Informed Consent in Paediatric Telemedicine: Challenge or Opportunity? A Scoping Review
Abstract
1. Introduction
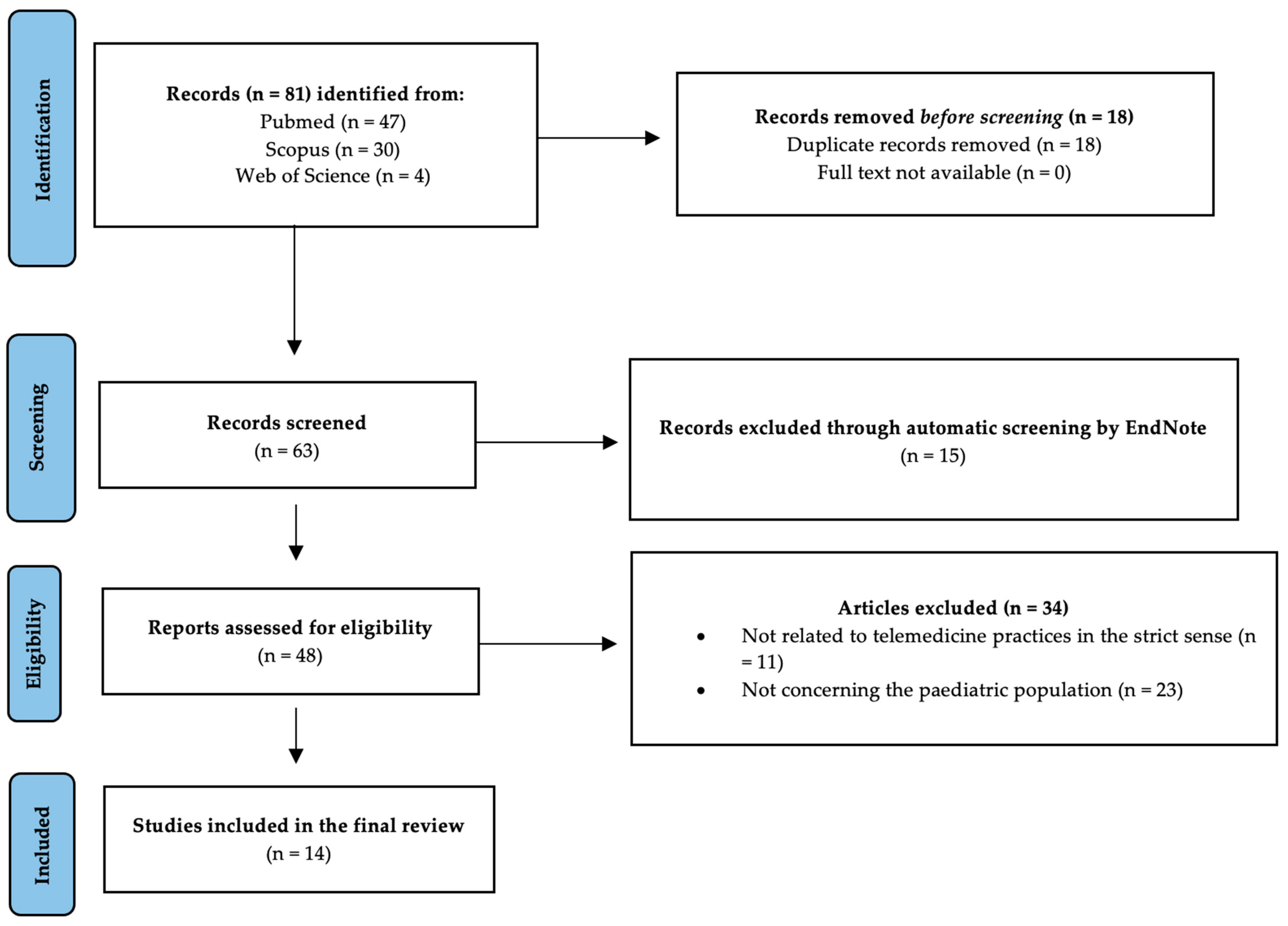
2. Materials and Methods
- Step 1.
- Identifying the research questions.
- Step 2.
- Identifying relevant studies.
- Step 3.
- Selecting studies.
- Step 4.
