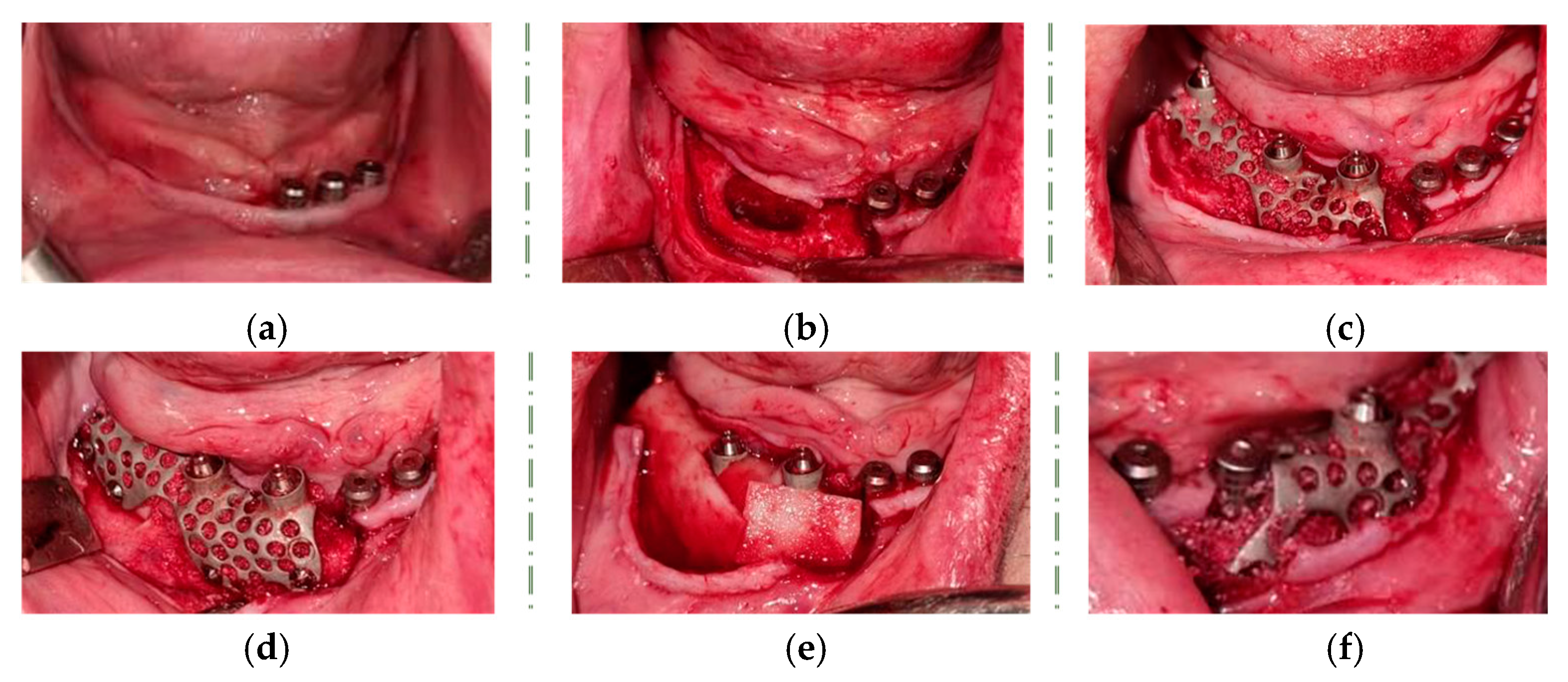
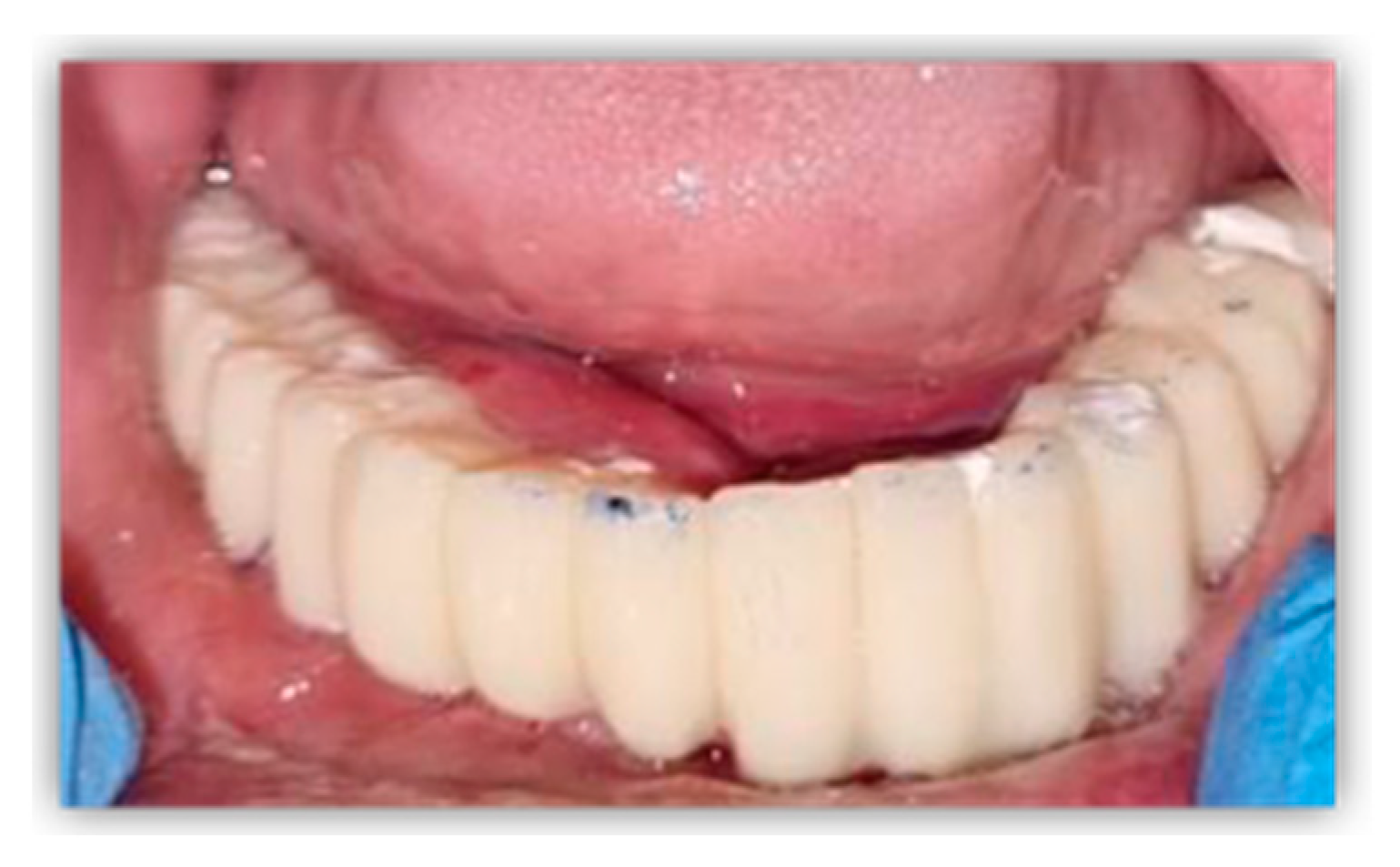
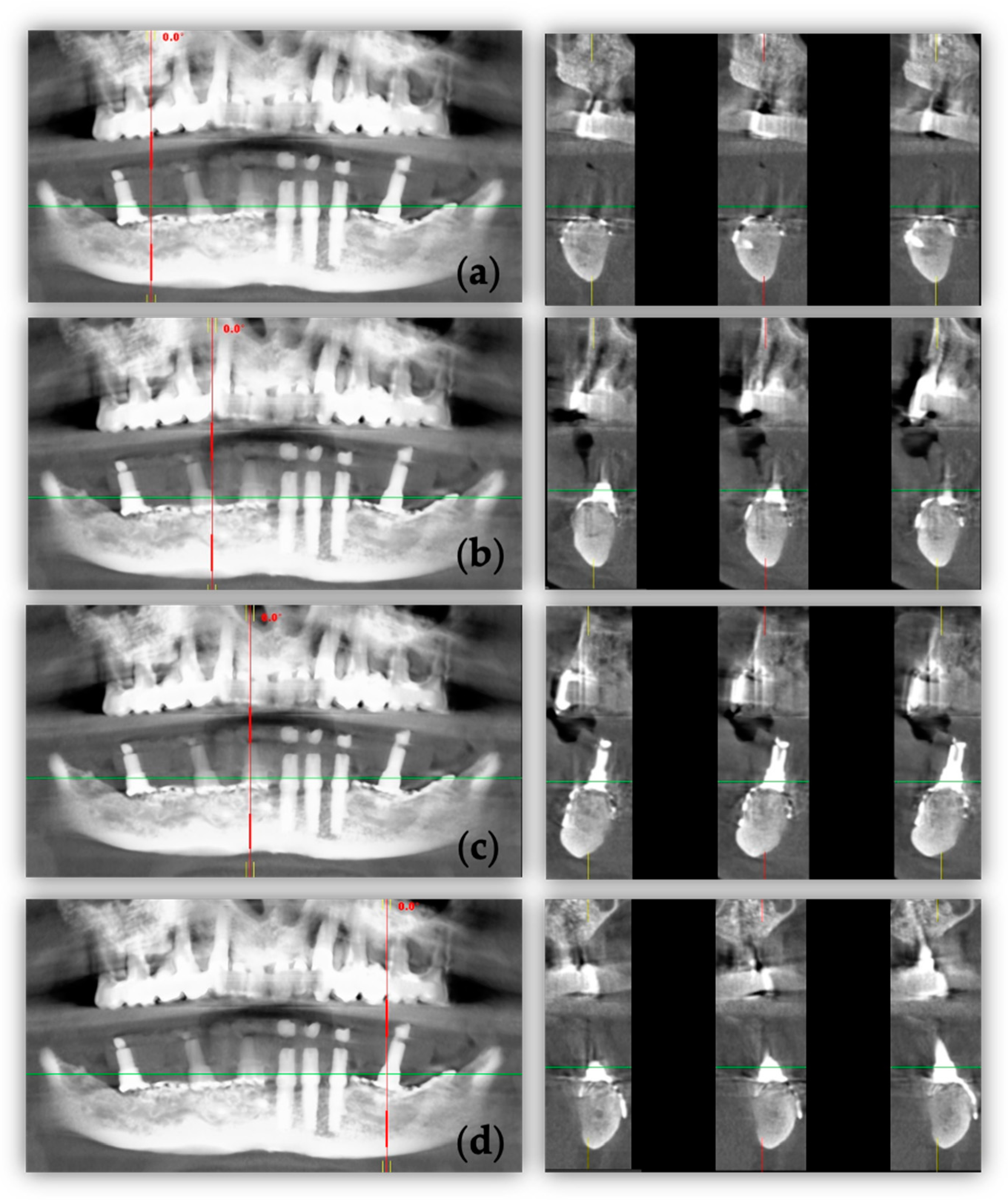
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bassir, S.H.; Alhareky, M.; Wangsrimongkol, B.; Jia, Y.; Karimbux, N. Systematic Review and Meta-Analysis of Hard Tissue Outcomes of Alveolar Ridge Preservation. Int. J. Oral. Maxillofac. Implants. 2018, 33, 979–994. [Google Scholar] [CrossRef]
- Chiapasco, M.; Casentini, P.; Tommasato, G.; Dellavia, C.; Del Fabbro, M. Customized CAD/CAM titanium meshes for the guided bone regeneration of severe alveolar ridge defects: Preliminary results of a retrospective clinical study in humans. Clin. Oral. Implants Res. 2021, 32, 498–510. [Google Scholar] [CrossRef] [PubMed]
- von Arx, T.; Hardt, N.; Wallkamm, B. The TIME technique: A new method for localized alveolar ridge augmentation prior to placement of dental implants. Int. J. Oral. Maxillofac. Implants 1996, 11, 387–394. [Google Scholar] [PubMed]
- Hasegawa, H.; Masui, S.; Ishihata, H.; Kaneko, T.; Ishida, D.; Endo, M.; Kanno, C.; Yamazaki, M.; Kitabatake, T.; Utsunomiya, S.; et al. Evaluation of a Newly Designed Microperforated Pure Titanium Membrane for Guided Bone Regeneration. Int. J. Oral. Maxillofac. Implants 2019, 34, 411–422. [Google Scholar] [CrossRef]
- Elias, C.; Lima, J.; Valiev, R.; Meyers, M. Biomedical applications of titanium and its alloys. JOM 2008, 60, 46–49. [Google Scholar] [CrossRef]
- Sidambe, A.T. Biocompatibility of Advanced Manufactured Titanium Implants-A Review. Materials 2014, 7, 8168–8188. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, Y.; Rao, S.; Ito, Y.; Tateishi, T. Corrosion resistance, mechanical properties, corrosion fatigue strength and cytocompatibility of new Ti alloys without Al and V. Biomaterials 1998, 19, 1197–1215. