Relationship between the Social Development Index and Self-Reported Periodontal Conditions
Abstract
1. Introduction
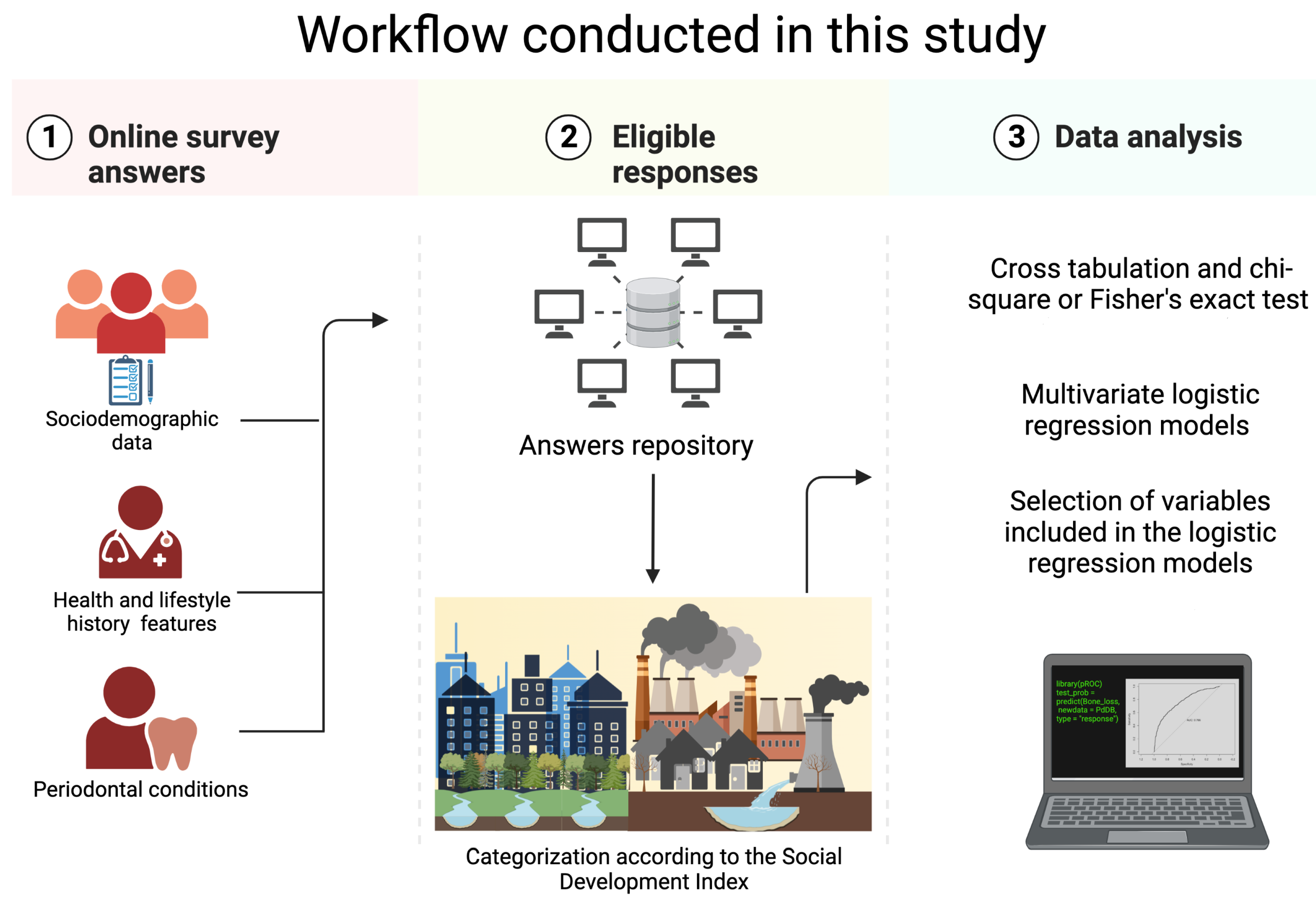
2. Materials and Methods
2.1. Study Population and Data Collection
2.2. Institutional Review Board Statement
2.3. Social Development Index
- Quality and available space in the home (QASH): The quality of housing is measured by the type of flooring, and the amount of living space is indicated by the number of people per bedroom, with two being the standard.
- Educational access (EduAcc): This indicator measures the proportion of people aged 18–59 who have completed secondary school or have received 13 years of schooling, which is considered a minimum standard for well-being.
- Access to social security and/or medical services (ASSMS): This indicator measures the coverage of any of the Mexican health systems.
- Durable goods (DGd): This indicator measures possession of material goods whose value is equal to or greater than 17.81 USD, or possession of at least three items such as a television, gas stove, computer, refrigerator, or washing machine.
- Sanitary adequacy (SAd): This indicator measures the availability of a water supply, toilet facilities, and access to a drainage system.
- Electricity access (EAcc): This indicator measures whether or not there is adequate access to electricity.