- Charting the data.
- Step 5.
- Collating, summarising, and reporting the results.
2.1. Identifying the Research Questions
2.2. Identifying Relevant Studies
2.2.1. Databases
2.2.2. Inclusion Criteria. The Application of the PCC Framework
2.2.3. Search Strategy
((“informed consent”[MeSH Terms] OR “informed consent”[All Fields])) AND ((“telemedicine”[MeSH Terms] OR “telemedicine”[All Fields] OR “remote consultation”[All Fields] OR “virtual consultation”[All Fields])) AND ((“paediatrics”[MeSH Terms] OR “paediatrics”[All Fields] OR “child”[All Fields] OR “adolescent”[MeSH Terms] OR “adolescent”[All Fields])) AND ((“parents”[MeSH Terms] OR “parents”[All Fields] OR “communication”[MeSH Terms] OR “communication”[All Fields])) AND “English”[Language] AND (“2013/01/01”[Date—Create]: “2023/01/31”[Date—Create])
(TITLE-ABS-KEY(“informed consent”) AND TITLE-ABS-KEY(“telemedicine” OR “remote consultation” OR “virtual consultation”) AND TITLE-ABS-KEY(“paediatrics” OR “child” OR “adolescent”) AND TITLE-ABS-KEY(“parents” OR “communication”) AND LANGUAGE (English) AND PUBYEAR > 2012 AND PUBYEAR < 2024)
(TS = (“informed consent”) AND TS = (“telemedicine” OR “remote consultation” OR “virtual consultation”) AND TS = (“paediatrics” OR “child” OR “adolescent”) AND TS = (“parents” OR “communication”) AND LANGUAGE: (English) AND PY = 2013 − 2023)
2.3. Identifying Relevant Studies
2.4. Charting the Data
- Author(s)
- Title
- Year of publication
- Geographical context
- Aim of the study
- Patients’ age
- Consent collection method
2.5. Collating, Summarising, and Reporting the Results
3. Discussion
4. Conclusions
(1) What are the major critical issues encountered in providing medical information to minor patients through telemedicine services and collecting their informed consent?
(2) If properly utilised, could telemedicine services facilitate adequate transmission of medical treatment information?
(3) Is it possible to propose an operational mode of delivery of medical information to the paediatric patient in telemedicine and consent collection?
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grootens-Wiegers, P.; Hein, I.M.; van den Broek, J.M.; de Vries, M.C. Medical decision-making in children and adolescents: Developmental and neuroscientific aspects. BMC Pediatr. 2017, 17, 120. [Google Scholar] [CrossRef] [PubMed]
- Hein, I.M. Children’s Competence to Consent to Medical Treatment or Research; Amsterdam University Press: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Young, M. EU Regulators Provide Guidance on Notice and Consent under GDPR. 14 December 2017. Children’s Privacy, European Union, Privacy Policies. Available online: https://www.insideprivacy.com/international/european-union/eu-regulators-provide-guidance-on-notice-and-consent-under-gdpr/ (accessed on 9 May 2023).
- Kichloo, A.; Albosta, M.; Dettloff, K.; Wani, F.; El-Amir, Z.; Singh, J.; Chugh, S. Telemedicine, the current COVID-19 pandemic and the future: A narrative review and perspectives moving forward in the USA. Fam. Med. Community Health 2020, 8, e000530. [Google Scholar] [CrossRef] [PubMed]
- Hyder, M.A.; Razzak, J. Telemedicine in the United States: An introduction for students and residents. J. Med. Internet Res. 2020, 22, e20839. [Google Scholar] [CrossRef] [PubMed]
- DePuccio, M.J.; Gaughan, A.A.; Shiu-Yee, K.; McAlearney, A.S. Doctoring from home: Physicians’ perspectives on the advantages of remote care delivery during the COVID-19 pandemic. PLoS ONE 2022, 17, e0269264. [Google Scholar] [CrossRef]
- Tully, L.; Case, L.; Arthurs, N.; Sorensen, J.; Marcin, J.P.; O’Malley, G. Barriers and facilitators for implementing paediatric telemedicine: Rapid review of user perspectives. Front. Pediatr. 2021, 9, 630365. [Google Scholar] [CrossRef]
- Utidjian, L.; Abramson, E. Pediatric telehealth: Opportunities and challenges. Pediat. Clin. 2016, 63, 367–378. [Google Scholar] [CrossRef]
- Mondal, H.; Haldar, R.; Mondal, S. Informed consent for telemedicine. J. Family Med. Prim. Care 2020, 9, 5402–5403. [Google Scholar] [CrossRef]
- Agency for Healthcare Research and Quality. How to Obtain Consent for Telehealth. 2020. Available online: https://www.ahrq.gov/health-literacy/improve/informed-consent/obtain.html (accessed on 15 February 2023).