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, J.M.; Barão, V.A.R. Is there scientific evidence favoring the substitution of commercially pure titanium with titanium alloys for the manufacture of dental implants? Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 71, 1201–1215. [Google Scholar] [CrossRef] [PubMed]
- Cucchi, A.; Sartori, M.; Aldini, N.N.; Vignudelli, E.; Corinaldesi, G. A Proposal of Pseudo-periosteum Classification After GBR by Means of Titanium-Reinforced d-PTFE Membranes or Titanium Meshes Plus Cross-Linked Collagen Membranes. Int. J. Periodontics Restor. Dent. 2019, 39, e157–e165. [Google Scholar] [CrossRef]
- Zhou, T.; Chen, S.; Ding, X.; Hu, Z.; Cen, L.; Zhang, X. Fabrication and Characterization of Collagen/PVA Dual-Layer Membranes for Periodontal Bone Regeneration. Front. Bioeng. Biotechnol. 2021, 9, 630977. [Google Scholar] [CrossRef]
- Hartmann, A.; Hildebrandt, H.; Schmohl, J.U.; Kämmerer, P.W. Evaluation of Risk Parameters in Bone Regeneration Using a Customized Titanium Mesh: Results of a Clinical Study. Implant Dent. 2019, 28, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Liu, J.; Du, M.; Zhang, S.; Liu, Y.; Yang, H.; Shi, R.; Guo, Y.; Song, F.; Zhao, Y.; et al. Customized Barrier Membrane (Titanium Alloy, Poly Ether-Ether Ketone and Unsintered Hydroxyapatite/Poly-l-Lactide) for Guided Bone Regeneration. Front. Bioeng. Biotechnol. 2022, 10, 916967. [Google Scholar] [CrossRef] [PubMed]
- Sagheb, K.; Schiegnitz, E.; Moergel, M.; Walter, C.; Al-Nawas, B.; Wagner, W. Clinical outcome of alveolar ridge augmentation with individualized CAD-CAM-produced titanium mesh. Int. J. Implant Dent. 2017, 3, 36. [Google Scholar] [CrossRef]