| Indicators | Abbreviation | Weigh |
|---|---|---|
| Quality and available | QASH | 0.338 |
| space in the home | ||
| Educational access | EduAcc | 0.244 |
| Access to social security | ASSMS | 0.291 |
| and/or medical services | ||
| Durable goods | DGd | 0.060 |
| Sanitary adequacy | SAd | 0.038 |
| Electricity access | EAcc | 0.029 |
| Value | Level |
|---|---|
| <0.7000 | Very low |
| 0.7001–0.8000 | Low |
| 0.8001–0.9000 | Medium |
| >0.9001 | High |
2.4. Statistical Analysis
3. Results
3.1. General Characteristics
3.2. Self-Reported Oral Health
3.2.1. Self-Reported Periodontal Conditions
3.2.2. Self-Reported Dental Treatment, Oral Hygiene, and Ability to Chew
3.3. Associations of Self-Reported Periodontal Conditions
4. Discussion
4.1. General Findings
4.2. Implications for Health Policy
4.3. Limitations of the Present Study
4.4. Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Questionnaire & Regression Variable Tables
| Abbreviation | Measurement | ||
|---|---|---|---|
| Group of Variable | Variable Name | (Variable Name) | Scale |
| Periodontal conditions | In the past three months, have you noticed a tooth that doesn’t seem to look good? | Tooth does not look right | 0. No 1. Yes |
| Have you noticed if you have any loose teeth? | Loose tooth | 0. No 1. Yes | |
| How many loose teeth do you have? | Number loose teeth | Number | |
| Have you ever had a loose tooth that you lost later? | Loose tooth later | 0. No 1. Yes | |
| Have you ever lost any teeth without an injury? | Lost tooth | 0. No 1. Yes | |
| Do you think you may have gum disease? | Gum disease | 0. No 1. Yes | |
| During the past year, have you had bleeding gums when you brush your teeth? | Bleeding gums | 0. No 1. Yes | |
| During the last year, have your gums been injured or infected? | Gum infected | 0. No 1. Yes | |
| Do you have bad breath? | Bad breath | 0. No 1. Yes | |
| During the last year, have you had abscessed teeth? | Abscessed teeth | 0. No 1. Yes | |
| Has a dentist ever told you that you have bone loss around your teeth? | Bone loss | 0. No 1. Yes | |
| Dental treatment | Did you receive dental care in the last 12 months? | Dental care | 0. No 1. Yes |
| Have you had an implant placed? | Implant placed | 0. No 1. Yes | |
| Have you ever had a gum treatment such as scraping or root planing, which is sometimes referred to as deep cleaning? | Gum treatment | 0. No 1. Yes |
| Abbreviation | Measurement | ||
|---|---|---|---|
| Group of Variable | Variable Name | (Variable Name) | Scale |
| Oral hygiene | Do you usually brush your teeth? | Brush your teeth | 0. No 1. Yes |
| How many times per day do you brush your teeth? | Times brush teeth | Times | |
| Do you brush your teeth before bed? | Brush before bed | 0. No 1. Yes | |
| Do you usually floss after brushing your teeth? | Floss use after | 0. No 1. Yes | |
| In addition to brushing your teeth, in the past seven days, how many times have you flossed between your teeth? | Times floss use | Times | |
| In addition to brushing your teeth, in the past seven days, how many times have you used a mouthwash or other liquid product to treat dental disease or problems? | Times mouthwash use | Times | |
| Ability to chew | How satisfied are you with your ability to chew food? | Satisfaction level | 0. Very satisfied |
| 1. Satisfied | |||
| 2. Unsatisfied | |||
| 3. Very unsatisfied |
| Dependent Variables | Type | Idependent Variables | Type |
|---|---|---|---|
| Loose tooth | Dichotomous | Sex | Dichotomous |
| Lost tooth later | Dichotomous | COVID-19 | Dichotomous |
| Bone loss | Dichotomous | Smoking | Dichotomous |
| Bleeding gums | Dichotomous | Physical activity low | Dichotomous |
| Gum disease | Dichotomous | Physical activity moderate | Dichotomous |
| Gum infected | Dichotomous | Physical activity high | Dichotomous |
| Sleep quality | Dichotomous | ||
| Metabolic syndrome | Dichotomous | ||
| Received periodontal care | Dichotomous | ||
| Dental care | Dichotomous | ||
| Brush your teeth | Dichotomous | ||
| Brush before bed | Dichotomous | ||
| Floss use after | Dichotomous | ||
| Very unsatisfied with ability to chew | Dichotomous | ||
| Unsatisfied with ability to chew | Dichotomous | ||
| Satisfied with ability to chew | Dichotomous | ||
| Very satisfied with ability to chew | Dichotomous | ||
| Age | Continuous | ||
| Body mass index | Continuous | ||
| Systolic blood pressure | Continuous | ||
| Diastolic blood pressure | Continuous | ||
| SDI global | Continuous | ||
| Educational access | Continuous | ||
| Quality and available | |||
| space in the home | Continuous | ||
| Durable goods | Continuous | ||
| Access to social security | |||
| and/or medical services | Continuous | ||
| Sanitary adequacy | Continuous | ||
| Electricity access | Continuous | ||
| Times floss use | Continuous | ||
| Times mouthwash use | Continuous |
References
- World Health Organization. Global Oral Health Status Report: Towards Universal Health Coverage for Oral Health by 2030; World Health Organization: Geneva, Switzerland, 2022; pp. 37–41. [Google Scholar]
- Islas-Granillo, H.; Borges-Yañez, S.A.; de Jesús Navarrete-Hernández, J.; Veras-Hernández, M.A.; Casanova-Rosado, J.F.; Minaya-Sánchez, M.; Casanova-Rosado, A.J.; Fernández-Barrera, M.Á.; Medina-Solís, C.E. Indicators of oral health in older adults with and without the presence of multimorbidity: A cross-sectional study. Clin. Interv. Aging. 2019, 14, 219. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.X.; Zhong, Y.J.; Dong, Q.Q.; Wong, H.M.; Wen, Y.F. Global, regional, and national burden of severe periodontitis, 1990–2019: An analysis of the Global Burden of Disease Study 2019. J. Clin. Periodontol. 2021, 48, 1165–1188. [Google Scholar] [CrossRef]
- Romano, F.; Perotto, S.; Bianco, L.; Parducci, F.; Mariani, G.M.; Aimetti, M. Self-perception of periodontal health and associated factors: A cross-sectional population-based study. Int. J. Environ. Res. Public Health 2020, 17, 2758. [Google Scholar] [CrossRef] [PubMed]