- Curfman, A.; McSwain, S.D.; Chuo, J.; Yeager-McSwain, B.; Schinasi, D.A.; Marcin, J.; Herendeen, N.; Chung, S.L.; Rheuban, K.; Olson, C.A. Pediatric telehealth in the COVID-19 pandemic era and beyond. Pediatrics 2021, 148. [Google Scholar] [CrossRef]
- McSwain, S.D.; Bernard, J.; Burke, B.L.; Cole, S.L.; Dharmar, M.; Hall-Barrow, J.; Krupinski, E.A. American Telemedicine Association operating procedures for pediatric telehealth. Telemed. E-Health 2017, 23, 699–706. [Google Scholar] [CrossRef]
- Neumann, C.M.; Schleifer, G.; Strassberger-Nerschbach, N.; Kamp, J.; Massoth, G.; Görtzen-Patin, A.; Wittmann, M. Digital Online Anaesthesia Patient Informed Consent before Elective Diagnostic Procedures or Surgery: Recent Practice in Children—An Exploratory ESAIC Survey (2021). J. Clin. Med. 2022, 11, 502. [Google Scholar] [CrossRef]
- Arksey, H.; O’Malley, L. Scoping studies: Towards a methodological framework. Int. J. Soc. Res. Methodol. 2005, 8, 19–32. [Google Scholar] [CrossRef]
- Peters, M.; Godfrey, C.; McInerney, P.; Soares, C.; Khalil, H.; Parker, D. The Joanna Briggs Institute Reviewers’ Manual 2015: Methodology for JBI Scoping Reviews; Joanna Briggs Institute: Adelaide, Australia, 2015. [Google Scholar]
- Munn, Z.; Peters, M.D.J.; Stern, C.; Tufanaru, C.; McArthur, A.; Aromataris, E. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med. Res. Methodol. 2018, 18, 143. [Google Scholar] [CrossRef] [PubMed]
- Gibelli, F.; Ricci, G.; Sirignano, A.; Turrina, S.; De Leo, D. The increasing centrality of robotic technology in the context of nursing care: Bioethical implications analyzed through a scoping review approach. J. Healthc. Eng. 2021, 1478025. [Google Scholar] [CrossRef] [PubMed]
- The Joanna Briggs Institute. The Joanna Briggs Institute Reviewers Manual; Joanna Briggs Institute: Adelaide, Australia, 2015; Chapter 11.2.5. [Google Scholar]
- Yager, P.H.; Clark, M.E.; Dapul, H.R.; Murphy, S.; Zheng, H.; Noviski, N. Reliability of circulatory and neurologic examination by telemedicine in a pediatric intensive care unit. J. Pediatr. 2014, 165, 962–966. [Google Scholar] [CrossRef]
- Hardy, V.; O’Connor, Y.; Heavin, C.; Mastellos, N.; Tran, T.; O’Donoghue, J.; Thompson, M. The added value of a mobile application of Community Case Management on referral, re-consultation and hospitalization rates of children aged under 5 years in two districts in Northern Malawi: Study protocol for a pragmatic, stepped-wedge cluster-randomized controlled trial. Trials 2017, 18, 475. [Google Scholar] [CrossRef]
- Ramelet, A.S.; Fonjallaz, B.; Rio, L.; Zoni, S.; Ballabeni, P.; Rapin, J.; Hofer, M. Impact of a nurse led telephone intervention on satisfaction and health outcomes of children with inflammatory rheumatic diseases and their families: A crossover randomized clinical trial. BMC Pediatr. 2017, 17, 168. [Google Scholar] [CrossRef]
- Rhodes, E.T.; Vernacchio, L.; Mitchell, A.A.; Fischer, C.; Giacalone, P.; Ludwig, D.S.; Ebbeling, C.B. A telephone intervention to achieve differentiation in dietary intake: A randomized trial in paediatric primary care. Pediatr. Obes. 2017, 12, 494–501. [Google Scholar] [CrossRef]