- Ciocca, L.; Ragazzini, S.; Fantini, M.; Corinaldesi, G.; Scotti, R. Work flow for the prosthetic rehabilitation of atrophic patients with a minimal-intervention CAD/CAM approach. J. Prosthet. Dent. 2015, 114, 22–26. [Google Scholar] [CrossRef]
- Cucchi, A.; Bianchi, A.; Calamai, P.; Rinaldi, L.; Mangano, F.; Vignudelli, E.; Corinaldesi, G. Clinical and volumetric outcomes after vertical ridge augmentation using computer-aided-design/computer-aided manufacturing (CAD/CAM) customized titanium meshes: A pilot study. BMC Oral. Health 2020, 20, 219. [Google Scholar] [CrossRef]
- Cucchi, A.; Chierico, A.; Fontana, F.; Mazzocco, F.; Cinquegrana, C.; Belleggia, F.; Rossetti, P.; Soardi, C.M.; Todisco, M.; Luongo, R.; et al. Statements and Recommendations for Guided Bone Regeneration: Consensus Report of the Guided Bone Regeneration Symposium Held in Bologna, October 15 to 16, 2016. Implant Dent. 2019, 28, 388–399. [Google Scholar] [CrossRef] [PubMed]
- Gelețu, G.L.; Burlacu, A.; Murariu, A.; Andrian, S.; Golovcencu, L.; Baciu, E.R.; Maftei, G.; Onica, N. Customized 3D-Printed Titanium Mesh Developed for an Aesthetic Zone to Regenerate a Complex Bone Defect Resulting after a Deficient Odontectomy: A Case Report. Medicina 2022, 58, 1192. [Google Scholar] [CrossRef] [PubMed]
- Cucchi, A.; Vignudelli, E.; Franceschi, D.; Randellini, E.; Lizio, G.; Fiorino, A.; Corinaldesi, G. Vertical and horizontal ridge augmentation using customized CAD/CAM titanium mesh with versus without resorbable membranes. A randomized clinical trial. Clin. Oral. Implants Res. 2021, 32, 1411–1424. [Google Scholar] [CrossRef]
- Xie, Y.; Li, S.; Zhang, T.; Wang, C.; Cai, X. Titanium mesh for bone augmentation in oral implantology: Current application and progress. Int. J. Oral. Sci. 2020, 12, 37. [Google Scholar] [CrossRef]