- Nazir, M.; Al-Ansari, A.; Al-Khalifa, K.; Alhareky, M.; Gaffar, B.; Almas, K. Global prevalence of periodontal disease and lack of its surveillance. Sci. World J. 2020, 2020, 2146160. [Google Scholar] [CrossRef]
- Gasner, N.S.; Schure, R.S. Periodontal Disease; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Papapanou, P.N.; Sanz, M.; Buduneli, N.; Dietrich, T.; Feres, M.; Fine, D.H.; Flemmig, T.F.; Garcia, R.; Giannobile, W.V.; Graziani, F.; et al. Periodontitis: Consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89, S173–S182. [Google Scholar] [CrossRef] [PubMed]
- Martínez-García, M.; Hernández-Lemus, E. Periodontal Inflammation and Systemic Diseases: An Overview. Front. Physiol. 2021, 1, 1842. [Google Scholar] [CrossRef]
- Herrera, D.; Sanz, M.; Shapira, L.; Brotons, C.; Chapple, I.; Frese, T.; Graziani, F.; Hobbs, F.R.; Huck, O.; Hummers, E.; et al. Association between periodontal diseases and cardiovascular diseases, diabetes and respiratory diseases: Consensus report of the Joint Workshop by the European Federation of Periodontology (EFP) and the European arm of the World Organization of Family Doctors (WONCA Europe). J. Clin. Periodontol. 2023, 1, 819–841. [Google Scholar]
- Hyde, S.; Dupuis, V.; Mariri, B.P.; Dartevelle, S. Prevention of tooth loss and dental pain for reducing the global burden of oral diseases. Int. Dent. J. 2017, 67, 19–25. [Google Scholar] [CrossRef]
- Gurav, A.N. The association of periodontitis and metabolic syndrome. Dent. Res. J. 2014, 11, 1. [Google Scholar]
- Kuraji, R.; Sekino, S.; Kapila, Y.; Numabe, Y. Periodontal disease–related nonalcoholic fatty liver disease and nonalcoholic steatohepatitis: An emerging concept of oral-liver axis. Periodontol. 2000 2021, 87, 204–240. [Google Scholar] [CrossRef] [PubMed]
- Gomes-Filho, I.S.; Oliveira, M.T.; Cruz, S.S.d.; Cerqueira, E.d.M.M.; Trindade, S.C.; Vieira, G.O.; Couto Souza, P.H.; Adan, L.F.F.; Hintz, A.M.; Passos-Soares, J.d.S.; et al. Periodontitis is a factor associated with dyslipidemia. Oral Dis. 2021, 1, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Del Carmen, A.D.F.; Aída, B.Y.S. Risk Indicators of Tooth Loss Among Mexican Adult Population: A Cross-Sectional Study. Int. Dent. J. 2021, 71, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Carrizales-Sepúlveda, E.F.; Ordaz-Farías, A.; Vera-Pineda, R.; Flores-Ramírez, R. Periodontal disease, systemic inflammation and the risk of cardiovascular disease. Heart. Lung. Circ. 2018, 27, 1327–1334. [Google Scholar] [CrossRef] [PubMed]
- Buduneli, N. Environmental factors and periodontal microbiome. Periodontol. 2000 2021, 85, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Silvestre, F.J.; Márquez-Arrico, C.F. COVID-19 and Periodontitis: A Dangerous Association? Front. Pharmacol. 2021, 12, 789681. [Google Scholar] [CrossRef]
- Caffesse, R.G. A Latin American perspective of periodontology. Periodontol. 2000 2015, 67, 7–12. [Google Scholar] [CrossRef]
- Oppermann, R.V.; Haas, A.N.; Rösing, C.K.; Susin, C. Epidemiology of periodontal diseases in adults from Latin America. Periodontol. 2000 2015, 67, 13–33. [Google Scholar] [CrossRef]
- Romito, G.A.; Feres, M.; Gamonal, J.; Gomez, M.; Carvajal, P.; Pannuti, C.; Duque Duque, A.; Romanelli, H.; Rösing, C.K.; Aranguiz Freyhofer, V.; et al. Periodontal disease and its impact on general health in Latin America: LAOHA Consensus Meeting Report. Braz. Oral. Res. 2020, 34, e027. [Google Scholar] [CrossRef]
- Pérez-Núñez, R.; Vargas-Palacios, A.; Ochoa-Moreno, I.; Medina-Solis, C.E. Household expenditure in dental health care: National estimations in Mexico for 2000, 2002, and 2004. J. Public. Health Dent. 2007, 67, 234–242. [Google Scholar] [CrossRef]
- Medina-Solís, C.E.; García-Cortés, J.O.; Robles-Minaya, J.L.; Casanova-Rosado, J.F.; Mariel-Cárdenas, J.; del Socorro Ruiz-Rodríguez, M.; de Jesús Navarrete-Hernández, J.; Ávila-Burgos, L.; Maupomé, G. Clinical and non-clinical variables associated with preventive and curative dental service utilisation: A cross-sectional study among adolescents and young adults in Central Mexico. BMJ Open 2019, 9, e027101. [Google Scholar] [CrossRef]
- Baltazar-Díaz, T.A.; Zamora-Pérez, A.L. Enfermedad periodontal y COVID-19: Factores de riesgo y mecanismos compartidos. Rev. Mex. Periodontol. 2021, 12, 12–17. [Google Scholar]
- SIVEPAB. Resultados del Sistema de Vigilancia Epidemiológica de Patologías Bucales. Dir. Gen. Epidemiol. Secr. Salud 2019, 1, 47–58. [Google Scholar]
- Villalobos-Rodelo, J.J.; Medina-Solís, C.E.; Maupomé, G.; Vallejos-Sánchez, A.A.; Lau-Rojo, L.; de León-Viedas, M.V.P. Socioeconomic and sociodemographic variables associated with oral hygiene status in Mexican schoolchildren aged 6 to 12 years. J. Periodontol. 2007, 78, 816–822. [Google Scholar] [CrossRef]
- Minaya-Sánchez, M.; Medina-Solís, C.E.; Vallejos-Sánchez, A.A.; Marquez-Corona, M.L.; Pontigo-Loyola, A.P.; Islas-Granillo, H.; Maupomé, G. Gingival recession and associated factors in a homogeneous Mexican adult male population: A cross-sectional study. Med. Oral Patol. Oral Cir. Bucal 2012, 17, e807. [Google Scholar] [CrossRef]
- López-Gómez, S.A.; González-López, B.S.; Scougall-Vilchis, R.J.; Pontigo-Loyola, A.P.; de Lourdes Márquez-Corona, M.; Villalobos-Rodelo, J.J.; Rueda-Ibarra, V.; Medina-Solís, C.E. Tooth loss in patients with and without diabetes: A large-scale, cross-sectional study of Mexican adults. J. Am. Dent. Assoc. 2020, 151, 276–286. [Google Scholar] [CrossRef]
- Herrera-Serna, B.Y.; Lara-Carrillo, E.; Toral-Rizo, V.H.; do Amaral, R.C.; Aguilera-Eguía, R.A. Relationship between the human development index and its components with oral cancer in Latin America. J. Epidemiol. Glob. Health 2019, 9, 223. [Google Scholar] [CrossRef] [PubMed]
- GBD 2017 Oral Disorders Collaborators; Bernabe, E.; Marcenes, W.; Hernandez, C.; Bailey, J.; Abreu, L.; Alipour, V.; Amini, S.; Arabloo, J.; Arefi, Z.; et al. Global, regional, and national levels and trends in burden of oral conditions from 1990 to 2017: A systematic analysis for the global burden of disease 2017 study. J. Dent. Res. 2020, 99, 362–373. [Google Scholar] [PubMed]
