Tozinameran (Pfizer, BioNTech) and Elasomeran (Moderna) Efficacy in COVID-19—A Systematic Review of Randomised Controlled Trial Studies
Abstract
:1. Introduction
- Beta (first diagnosed in the Republic of South Africa in September 2020),
- Gamma (first diagnosed in Brazil in December 2020),
- Delta (first diagnosed in India in December 2020),
- Omicron (first diagnosed in the Republic of South Africa and Botswana in November 2021).
2. Materials and Methods
2.1. Inclusion/Exclusion Criteria
- Randomised controlled trial (highest quality of the data).
- Double-blind.
- The population covered by the trial included healthy persons aged over 16.
- Patients were vaccinated with two doses of tozinameran (30 μg) or elasomeran (100 μg) vaccines.
- The effects were compared to a placebo.
- The measure of the vaccine’s efficacy was the proportion of persons with or without a confirmed diagnosis of COVID-19 (based on RT-PCR test) 7 days after the 2nd dose of the BNT162b2 vaccine and 14 days after the 2nd dose of the mRNA-1273 vaccine.
- The trial did not include randomisation and double-blinding.
- Patients did not receive two doses of either vaccine.
- The comparator was not a placebo.
- The vaccine doses were other than 30 μg of tozinameran and 100 μg of elasomeran.
2.2. Databases and Publication Search Methods
2.3. Bibliographic Analysis
2.4. Data Selection and Collection
2.5. Data Analysis
2.5.1. Assessment of the Risk of Bias and Missing Data
2.5.2. Assessment of the Quality of Clinical Trials—JADAD Scale
2.5.3. Certainty of Evidence
2.5.4. Outcome Measurement
- Vaccine efficacy was confirmed by the diagnosis of COVID-19 based on an RT-PCR test 7 days after the 2nd dose of the BNT162b2 vaccine and 14 days after the 2nd dose of the mRNA-1273 vaccine).
- The overall number of adverse events (AEs) such as erythema, tenderness, swelling, pain after injection, fever, headache, fatigue, myalgia, arthralgia, nausea, vomiting, and chills.