- Bertran Faus, A.; Cordero Bayo, J.; Velasco-Ortega, E.; Torrejon-Moya, A.; Fernández-Velilla, F.; García, F.; López-López, J. Customized Titanium Mesh for Guided Bone Regeneration with Autologous Bone and Xenograft. Materials 2022, 15, 6271. [Google Scholar] [CrossRef]
- Al-Ardah, A.J.; Alqahtani, N.; AlHelal, A.; Goodacre, B.J.; Swamidass, R.; Garbacea, A.; Lozada, J. Using Virtual Ridge Augmentation and 3-Dimensional Printing to Fabricate a Titanium Mesh Positioning Device: A Novel Technique Letter. J. Oral. Implantol. 2018, 44, 293–299. [Google Scholar] [CrossRef]
- Rakhmatia, Y.D.; Ayukawa, Y.; Furuhashi, A.; Koyano, K. Microcomputed tomographic and histomorphometric analyses of novel titanium mesh membranes for guided bone regeneration: A study in rat calvarial defects. Int. J. Oral. Maxillofac. Implants 2014, 29, 826–835. [Google Scholar] [CrossRef] [PubMed]
- Briguglio, F.; Falcomatà, D.; Marconcini, S.; Fiorillo, L.; Briguglio, R.; Farronato, D. The Use of Titanium Mesh in Guided Bone Regeneration: A Systematic Review. Int. J. Dent. 2019, 2019, 9065423. [Google Scholar] [CrossRef]
- Gutta, R.; Baker, R.A.; Bartolucci, A.A.; Louis, P.J. Barrier membranes used for ridge augmentation: Is there an optimal pore size? J. Oral. Maxillofac. Surg. 2009, 67, 1218–1225. [Google Scholar] [CrossRef] [PubMed]
- Celletti, R.; Davarpanah, M.; Etienne, D.; Pecora, G.; Tecucianu, J.F.; Djukanovic, D.; Donath, K. Guided tissue regeneration around dental implants in immediate extraction sockets: Comparison of e-PTFE and a new titanium membrane. Int. J. Periodontics. Restorative. Dent. 1994, 14, 242–253. [Google Scholar]