- Vettore, M.V.; Rebelo Vieira, J.M.; FF Gomes, J.; Martins, N.M.; Freitas, Y.N.; Lamarca, G.d.A.; Rebelo, M.A. Individual-and City-Level Socioeconomic Factors and Tooth Loss among Elderly People: A Cross-Level Multilevel Analysis. Int. J. Environ. Res. Public Health 2020, 17, 2345. [Google Scholar] [CrossRef] [PubMed]
- Roberto, L.L.; Silveira, M.F.; De Paula, A.M.B.; Ferreira e Ferreira, E.; Martins, A.M.E.d.B.L.; Haikal, D.S. Contextual and individual determinants of tooth loss in adults: A multilevel study. BMC Oral Health 2020, 20, 73. [Google Scholar] [CrossRef]
- Anand, S.; Sen, A. Human Development Index: Methodology and Measurement. United Nations Dev. Programme 1994, 1, 2–14. [Google Scholar]
- Hobdell, M.; Oliveira, E.; Bautista, R.; Myburgh, N.; Lalloo, R.; Narendran, S.; Johnson, N. Oral diseases and socio-economic status (SES). Br. Dent. J. 2003, 194, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Permanyer, I.; Smits, J. Inequality in human development across the globe. Popul. Dev. Rev. 2020, 46, 583–601. [Google Scholar] [CrossRef]
- EVALUA-DF. Metodología para la construcción del Índice de Desarrollo Social de Unidades territoriales del Distrito Federal. In Índice del Desarrollo Social de las Unidades Territoriales del Distrito Federal, delegación, colonia y manzana 2011, 1st ed.; Consejo de Evaluación del Desarrollo Social del Distrito Federal. Gobierno del Distrito Federal: Ciudad de México, Mexico, 2011; Volume 1, pp. 14–43. [Google Scholar]
- Gilbert, A.; Nuttall, N. Self-reporting of periodontal health status. Br. Dent. J. 1999, 186, 241–244. [Google Scholar] [CrossRef] [PubMed]
- Buhlin, K.; Gustafsson, A.; Andersson, K.; Håkansson, J.; Klinge, B. Validity and limitations of self-reported periodontal health. Community Dent. Oral Epidemiol. 2002, 30, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Cyrino, R.M.; Miranda Cota, L.O.; Pereira Lages, E.J.; Bastos Lages, E.M.; Costa, F.O. Evaluation of self-reported measures for prediction of periodontitis in a sample of Brazilians. J. Periodontol. 2011, 82, 1693–1704. [Google Scholar] [CrossRef]
- Khader, Y.; Alhabashneh, R.; Alhersh, F. Development and validation of a self-reported periodontal disease measure among Jordanians. Int. Dent. J. 2015, 65, 203–210. [Google Scholar] [CrossRef]
- Foster Page, L.; Thomson, W.; Broadbent, J. Validity of self-reported periodontal questions in a New Zealand cohort. Clin. Oral Investig. 2016, 20, 563–569. [Google Scholar] [CrossRef]
- Blicher, B.; Joshipura, K.; Eke, P. Validation of self-reported periodontal disease: A systematic review. J. Dent. Res. 2005, 84, 881–890. [Google Scholar] [CrossRef]
- Yamamoto, T.; Koyama, R.; Tamaki, N.; Maruyama, T.; Tomofuji, T.; Ekuni, D.; Yamanaka, R.; Azuma, T.; Morita, M. Validity of a questionnaire for periodontitis screening of Japanese employees. J. Occup. Health 2009, 51, 137–143. [Google Scholar] [CrossRef]
- Ramos, R.Q.; Bastos, J.L.; Peres, M.A. Diagnostic validity of self-reported oral health outcomes in population surveys: Literature review. Rev. Bras. Epidemiol. 2013, 16, 716–728. [Google Scholar] [CrossRef]
- Abbood, H.M.; Hinz, J.; Cherukara, G.; Macfarlane, T.V. Validity of self-reported periodontal disease: A systematic review and meta-analysis. J. Periodontol. 2016, 87, 1474–1483. [Google Scholar] [CrossRef] [PubMed]
- Fleming, E. A Self-Report to 2-Domain Questions May Accurately Screen for Periodontitis. J. Evid. Based. Dent. Pract. 2017, 17, 271–273. [Google Scholar] [CrossRef] [PubMed]
- Guardado-Luevanos, I.; Bologna-Molina, R.; Zepeda-Nuño, J.S.; Isiordia-Espinoza, M.; Molina-Frechero, N.; González-González, R.; Pérez-Pérez, M.; López-Verdín, S. Self-Reported Periodontal Disease and Its Association with SARS-CoV-2 Infection. Int. J. Environ. Res. Public Health 2022, 19, 10306. [Google Scholar] [CrossRef] [PubMed]
- Larvin, H.; Wilmott, S.; Wu, J.; Kang, J. The impact of periodontal disease on hospital admission and mortality during COVID-19 pandemic. Front. Med. 2020, 7, 604980. [Google Scholar] [CrossRef] [PubMed]
- Folayan, M.O.; Zuniga, R.A.A.; Ezechi, O.C.; Brown, B.; Nguyen, A.L.; Aly, N.M.; Ellakany, P.; Idigbe, I.E.; Khan, A.T.A.; Lawal, F.B.; et al. Associations between Emotional Distress, Sleep Changes, Decreased Tooth Brushing Frequency, Self-Reported Oral Ulcers and SARS-Cov-2 Infection during the First Wave of the COVID-19 Pandemic: A Global Survey. Int. J. Environ. Res. Public Health 2022, 19, 11550. [Google Scholar] [CrossRef]
- Larvin, H.; Wilmott, S.; Kang, J.; Aggarwal, V.; Pavitt, S.; Wu, J. Additive effect of periodontal disease and obesity on COVID-19 outcomes. J. Dent. Res. 2021, 100, 1228–1235. [Google Scholar] [CrossRef]
- Martínez-García, M.; Gutierrez-Esparza, G.O.; Roblero-Godinez, J.C.; Marín-Pérez, D.V.; Montes-Ruiz, C.L.; Vallejo, M.; Hernández-Lemus, E. Cardiovascular Risk Factors and Social Development Index. Front. Cardiovasc. Med. 2021, 8, 68. [Google Scholar] [CrossRef]
- Gutiérrez-Esparza, G.O.; Infante Vázquez, O.; Vallejo, M.; Hernández-Torruco, J. Prediction of Metabolic Syndrome in a Mexican Population Applying Machine Learning Algorithms. Symmetry 2020, 12, 581. [Google Scholar] [CrossRef]
- Gutiérrez-Esparza, G.O.; Ramírez-delReal, T.A.; Martínez-García, M.; Infante Vázquez, O.; Vallejo, M.; Hernández-Torruco, J. Machine and Deep Learning Applied to Predict Metabolic Syndrome without a Blood Screening. Appl. Sci. 2021, 11, 4334. [Google Scholar] [CrossRef]
- Martínez-García, M.; Castrejón-Pérez, R.C.; Rodríguez-Hernández, A.P.; Sandoval-Motta, S.; Vallejo, M.; Borges-Yáñez, S.A.; Hernández-Lemus, E. Incidence of Arterial Hypertension in People With Periodontitis and Characterization of the Oral and Subgingival Microbiome: A Study Protocol. Front. Cardiovasc. Med. 2021, 8, 763293. [Google Scholar] [CrossRef]
- Eke, P.I.; Genco, R.J. CDC Periodontal Disease Surveillance Project: Background, Objectives, and Progress Report; CDC: Atlanta, GA, USA, 2007.