- The number of serious adverse events (SAEs) (life-threatening) such as hypersensitivity reactions, dermal filler reactions, Bell’s palsy, thromboembolism, and pericarditis.
2.5.5. Quantitative Synthesis of Included Trials
2.5.6. Assessment of the Heterogeneity of the Studies
3. Results
3.1. Study Selection
3.2. Characteristics of Studies Included in the Analysis
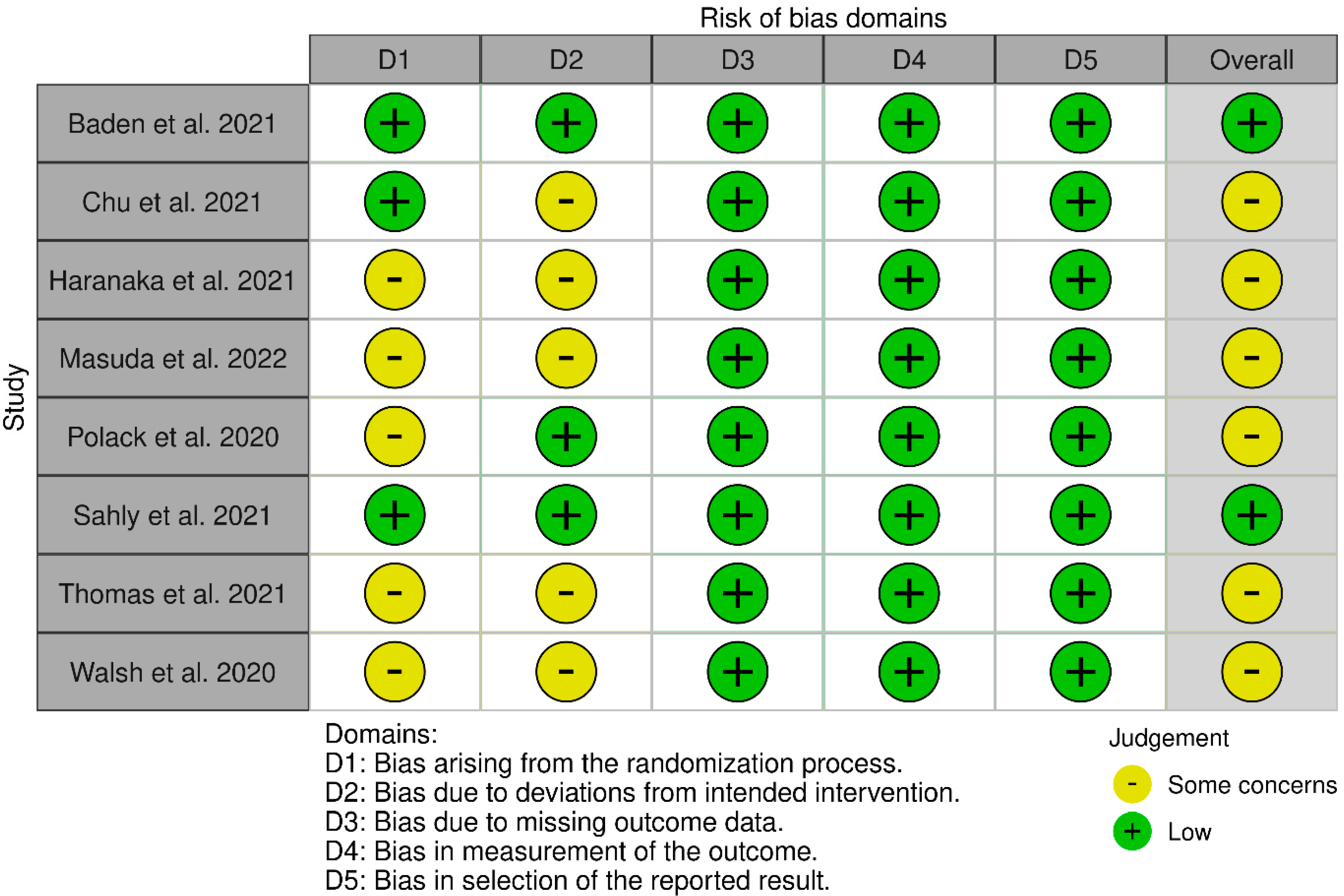
3.3. Assessment of the Quality of Studies Included in the Analysis
3.4. Results of the Meta-Analysis
3.5. Clinical Response
3.6. Adverse Events
3.7. Serious Adverse Events
3.8. Sensitivity Analysis
3.9. Sensitivity Analysis Based on Two Doses of the Vaccines
3.10. Sensitivity Analysis concerning Elasomeran
3.11. Publication Quantity and Citation Metrics
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FDA Approves First COVID-19 Vaccine. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-first-covid-19-vaccine (accessed on 5 June 2022).
- Coronavirus (COVID-19) Update: FDA Takes Key Action by Approving Second COVID-19 Vaccine. Available online: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-takes-key-action-approving-second-covid-19-vaccine (accessed on 5 June 2022).
- Spikevax Chmp Annex I Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/spikevax-previously-covid-19-vaccine-moderna-epar-product-information_en.pdf (accessed on 7 August 2022).
- SARS-CoV-2 Variants of Concern as of 20 January 2022. Available online: https://www.ecdc.europa.eu/en/covid-19/variants-concern (accessed on 23 January 2022).
- He, F.; Deng, Y.; Li, W. Coronavirus Disease 2019: What We Know? J. Med. Virol. 2020, 92, 719–725. [Google Scholar] [CrossRef]
- Chmp Annex I Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/comirnaty-epar-product-information_en.pdf (accessed on 7 August 2022).