- Her, S.; Kang, T.; Fien, M.J. Titanium mesh as an alternative to a membrane for ridge augmentation. J. Oral. Maxillofac. Surg. 2012, 70, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, I.; Funaki, K.; Yamauchi, K.; Kodama, T.; Takahashi, T. Alveolar ridge reconstruction with titanium mesh and autogenous particulate bone graft: Computed tomography-based evaluations of augmented bone quality and quantity. Clin. Implant Dent. Relat. Res. 2012, 14, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Ciocca, L.; Lizio, G.; Baldissara, P.; Sambuco, A.; Scotti, R.; Corinaldesi, G. Prosthetically CAD-CAM-Guided Bone Augmentation of Atrophic Jaws Using Customized Titanium Mesh: Preliminary Results of an Open Prospective Study. J. Oral. Implantol. 2018, 44, 131–137. [Google Scholar] [CrossRef]
- Katanec, D.; Granić, M.; Majstorović, M.; Trampus, Z.; Pandurić, D.G. Use of recombinant human bone morphogenetic protein (rhBMP2) in bilateral alveolar ridge augmentation: Case report. Coll. Antropol. 2014, 38, 325–330. [Google Scholar]
- Ribeiro Filho, S.A.; Francischone, C.E.; de Oliveira, J.C.; Ribeiro, L.Z.; do Prado, F.Z.; Sotto-Maior, B.S. Bone augmentation of the atrophic anterior maxilla for dental implants using rhBMP-2 and titanium mesh: Histological and tomographic analysis. Int. J. Oral. Maxillofac. Surg. 2015, 44, 1492–1498. [Google Scholar] [CrossRef]
- Buser, D.; Dula, K.; Belser, U.C.; Hirt, H.P.; Berthold, H. Localized ridge augmentation using guided bone regeneration. II. Surgical procedure in the mandible. Int. J. Periodontics. Restor. Dent. 1995, 15, 10–29. [Google Scholar] [PubMed]
- Antoun, H.; Sitbon, J.M.; Martinez, H.; Missika, P. A prospective randomized study comparing two techniques of bone augmentation: Onlay graft alone or associated with a membrane. Clin. Oral. Implants Res. 2001, 12, 632–639. [Google Scholar] [CrossRef] [PubMed]