- Gilbert, G.H.; Litaker, M.S. Validity of self-reported periodontal status in the Florida dental care study. J. Periodontol. 2007, 78, 1429–1438. [Google Scholar] [CrossRef] [PubMed]
- Eke, P.; Dye, B.; Wei, L.; Slade, G.; Thornton-Evans, G.; Beck, J.; Taylor, G.; Borgnakke, W.; Page, R.; Genco, R. Self-reported measures for surveillance of periodontitis. J. Dent. Res. 2013, 92, 1041–1047. [Google Scholar] [CrossRef] [PubMed]
- García-Pérez, Á.; Borges-Yáñez, S.A.; Jiménez-Corona, A.; Jiménez-Corona, M.E.; Ponce-de León, S. Self-report of gingival problems and periodontitis in indigenous and non-indigenous populations in Chiapas, Mexico. Int. Dent. J. 2016, 66, 105–112. [Google Scholar] [CrossRef]
- Quiroz, V.; Reinero, D.; Hernández, P.; Contreras, J.; Vernal, R.; Carvajal, P. Development of a self-report questionnaire designed for population-based surveillance of gingivitis in adolescents: Assessment of content validity and reliability. J. Appl. Oral Sci. 2017, 25, 404–411. [Google Scholar] [CrossRef]
- Kim, H.N.; Jang, Y.E.; Kim, C.B.; Kim, N.H. Socioeconomic status and self-reported periodontal symptoms in community-dwelling individuals: Data from the Korea Community Health Surveys of 2011 and 2013. Int. Dent. J. 2018, 68, 411–419. [Google Scholar] [CrossRef]
- Jang, Y.E.; Kim, C.B.; Kim, N.H. Influence of dental insurance coverage on access to preventive periodontal care in middle-aged and elderly populations: Analysis of representative Korean Community Health Survey Data (2011–2015). Int. Dent. J. 2019, 69, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Callhoff, J.; Dietrich, T.; Chubrieva, M.; Klotsche, J.; Zink, A. A patient-reported questionnaire developed in a German early arthritis cohort to assess periodontitis in patients with rheumatoid arthritis. Arthritis Res. Ther. 2019, 21, 197. [Google Scholar] [CrossRef]
- Veynachter, T.; Orti, V.; Moulis, E.; Rousseau, H.; Thilly, N.; Anagnostou, F.; Jeanne, S.; Bisson, C. Prevalence and Associated Factors of Self-Reported Gingival Bleeding: A Multicenter Study in France. Int. J. Environ. Res. Public Health 2020, 17, 8563. [Google Scholar] [CrossRef]
- Micu, I.C.; Bolboacă, S.D.; Caracostea, G.V.; Gligor, D.; Ciurea, A.; Iozon, S.; Soancă, A.; Mureșan, D.; Roman, A. Self-reported and clinical periodontal conditions in a group of Eastern European postpartum women. PLoS ONE 2020, 15, e0237510. [Google Scholar] [CrossRef]
- Perdoncini, N.N.; Furquim, C.P.; Bonfim, C.M.S.; Soares, G.M.S.; Torres-Pereira, C.C. Self-perception of periodontal health status among individuals with Fanconi anemia. Hematol. Transfus. Cell Ther. 2021, 43, 453–458. [Google Scholar] [CrossRef]
- Hernández, M.H.R.; Campillo, M.R.; Nachón-García, M.G. Modelos de autorreportes para detección de enfermedades periodontales: Revisión sistemática. Uvserva 2021, 1, 169–185. [Google Scholar] [CrossRef]
- Sorunke, M.E.; Onigbinde, O.O.; Oyapero, A.; Coker, O.A. Self-Reported Periodontal Disease and its Association with Dental Anxiety in Lagos, Nigeria. Pesqui. Bras. Odontopediatria Clin. Integr. 2022, 22, e210051. [Google Scholar] [CrossRef]
- Benítez-Pérez, H.; Herrera, L.A.; López-Arellano, O.; Revuelta-Herrera, A.; Rosales-Tapia, A.R.; Suárez-Lastra, M.; Kershenobich, D.; Ruiz-Gutiérrez, R. Probability of hospitalisation and death among COVID-19 patients with comorbidity during outbreaks occurring in Mexico City. J. Glob. Health 2022, 12, 05038. [Google Scholar]
- Cerón Vargas, J.A.; Raccanello, K. Índice de desarrollo social de la Ciudad de México como herramienta de focalización de la política social. Retos Dir. 2018, 12, 64–86. [Google Scholar]
- Luengas-Aguirre, M.; Sáenz-Martínez, L.; Tenorio-Torres, G.; Garcilazo-Gómez, A.; Díaz-Franco, M. Aspectos sociales y biológicos del edentulismo en México: Un problema visible de las inequidades en salud. Cienc. Clin. 2015, 16, 29–36. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2012; Volume 10. [Google Scholar]
- Sánchez-García, S.; Heredia-Ponce, E.; Cruz-Hervert, P.; Juárez-Cedillo, T.; Cárdenas-Bahena, Á.; García-Peña, C. Oral health status in older adults with social security in Mexico City: Latent class analysis. J. Clin. Exp. Dent. 2014, 6, e29. [Google Scholar] [CrossRef] [PubMed]
- Castrejón-Pérez, R.C.; Borges-Yáñez, S.A.; Gutiérrez-Robledo, L.M.; Ávila-Funes, J.A. Oral health conditions and frailty in Mexican community-dwelling elderly: A cross sectional analysis. BMC Public Health 2012, 12, 773. [Google Scholar] [CrossRef]
- Castrejón-Pérez, R.C.; Borges-Yáñez, S.A.; Irigoyen-Camacho, M.E.; Cruz-Hervert, L.P. Negative impact of oral health conditions on oral health related quality of life of community dwelling elders in Mexico city, a population based study. Geriatr. Gerontol. Int. 2017, 17, 744–752. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Han, D.H. Early-life socioeconomic position and periodontal status in Korean adults. Community Dent. Oral Epidemiol. 2016, 44, 11–23. [Google Scholar] [CrossRef]
- Al-Sudani, F.Y.; Vehkalahti, M.M.; Suominen, A.L. Association of current employment status with oral health-related behaviors: Findings from the Finnish Health 2000 Survey. Eur. J. Oral Sci. 2016, 124, 368–376. [Google Scholar] [CrossRef]
- Bastos, T.F.; Medina, L.d.P.B.; Sousa, N.F.d.S.; Lima, M.G.; Malta, D.C.; Barros, M.B.d.A. Income inequalities in oral health and access to dental services in the Brazilian population: National Health Survey, 2013. Rev. Bras. Epidemiol. 2019, 22, E190015. [Google Scholar] [CrossRef] [PubMed]