- Zabor, E.C.; Kaizer, A.M.; Hobbs, B.P. Randomized Controlled Trials. Chest 2020, 158, S79–S87. [Google Scholar] [CrossRef] [PubMed]
- Averitt, A.J.; Weng, C.; Ryan, P.; Perotte, A. Translating Evidence into Practice: Eligibility Criteria Fail to Eliminate Clinically Significant Differences between Real-World and Study Populations. npj Digit. Med. 2020, 3, 67. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Boutron, I.; Page, M.J.; Higgins, J.P.T.; Altman, D.G.; Lundh, A.; Hróbjartsson, A. Chapter 7: Considering Bias and Conflicts of Interest among the Included Studies. Available online: https://www.training.cochrane.org/handbook/current/chapter-07 (accessed on 4 June 2022).
- Deeks, J.J.; Higgins, J.P.T.; Altman, D.G. Chapter 10: Analysing Data and Undertaking Meta-Analyses. Available online: https://www.training.cochrane.org/handbook/current/chapter-10 (accessed on 4 June 2022).
- Jadad, A.R.; Moore, R.A.; Carroll, D.; Jenkinson, C.; Reynolds, D.J.M.; Gavaghan, D.J.; McQuay, H.J. Assessing the Quality of Reports of Randomised Clinical Trials: Is Blinding Necessary? Control. Clin. Trials 1996, 17, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.; Thomas, J. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (Updated February 2021). Available online: https://training.cochrane.org/handbook (accessed on 5 June 2022).
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 MRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.J.; Moreira, E.D.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Polack, F.P.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 MRNA COVID-19 Vaccine through 6 Months. N. Engl. J. Med. 2021, 385, 1761–1773. [Google Scholar] [CrossRef] [PubMed]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the MRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Chu, L.; McPhee, R.; Huang, W.; Bennett, H.; Pajon, R.; Nestorova, B.; Leav, B. A Preliminary Report of a Randomized Controlled Phase 2 Trial of the Safety and Immunogenicity of MRNA-1273 SARS-CoV-2 Vaccine. Vaccine 2021, 39, 2791–2799. [Google Scholar] [CrossRef] [PubMed]
- El Sahly, H.M.; Baden, L.R.; Essink, B.; Doblecki-Lewis, S.; Martin, J.M.; Anderson, E.J.; Campbell, T.B.; Clark, J.; Jackson, L.A.; Fichtenbaum, C.J.; et al. Efficacy of the MRNA-1273 SARS-CoV-2 Vaccine at Completion of Blinded Phase. N. Engl. J. Med. 2021, 385, 1774–1785. [Google Scholar] [CrossRef] [PubMed]
- Walsh, E.E.; Frenck, R.W.; Falsey, A.R.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Mulligan, M.J.; Bailey, R.; et al. Safety and Immunogenicity of Two RNA-Based COVID-19 Vaccine Candidates. N. Engl. J. Med. 2020, 383, 2439–2450. [Google Scholar] [CrossRef] [PubMed]
- Haranaka, M.; Baber, J.; Ogama, Y.; Yamaji, M.; Aizawa, M.; Kogawara, O.; Scully, I.; Lagkadinou, E.; Özlem, T.; Şahin, U.; et al. A Randomized Study to Evaluate Safety and Immunogenicity of the BNT162b2 COVID-19 Vaccine in Healthy Japanese Adults. Nat. Commun. 2021, 12, 7105. [Google Scholar] [CrossRef]