- Delisle Nyström, C.; Sandin, S.; Henriksson, P.; Henriksson, H.; Maddison, R.; Löf, M. A 12-month follow-up of a mobile-based (mHealth) obesity prevention intervention in pre-school children: The MINISTOP randomized controlled trial. BMC Public Health 2018, 18, 658. [Google Scholar] [CrossRef]
- Franke, K.H.; Krumkamp, R.; Mohammed, A.; Sarpong, N.; Owusu-Dabo, E.; Brinkel, J.; Kreuels, B. A mobile phone based tool to identify symptoms of common childhood diseases in Ghana: Development and evaluation of the integrated clinical algorithm in a cross-sectional study. BMC Med. Inform Decis. Mak. 2018, 18, 23. [Google Scholar] [CrossRef]
- Sgandurra, G.; Beani, E.; Giampietri, M.; Rizzi, R.; Cioni, G. Early intervention at home in infants with congenital brain lesion with CareToy revised: A RCT protocol. BMC Pediatr. 2018, 18, 295. [Google Scholar] [CrossRef]
- Simone, M.; Viterbo, R.G.; Margari, L.; Iaffaldano, P. Computer-assisted rehabilitation of attention in pediatric multiple sclerosis and ADHD patients: A pilot trial. BMC Neurol. 2018, 18, 82. [Google Scholar] [CrossRef] [PubMed]
- Strickler, A.S.; Palma, J.; Charris, R.; Candia, T.; Grez, M.; González, B.; Rivera, V. Contribution of the use of basic telemedicine tools to the care of children and adolescents with juvenile idiopathic arthritis at the Puerto Montt Hospital, Chile. Rev. Chil. Pediatr. 2018, 89, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Ramkumar, V.; Nagarajan, R.; Shankarnarayan, V.C.; Kumaravelu, S.; Hall, J.W. Implementation and evaluation of a rural community-based pediatric hearing screening program integrating in-person and tele-diagnostic auditory brainstem response (ABR). BMC Health Serv. Res. 2019, 19, 1. [Google Scholar] [CrossRef] [PubMed]
- Browne, S.; Kechadi, M.T.; O’Donnell, S.; Dow, M.; Tully, L.; Doyle, G.; O’Malley, G. Mobile health apps in pediatric obesity treatment: Process outcomes from a feasibility study of a multicomponent intervention. JMIR mHealth uHealth 2020, 8, e16925. [Google Scholar] [CrossRef]
- Kobel, M.; Kalden, P.; Michaelis, A.; Markel, F.; Mensch, S.; Weidenbach, M.; Paech, C. Accuracy of the Apple Watch iECG in children with and without congenital heart disease. Pediatr. Cardiol. 2022, 43, 191–196. [Google Scholar] [CrossRef]
- Smith, P.; Ehlers, A.; Carr, E.; Clark, D.; Dalgleish, T.; Forbes, G.; Meiser-Stedman, R. Therapist-supported online cognitive therapy for post-traumatic stress disorder (PTSD) in young people: Protocol for an early-stage, parallel-group, randomised controlled study (OPTYC trial). BMJ Open 2022, 12, e054852. [Google Scholar] [CrossRef]
- Sonney, J.; Ward, T.; Thompson, H.J.; Kientz, J.A.; Segrin, C. Improving Asthma Care Together (IMPACT) mobile health intervention for school-age children with asthma and their parents: A pilot randomised controlled trial study protocol. BMJ Open 2022, 12, e059791. [Google Scholar] [CrossRef]
- Ministero della Salute. National Guidelines on Telemedicine. 2012. Available online: https://www.salute.gov.it/imgs/C_17_pubblicazioni_2129_allegato.pdf (accessed on 15 February 2023).