- Fagundes, M.L.B.; Bastos, L.F.; Amaral Júnior, O.L.d.; Menegazzo, G.R.; Cunha, A.R.d.; Stein, C.; Abreu, L.G.; Hugo, F.N.; Giordani, J.M.d.A.; Malta, D.C.; et al. Socioeconomic inequalities in the use of dental services in Brazil: An analysis of the 2019 National Health Survey. Rev. Bras. Epidemiol. 2021, 24, e210004. [Google Scholar] [CrossRef] [PubMed]
- Ju, X.; Mejia, G.C.; Wu, Q.; Luo, H.; Jamieson, L.M. Use of oral health care services in the United States: Unequal, inequitable—A cross-sectional study. BMC Oral Health 2021, 21, 370. [Google Scholar] [CrossRef]
- Koyama, S.; Aida, J.; Mori, Y.; Okawa, S.; Odani, S.; Miyashiro, I. COVID-19 Effects on Income and Dental Visits: A Cross-sectional Study. JDR Clin. Trans. Res. 2022, 7, 307–314. [Google Scholar] [CrossRef]
- Meehan, K.; Jurjevich, J.R.; Chun, N.M.; Sherrill, J. Geographies of insecure water access and the housing–water nexus in US cities. Proc. Natl. Acad. Sci. USA 2020, 117, 28700–28707. [Google Scholar] [CrossRef] [PubMed]
- Folayan, M.O.; Obiyan, M.O.; Olaleye, A.O. Association between water, sanitation, general hygiene and oral hygiene practices of street-involved young people in Southwest Nigeria. BMC Oral Health 2020, 20, 32. [Google Scholar] [CrossRef]
- Heijnen, M.; Cumming, O.; Peletz, R.; Chan, G.K.S.; Brown, J.; Baker, K.; Clasen, T. Shared sanitation versus individual household latrines: A systematic review of health outcomes. PLoS ONE 2014, 9, e93300. [Google Scholar] [CrossRef]
- Nicolau, B.; Marcenes, W.; Hardy, R.; Sheiham, A. A life-course approach to assess the relationship between social and psychological circumstances and gingival status in adolescents. J. Clin. Periodontol. 2003, 30, 1038–1045. [Google Scholar] [CrossRef]
- Burt, B.A. Concepts of risk in dental public health. Community Dent. Oral Epidemiol. 2005, 33, 240–247. [Google Scholar] [CrossRef]
- de França Doria, M.; Segurado, P.; Korc, M.; Heller, L.; Jimenez Cisneros, B.; Hunter, P.R.; Forde, M. Preliminary assessment of COVID-19 implications for the water and sanitation sector in Latin America and the Caribbean. Int. J. Environ. Res. Public Health 2021, 18, 11703. [Google Scholar] [CrossRef]
- Ramirez-Rubio, O.; Daher, C.; Fanjul, G.; Gascon, M.; Mueller, N.; Pajín, L.; Plasencia, A.; Rojas-Rueda, D.; Thondoo, M.; Nieuwenhuijsen, M.J. Urban health: An example of a health in all policies approach in the context of SDGs implementation. Global. Health 2019, 15, 87. [Google Scholar] [CrossRef] [PubMed]
- Ruano, A.L.; Rodríguez, D.; Rossi, P.G.; Maceira, D. Understanding inequities in health and health systems in Latin America and the Caribbean: A thematic series. Int. J. Equity Health 2021, 20, 94. [Google Scholar] [CrossRef] [PubMed]
- Cifuentes, E.; Suárez, L.; Solano, M.; Santos, R. Diarrheal diseases in children from a water reclamation site in Mexico city. Environ. Health Perspect. 2002, 110, A619–A624. [Google Scholar] [CrossRef]
- Munoz-Pina, C.; Rivera, M.; Morales, R.; Aguirre, A. Poverty and the environment in Mexico—The right to a healthy environment. In Proceedings of the The Fourth GGKP Annual Conference, Jeju Island, Republic of Korea, 6–7 September 2016; pp. 1–15. [Google Scholar]
- Manrique-Espinoza, B.; Salinas-Rodríguez, A.; Mojarro-Íñiguez, M.; Téllez-Rojo, M.; Pérez-Núñez, R.; Ventura-Alfaro, C. Tooth loss and dental healthcare coverage in older rural Mexican adults living in poverty. J. Am. Geriatr. Soc. 2010, 58, 804–805. [Google Scholar] [CrossRef] [PubMed]
- Cruz Palma, G.; Nakagoshi Cepeda, A.A.; Quiroga García, M.Á.; Palomares Gorham, P.I.; Galindo Lartigue, C.; González Meléndez, R. Sustentabilidad en los servicios de salud bucal en México. Odontología Vital 2018, 1, 39–42. [Google Scholar]
- Colchero, M.A.; Gómez, R.; Bautista-Arredondo, S. Characterization of the “primary health care cascade” in public services in Mexico in localities with less than 100 000 inhabitants. Salud. Publica. Mex. 2019, 61, 734–741. [Google Scholar] [CrossRef]
- Peres, M.A.; Macpherson, L.M.; Weyant, R.J.; Daly, B.; Venturelli, R.; Mathur, M.R.; Listl, S.; Celeste, R.K.; Guarnizo-Herreño, C.C.; Kearns, C.; et al. Oral diseases: A global public health challenge. Lancet 2019, 394, 249–260. [Google Scholar] [CrossRef]
- Srinarupat, J.; Oshiro, A.; Zaitsu, T.; Prasertsom, P.; Niyomsilp, K.; Kawaguchi, Y.; Aida, J. Inequalities in periodontal disease according to insurance schemes in thailand. Int. J. Environ. Res. Public Health 2021, 18, 5945. [Google Scholar] [CrossRef]
- Ronis, D.L.; Lang, W.P.; Farghaly, M.M.; Passow, E. Tooth brushing, flossing, and preventive dental visits by Detroit-area residents in relation to demographic and socioeconomic factors. J. Public. Health Dent. 1993, 53, 138–145. [Google Scholar] [CrossRef]
- Moraes, R.B.; Marques, B.B.; Cocco, D.M.P.; Knorst, J.K.; Tomazoni, F.; Ardenghi, T.M. Effect of environmental and socioeconomic factors on the use of dental floss among children: A hierarchical approach. Braz. Oral Res. 2019, 33, e096. [Google Scholar] [CrossRef]
- Yilmaz Cirakoglu, N.; Gokcek, M. The impact of socioeconomic factors and oral hygiene habits on knowledge level of oral health and procedures: The questionnaire based research. Clin. Exp. Health. Sci. 2021, 1, 105–112. [Google Scholar] [CrossRef]