- Masuda, T.; Murakami, K.; Sugiura, K.; Sakui, S.; Philip Schuring, R.; Mori, M. A Phase 1/2 Randomised Placebo-Controlled Study of the COVID-19 Vaccine MRNA-1273 in Healthy Japanese Adults: An Interim Report. Vaccine 2022, 40, 2044–2052. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.-J.; Chan, K.-H.; Fan-Ngai Hung, I.; Fan, Y.-J.; Chan, K.-H.; Bradfute, S.B.; Anthony, S. Safety and Efficacy of COVID-19 Vaccines: A Systematic Review and Meta-Analysis of Different Vaccines at Phase 3 Vaccines: A Systematic Review and Meta-Analysis of Different Vaccines at Phase 3. Vaccines 2021, 9, 989. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Yuan, Y.; Zhou, Y.; Deng, Z.; Zhao, J.; Feng, F.; Zou, H.; Sun, C. Safety of SARS-CoV-2 Vaccines: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Infect. Dis. Poverty 2021, 10, 94. [Google Scholar] [CrossRef] [PubMed]


| MEDLINE via PubMed | Efficacy/Effectiveness Study | Adverse Events Study | Type of Study | Language | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of Papers | Number of Preprints | RCT | Observational | Meta-Analysis | Systematic Review | Case Reports | Clinical Trial | Editorial | English | |||
| Tozinameran | 4680 | 88 | 693 | 313 | 45 | 225 | 32 | 75 | 635 | 86 | 36 | 4612 |
| Elasomeran | 1643 | 70 | 269 | 138 | 30 | 68 | 24 | 55 | 250 | 54 | 17 | 1620 |
| Type of Vaccine | Journal | Citation Number (Web of Science) | |
|---|---|---|---|
| Baden et al. 2021 [16] | Elasomeran | New England Journal of Medicine | 4566 |
| Chu et al. 2021 [17] | Elasomeran | Vaccine | 111 |
| Haranaka et al. 2021 [20] | Tozinameran | Nature Communications | 9 |
| Masuda et al. 2022 [21] | Elasomeran | Vaccine | 2 |
| Polack et al. 2020 [14] | Tozinameran | New England Journal of Medicine | 6825 |
| Sahly et al. 2021 [18] | Elasomeran | New England Journal of Medicine | 202 |
| Thomas et al. 2021 [15] | Tozinameran | New England Journal of Medicine | 462 |
| Walsh et al. 2020 [19] | Tozinameran | New England Journal of Medicine | 1312 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ratajczak, P.; Banach, Z.; Kopciuch, D.; Paczkowska, A.; Zaprutko, T.; Krawczyk, J.; Maciuszek-Bartkowska, B.; Kus, K. Tozinameran (Pfizer, BioNTech) and Elasomeran (Moderna) Efficacy in COVID-19—A Systematic Review of Randomised Controlled Trial Studies. Healthcare 2023, 11, 1532. https://doi.org/10.3390/healthcare11111532
Ratajczak P, Banach Z, Kopciuch D, Paczkowska A, Zaprutko T, Krawczyk J, Maciuszek-Bartkowska B, Kus K. Tozinameran (Pfizer, BioNTech) and Elasomeran (Moderna) Efficacy in COVID-19—A Systematic Review of Randomised Controlled Trial Studies. Healthcare. 2023; 11(11):1532. https://doi.org/10.3390/healthcare11111532
Chicago/Turabian StyleRatajczak, Piotr, Zuzanna Banach, Dorota Kopciuch, Anna Paczkowska, Tomasz Zaprutko, Józef Krawczyk, Barbara Maciuszek-Bartkowska, and Krzysztof Kus. 2023. "Tozinameran (Pfizer, BioNTech) and Elasomeran (Moderna) Efficacy in COVID-19—A Systematic Review of Randomised Controlled Trial Studies" Healthcare 11, no. 11: 1532. https://doi.org/10.3390/healthcare11111532
APA StyleRatajczak, P., Banach, Z., Kopciuch, D., Paczkowska, A., Zaprutko, T., Krawczyk, J., Maciuszek-Bartkowska, B., & Kus, K. (2023). Tozinameran (Pfizer, BioNTech) and Elasomeran (Moderna) Efficacy in COVID-19—A Systematic Review of Randomised Controlled Trial Studies. Healthcare, 11(11), 1532. https://doi.org/10.3390/healthcare11111532