- Cordaro, L.; Amadé, D.S.; Cordaro, M. Clinical results of alveolar ridge augmentation with mandibular block bone grafts in partially edentulous patients prior to implant placement. Clin. Oral. Implants Res. 2002, 13, 103–111. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, T.; Cai, X. The application of a newly designed L-shaped titanium mesh for GBR with simultaneous implant placement in the esthetic zone: A retrospective case series study. Clin. Implant Dent. Relat. Res. 2019, 21, 862–872. [Google Scholar] [CrossRef] [PubMed]
- Poli, P.P.; Beretta, M.; Cicciù, M.; Maiorana, C. Alveolar ridge augmentation with titanium mesh. A retrospective clinical study. Open Dent. J. 2014, 8, 148–158. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Onică, N.; Onică, C.A.; Baciu, E.-R.; Vasluianu, R.-I.; Ciofu, M.; Balan, M.; Gelețu, G.L. Advanced Techniques for Bone Restoration and Immediate Loading after Implant Failure: A Case Report. Healthcare 2023, 11, 1608. https://doi.org/10.3390/healthcare11111608
Onică N, Onică CA, Baciu E-R, Vasluianu R-I, Ciofu M, Balan M, Gelețu GL. Advanced Techniques for Bone Restoration and Immediate Loading after Implant Failure: A Case Report. Healthcare. 2023; 11(11):1608. https://doi.org/10.3390/healthcare11111608
Chicago/Turabian StyleOnică, Neculai, Cezara Andreea Onică, Elena-Raluca Baciu, Roxana-Ionela Vasluianu, Mihai Ciofu, Mihail Balan, and Gabriela Luminița Gelețu. 2023. "Advanced Techniques for Bone Restoration and Immediate Loading after Implant Failure: A Case Report" Healthcare 11, no. 11: 1608. https://doi.org/10.3390/healthcare11111608
APA StyleOnică, N., Onică, C. A., Baciu, E.-R., Vasluianu, R.-I., Ciofu, M., Balan, M., & Gelețu, G. L. (2023). Advanced Techniques for Bone Restoration and Immediate Loading after Implant Failure: A Case Report. Healthcare, 11(11), 1608. https://doi.org/10.3390/healthcare11111608