- Berchier, C.; Slot, D.; Haps, S.; Van der Weijden, G. The efficacy of dental floss in addition to a toothbrush on plaque and parameters of gingival inflammation: A systematic review. Int. J. Dent. Hyg. 2008, 6, 265–279. [Google Scholar] [CrossRef] [PubMed]
- Merchant, A.T.; Pitiphat, W.; Rimm, E.B.; Joshipura, K. Increased physical activity decreases periodontitis risk in men. Eur. J. Epidemiol. 2003, 18, 891–898. [Google Scholar] [CrossRef] [PubMed]
- Al-Zahrani, M.S.; Borawski, E.A.; Bissada, N.F. Increased physical activity reduces prevalence of periodontitis. J. Dent. 2005, 33, 703–710. [Google Scholar] [CrossRef]
- Di Francescomarino, S.; Sciartilli, A.; Di Valerio, V.; Di Baldassarre, A.; Gallina, S. The effect of physical exercise on endothelial function. Sports Med. 2009, 39, 797–812. [Google Scholar] [CrossRef]
- Shimazaki, Y.; Egami, Y.; Matsubara, T.; Koike, G.; Akifusa, S.; Jingu, S.; Yamashita, Y. Relationship between obesity and physical fitness and periodontitis. J. Periodontol. 2010, 81, 1124–1131. [Google Scholar] [CrossRef]
- Bawadi, H.; Khader, Y.; Haroun, T.; Al-Omari, M.; Tayyem, R. The association between periodontal disease, physical activity and healthy diet among adults in Jordan. J. Periodontal Res. 2011, 46, 74–81. [Google Scholar] [CrossRef]
- Andrade, E.F.; Silva, V.d.O.; Moura, N.O.d.; Foureaux, R.d.C.; Orlando, D.R.; Moura, R.F.d.; Pereira, L.J. Physical exercise improves glycemic and inflammatory profile and attenuates progression of periodontitis in diabetic rats (HFD/STZ). Nutrients 2018, 10, 1702. [Google Scholar] [CrossRef]
- Hwang, S.Y.; Jang, J.H.; Park, J.E. Association between healthy lifestyle (diet quality, physical activity, normal body weight) and periodontal diseases in Korean adults. Int. J. Environ. Res. Public Health 2022, 19, 3871. [Google Scholar] [CrossRef]
- Iwasaki, M.; Yoshihara, A.; Suwama, K.; Zaitsu, T.; Suzuki, S.; Ihira, H.; Sawada, N.; Aida, J. A cross-sectional study of the association between periodontitis and physical activity in the Japanese population. J. Periodontal Res. 2023, 58, 350–359. [Google Scholar] [CrossRef]
- Agarwal, A.; Jindal, D.; Ajay, V.S.; Kondal, D.; Mandal, S.; Ghosh, S.; Ali, M.; Singh, K.; Huffman, M.D.; Tandon, N.; et al. Association between socioeconomic position and cardiovascular disease risk factors in rural north India: The Solan Surveillance Study. PLoS ONE 2019, 14, e0217834. [Google Scholar] [CrossRef] [PubMed]
- Watt, R.G. From victim blaming to upstream action: Tackling the social determinants of oral health inequalities. Community Dent. Oral Epidemiol. 2007, 35, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Butera, A.; Lovati, E.; Simone, R.; Segù, M.; Scribante, A.; Lanteri, V.; Chiesa, A.; Granata, M.; Ruggero, R.Y.B. Professional and home-management in non-surgical periodontal therapy to evaluate the percentage of glycated hemoglobin in type 1 diabetes patients. Int. J. Clin. Dent. 2021, 14, 41–53. [Google Scholar]
- Sanz, M.; Ceriello, A.; Buysschaert, M.; Chapple, I.; Demmer, R.T.; Graziani, F.; Herrera, D.; Jepsen, S.; Lione, L.; Madianos, P.; et al. Scientific evidence on the links between periodontal diseases and diabetes: Consensus report and guidelines of the joint workshop on periodontal diseases and diabetes by the International Diabetes Federation and the European Federation of Periodontology. Diabetes Res. Clin. Pract. 2018, 137, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Hernandez, L.; Perez-Sastre, M.A. Social inequalities in the progression of COVID-19 in the Mexican population. Rev. Panam. Salud Publica 2020, 44, e106. [Google Scholar] [CrossRef]
- Campisi, G.; Bizzoca, M.E.; Lo Muzio, L. COVID-19 and periodontitis: Reflecting on a possible association. Head Face Med. 2021, 17, 16. [Google Scholar] [CrossRef]
- Wang, Y.; Deng, H.; Pan, Y.; Jin, L.; Hu, R.; Lu, Y.; Deng, W.; Sun, W.; Chen, C.; Shen, X.; et al. Periodontal disease increases the host susceptibility to COVID-19 and its severity: A Mendelian randomization study. J. Transl. Med. 2021, 19, 528. [Google Scholar] [CrossRef]
- Meng, Z.; Ma, Y.; Li, W.; Deng, X. Association between periodontitis and COVID-19 infection: A two-sample Mendelian randomization study. PeerJ 2023, 11, e14595. [Google Scholar] [CrossRef]
- Gupta, S.; Mohindra, R.; Singla, M.; Khera, S.; Sahni, V.; Kanta, P.; Soni, R.K.; Kumar, A.; Gauba, K.; Goyal, K.; et al. The clinical association between Periodontitis and COVID-19. Clin. Oral Investig. 2022, 26, 1361–1374. [Google Scholar] [CrossRef]
- Qi, M.; Sun, W.; Wang, K.; Li, W.; Lin, J.; Gong, J.; Wang, L. Periodontitis and COVID-19: Immunological Characteristics, Related Pathways, and Association. Int. J. Mol. Sci. 2023, 24, 3012. [Google Scholar] [CrossRef]
- Iwasaki, M.; Usui, M.; Ariyoshi, W.; Nakashima, K.; Nagai-Yoshioka, Y.; Inoue, M.; Kobayashi, K.; Nishihara, T. Interruption of regular dental visits during the COVID-19 pandemic due to concerns regarding dental visits was associated with periodontitis in Japanese office workers. J. Periodontal Res. 2021, 56, 1091–1098. [Google Scholar] [CrossRef] [PubMed]
- Matsuyama, Y.; Aida, J.; Takeuchi, K.; Koyama, S.; Tabuchi, T. Dental pain and worsened socioeconomic conditions due to the COVID-19 pandemic. J. Dent. Res. 2021, 100, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Stennett, M.; Tsakos, G. The impact of the COVID-19 pandemic on oral health inequalities and access to oral healthcare in England. Br. Dent. J. 2022, 232, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Dickson-Swift, V.; Kangutkar, T.; Knevel, R.; Down, S. The impact of COVID-19 on individual oral health: A scoping review. BMC Oral Health 2022, 22, 422. [Google Scholar] [CrossRef]