| Main Concept | Alternate Keywords | Subject Headings (MeSH) | |
|---|---|---|---|
| Population | Children | Paediatrics; child | Paediatrics; Adolescent |
| Parents | / | Parents | |
| Concept | Acquiring informed consent | Informed consent | Informed Consent |
| Communication (between parents and children) | / | Communication | |
| Context | Telemedicine | Remote consultation; virtual consultation | Telemedicine |
| Reference | Title | Year | Geographical Context | Aim of the Study | Patients’ Age | Consent Collection Method |
|---|---|---|---|---|---|---|
| Yager et al. [19] | Reliability of circulatory and neurologic examination by telemedicine in a Pediatric Intensive Care Unit | 2014 | USA | Randomised prospective study in a 14-bed PICU in a tertiary care, academic-affiliated institution having the objective of comparing telemedicine with face-to-face assessment of patients undergoing circulatory or neurological examinations | Between 2 months and 19 years | Informed consent was obtained from all patients (or parents/guardians) before participation in the study after reading and understanding a written information sheet containing objectives, risks, and benefits |
| Hardy et al. [20] | The added value of a mobile application of Community Case Management on referral, re-consultation and hospitalization rates of children aged under 5 years in two districts in Northern Malawi: study protocol for a pragmatic, stepped-wedge cluster-randomised controlled trial | 2017 | Malawi | Stepped-wedge cluster-randomised trial with a pragmatic approach conducted to evaluate the impact of the SL eCCM App (Supporting LIFE electronic Community Case Management Application) on rates of urgent referral, re-consultation, and hospitalization of children within 7 days of the index visit | Between 2 months and 5 years | Given the age of the children, consent was obtained from caregivers prior to the start of the study after reading a written consent form |
| Ramelet et al. [21] | Impact of a nurse led telephone intervention on satisfaction and health outcomes of children with inflammatory rheumatic diseases and their families: a crossover randomised clinical trial | 2017 | Switzerland | Multicentre, randomised, longitudinal crossover study conducted in paediatric outpatients with newly diagnosed inflammatory rheumatic diseases in order to compare telenursing services with traditional medical care services | Under 16 years | Consent was obtained directly from children (if over 11 years old) or from parents through a written form before the trial began |
| Rhodes et al. [22] | A telephone intervention to achieve differentiation in dietary intake: a randomised trial in paediatric primary care | 2017 | USA | Randomised trial aimed at evaluating whether dietary advice based on two healthy nutrition programs can be effectively provided to families of obese children by telephone | Between 5 and 10 years | Consent was obtained directly from children (if over 7 years old) or from parents after an information activity delivered by telephone |
| Nyström et al. [23] | A 12-month follow-up of a mobile-based (mHealth) obesity prevention intervention in pre-school children: the MINISTOP randomised controlled trial | 2018 | Sweden | Two-arm parallel randomised controlled trial aimed at testing the effectiveness of an app to combat obesity in preschool children | 4.5 years | Consent was obtained from parents |
| Franke et al. [24] | A mobile phone based tool to identify symptoms of common childhood diseases in Ghana: development and evaluation of the integrated clinical algorithm in a cross-sectional study | 2018 | Ghana | Study aimed at the development and evaluation of an algorithm-based diagnostic tool, applicable on mobile phones, to support parents/guardians in providing appropriate care to sick children | Between 1 month and 15 years | Written informed consent was obtained from the parents/guardians before the start of the study |
| Sgandurra et al. [25] | Early intervention at home in infants with congenital brain lesion with CareToy revised: a RCT protocol | 2018 | Italy | Randomised controlled trial aiming to evaluate the efficacy of CT-R (a medical device that delivers an early, intensive, customised, intervention program, carried out at home by parents but remotely managed by expert and trained clinicians) compared to Infant Massage (IM) intervention in a sample of infants at high-risk for cerebral palsy, | Preterm or full-term infants with brain lesions, in first year of life | Parents signed two informed consent forms (one for each phase of the study) after receiving detailed information in both written and oral form |