| Sociomedical Features | Very Low (n = 134) | Low (n = 462) | Medium (n = 305) | High (n = 393) | p Value |
|---|---|---|---|---|---|
| Female * | 61.94 | 64.72 | 65.25 | 66.67 | 0.790 |
| Male * | 38.06 | 35.28 | 34.75 | 33.33 | |
| Age, years ** | 40 (32–48) | 43 (33–50) | 43 (35–50) | 45 (36–51) | <0.05 |
| Elementary school * | 0.74 | 0.22 | 0.66 | 0.25 | 0.545 |
| Middle school * | 11.94 | 5.19 | 4.92 | 2.54 | <0.001 |
| High school * | 40.30 | 36.80 | 29.18 | 21.12 | <0.001 |
| University studies * | 38.81 | 47.62 | 50.82 | 52.42 | <0.001 |
| Postgraduate * | 8.20 | 10.17 | 14.42 | 23.66 | 0.031 |
| COVID-19 * | 21.64 | 24.89 | 19.02 | 13.99 | <0.001 |
| Smoking * | 18.66 | 22.29 | 16.72 | 17.05 | 0.852 |
| MetS diagnostic * | 6.72 | 4.33 | 3.61 | 4.58 | 0.543 |
| Physical activity (Low) * | 16.42 | 19.70 | 19.34 | 17.05 | 0.677 |
| Physical activity (Moderate) * | 50 | 46.10 | 44.92 | 48.85 | 0.636 |
| Physical activity (High) * | 33.58 | 34.20 | 35.74 | 34.10 | 0.959 |
| Sleep quality (Bad) * | 38.81 | 46.54 | 44.26 | 47.84 | 0.299 |
| Oral Health Features | Very Low (n = 134) | Low (n = 462) | Medium (n = 305) | High (n = 393) | p Value |
|---|---|---|---|---|---|
| Periodontal conditions | |||||
| Tooth does not look right * | 38.06 | 31.60 | 33.44 | 30.28 | <0.001 |
| Loose tooth * | 11.19 | 9.09 | 10.16 | 10.18 | 0.885 |
| Loose tooth later * | 8.21 | 5.63 | 6.56 | 6.87 | 0.725 |
| Lost tooth * | 44.78 | 41.77 | 40.66 | 40.97 | 0.864 |
| Gum disease * | 19.40 | 25.54 | 31.80 | 25.45 | <0.05 |
| Bleeding gums * | 48.51 | 52.38 | 50.82 | 47.84 | <0.001 |
| Gum infected * | 16.42 | 20.56 | 19.67 | 22.14 | <0.05 |
| Bad breath * | 23.13 | 25.97 | 27.54 | 21.88 | <0.001 |
| Abscessed teeth * | 14.93 | 15.80 | 12.13 | 12.47 | <0.001 |
| Bone loss * | 8.96 | 9.09 | 13.44 | 14.50 | <0.05 |
| Dental treatment | |||||
| Dental care * | 45.52 | 52.38 | 50.16 | 54.20 | 0.333 |
| Implant placed * | 14.18 | 11.47 | 15.08 | 12.98 | 0.517 |
| Gum treatment * | 17.16 | 22.73 | 25.25 | 21.88 | <0.05 |
| Oral hygiene | |||||
| Brush your teeth * | 98.51 | 97.62 | 97.38 | 97.96 | 0.881 |
| Times brush teeth ** | 2 (1) | 2 (1) | 2 (1) | 2 (1) | 0.061 |
| Brush before bed * | 76.12 | 78.14 | 80.33 | 86.26 | <0.05 |
| Floss use after * | 6.72 | 16.23 | 20.33 | 20.61 | 0.259 |
| Times floss use ** | 0 (0–2) | 1 (0–3) | 1 (0–3) | 1 (0–4) | <0.001 |
| Times mouthwash use ** | 0 (0–3) | 0 (0–3) | 0 (0–3) | 0 (0–3) | 0.240 |
| Ability to chew | |||||
| (Satisfaction level) | |||||
| Very unsatisfied * | 2.24 | 1.30 | 1.97 | 0.76 | 0.160 |
| Unsatisfied * | 8.96 | 9.96 | 9.51 | 8.40 | 0.032 |
| Satisfied * | 55.97 | 55.19 | 47.87 | 57 | <0.001 |
| Very Satisfied * | 32.84 | 33.55 | 40.66 | 33.84 | <0.001 |
| Variables | Estimate | OR (95% C.I.) | p-Value |
|---|---|---|---|
| Intercept | −3.443 | 0.032 (−4.955, −1.982) | 5.47 |
| SDI global | 1.985 | 7.276 (0.258, 3.744) | 0.025 |
| Physical activity (Low) † | −0.683 | 0.505 (−1.248, −0.159) | 0.013 |
| Physical activity (Moderate) † | −0.535 | 0.586 (−0.913, −0.159) | 0.005 |
| Times floss use | 0.076 | 1.079 (0.034, 0.118) | 3.3 |
| Satisfaction level (Unsatisfied) ‡ | 0.736 | 2.088 (0.231, 1.219) | 0.003 |
| Satisfaction level (Very satisfied) ‡ | −0.734 | 0.480 (−1.187, −0.306) | 0.001 |
| Satisfaction level (Very Unsatisfied) ‡ | 1.770 | 5.869 (0.742, 2.759) | 4.75 |
| Variables | Estimate | OR (95% C.I.) | p-Value |
|---|---|---|---|
| Intercept | −2.750 | 0.064 (−3.676, −1.851) | 3.34 |
| QASH | 1.299 | 3.666 (0.145, 2.460) | 0.028 |
| Physical activity (low) † | −0.681 | 0.506 (−1.246, −0.157) | 0.014 |
| Physical activity (moderate) † | −0.535 | 0.586 (−0.914, −0.160) | 0.005 |
| Times floss use | 0.077 | 1.080 (0.034, 0.118) | 3.14 |
| Satisfaction level (unsatisfied) ‡ | 0.737 | 2.090 (0.232, 1.220) | 0.003 |
| Satisfaction level (very satisfied) ‡ | −0.732 | 0.480 (−1.186, −0.304) | 0.001 |
| Satisfaction level (very unsatisfied) ‡ | 1.766 | 5.847 (0.739, 2.754) | 4.88 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-García, M.; Rodríguez-Hernández, A.-P.; Gutiérrez-Esparza, G.O.; Castrejón-Pérez, R.C.; Hernández-Lemus, E.; Borges-Yáñez, S.A. Relationship between the Social Development Index and Self-Reported Periodontal Conditions. Healthcare 2023, 11, 1548. https://doi.org/10.3390/healthcare11111548
Martínez-García M, Rodríguez-Hernández A-P, Gutiérrez-Esparza GO, Castrejón-Pérez RC, Hernández-Lemus E, Borges-Yáñez SA. Relationship between the Social Development Index and Self-Reported Periodontal Conditions. Healthcare. 2023; 11(11):1548. https://doi.org/10.3390/healthcare11111548
Chicago/Turabian StyleMartínez-García, Mireya, Adriana-Patricia Rodríguez-Hernández, Guadalupe O. Gutiérrez-Esparza, Roberto Carlos Castrejón-Pérez, Enrique Hernández-Lemus, and Socorro Aída Borges-Yáñez. 2023. "Relationship between the Social Development Index and Self-Reported Periodontal Conditions" Healthcare 11, no. 11: 1548. https://doi.org/10.3390/healthcare11111548
APA StyleMartínez-García, M., Rodríguez-Hernández, A.-P., Gutiérrez-Esparza, G. O., Castrejón-Pérez, R. C., Hernández-Lemus, E., & Borges-Yáñez, S. A. (2023). Relationship between the Social Development Index and Self-Reported Periodontal Conditions. Healthcare, 11(11), 1548. https://doi.org/10.3390/healthcare11111548