| Simone et al. [26] | Computer-assisted rehabilitation of attention in pediatric multiple sclerosis and ADHD patients: a pilot trial | 2018 | Italy | Pilot double-blind randomised controlled trial to evaluate the efficacy of a home-based computerised-program for retraining attention in two cohorts of POMS (Paediatric Onset Multiple Sclerosis) and ADHD (Attention Deficit/Hyperactivity Disorder) patients | Under 18 years | Informed consent form was signed by the parents of the participants |
| Strickler et al. [27] | Contribution of the use of basic telemedicine tools to the care of children and adolescents with juvenile idiopathic arthritis at the Puerto Montt Hospital, Chile | 2018 | Chile | Retrospective study consisting of a review of the medical records of children over 14 years of age with juvenile idiopathic arthritis undergoing clinical monitoring via a mixed system (face-to-face and telemedicine visits) | Under 18 years | Informed consent to the study was provided directly by the patients |
| Ramkumar et al. [28] | Implementation and evaluation of a rural community-based pediatric hearing screening program integrating in-person and tele-diagnostic auditory brainstem response (ABR) | 2019 | India | Evaluation of the effectiveness of a paediatric hearing screening programme by integrating two diagnostic ABR (Auditory Brainstem Response) test models: one using a telemedicine approach and the other a traditional test | Under 5 years | Written and verbal informed consent was obtained from parents |
| Browne et al. [29] | Mobile Health Apps in Pediatric Obesity Treatment: Process Outcomes From a Feasibility Study of a Multicomponent Intervention | 2020 | Republic of Ireland | Evaluation of the usability of 2 m-Health (mobile health) applications as an adjunct to traditional treatment for obesity | Between 9 and 16 years | Informed consent given after reading information leaflet by children and parents |
| Kobel et al. [30] | Accuracy of the Apple Watch iECG in Children With and Without Congenital Heart Disease | 2022 | Germany | Evaluation of the agreement of measured values of rate, interval, and amplitude with those obtained by a diagnostic quality ECG recording to an Apple Watch iECG in children with and without congenital heart disease | Between 0 and 16 years | Consent obtained from parents |
| Smith et al. [31] | Therapist-supported online cognitive therapy for post-traumatic stress disorder (PTSD) in young people: Protocol for an early-stage, parallel-group, randomised controlled study (OPTYC trial) | 2022 | UK | Early-stage trial aimed at gathering data on feasibility, acceptability, and initial indications of clinical efficacy of internet-delivered cognitive therapy for post-traumatic stress disorder in young people | Between 12 and 17 years | For participants under 16 years of age, informed consent was provided by the parents/guardians after asking for the patients’ consent. Participants aged 16 years and older gave consent independently, without parental involvement |
| Sonney et al. [32] | Improving Asthma Care Together (IMPACT) mobile health intervention for school-age children with asthma and their parents: a pilot randomised controlled trial study protocol | 2022 | USA | Pilot randomised controlled trial aimed at determining the feasibility, acceptability, and preliminary efficacy of the IMPACT intervention, a novel shared management system composed of a mobile health (mHealth) application, symptom watch, and tailored health intervention that pairs parent and child together as an asthma management team | Between 7 and 11 years | Both study participants (children) and parents were asked to give consent. Parents accessed an electronic information form with electronic signature, and children accessed a short video explaining the purpose and modalities of the study. The study team then contacted the participants to answer any questions and discuss consent. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ricci, G.; Gibelli, F.; Bailo, P.; Caraffa, A.M.; Nittari, G.; Sirignano, A. Informed Consent in Paediatric Telemedicine: Challenge or Opportunity? A Scoping Review. Healthcare 2023, 11, 1430. https://doi.org/10.3390/healthcare11101430
Ricci G, Gibelli F, Bailo P, Caraffa AM, Nittari G, Sirignano A. Informed Consent in Paediatric Telemedicine: Challenge or Opportunity? A Scoping Review. Healthcare. 2023; 11(10):1430. https://doi.org/10.3390/healthcare11101430
Chicago/Turabian StyleRicci, Giovanna, Filippo Gibelli, Paolo Bailo, Anna Maria Caraffa, Giulio Nittari, and Ascanio Sirignano. 2023. "Informed Consent in Paediatric Telemedicine: Challenge or Opportunity? A Scoping Review" Healthcare 11, no. 10: 1430. https://doi.org/10.3390/healthcare11101430
APA StyleRicci, G., Gibelli, F., Bailo, P., Caraffa, A. M., Nittari, G., & Sirignano, A. (2023). Informed Consent in Paediatric Telemedicine: Challenge or Opportunity? A Scoping Review. Healthcare, 11(10), 1430. https://doi.org/10.3390/healthcare11101430






