1. Introduction
Rectal cancer is one of the leading causes of cancer-related death worldwide [
1,
2]. With advances in colorectal surgery and adjuvant therapy in recent years, patients’ survival has greatly improved [
3,
4]. Preoperative neoadjuvant chemoradiotherapy (nCRT) followed by total mesenteric excision (TME) has become a standard mode of treatment for locally advanced rectal cancer (LARC) [
5,
6]. Nevertheless, how to manage patients with lateral lymph node metastasis (LLNM) is still a tough problem.
There are two main lymphatic drainage pathways in the lower rectum. The major one is through mesorectal nodes, superior rectal nodes to inferior mesenteric artery (IMA) nodes. TME surgery, first proposed by Heald in 1982, has become the standard surgical technique [
7], which can completely remove rectal cancer lesions and mesorectal nodes. Another pathway is through lateral lymph nodes (LLNs) [
8]. This part of the lymph nodes is beyond the scope of the TME.
Previous studies revealed that the LLNM rate of LARC is approximately 10–25% [
9,
10,
11]. Patients with LLNM have a poor prognosis and a high local recurrence (LR) rate [
12]. However, colorectal surgeons have different views on LLNs. In Western countries, nCRT plus TME is recommended for these patients because LLNM is considered a systemic metastatic disease [
5,
6]. However, in Japan, LLNM is considered a regional disease [
9]; therefore, the Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer recommend that lateral pelvic lymph node dissection (LLND) should be performed for T3/T4 rectal cancer below the peritoneal reflection, regardless of whether LLNM was suspected [
13]. Therefore, the field of LLNs in rectal cancer is complex and remains controversial.
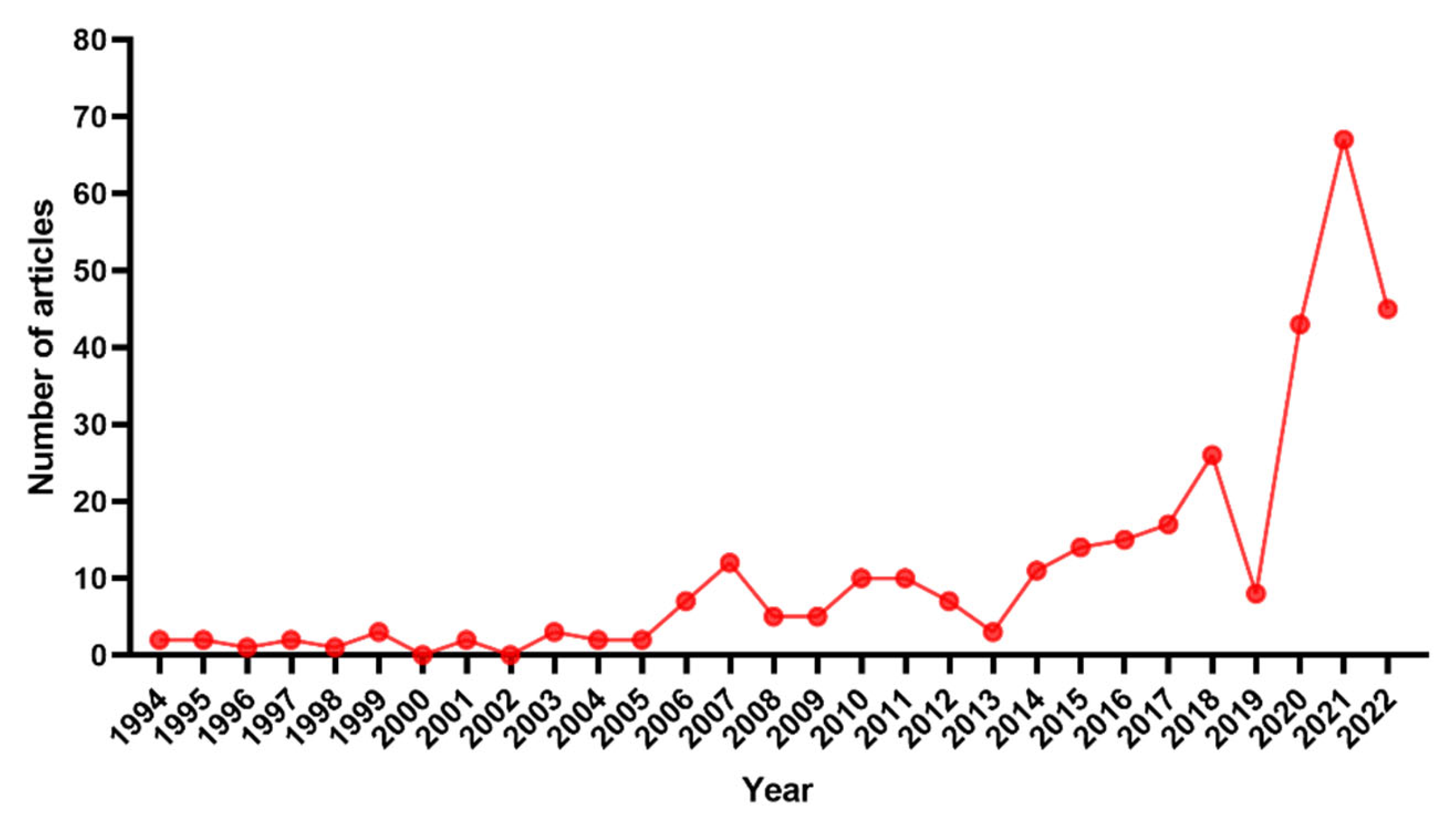
Currently, there are still many studies on LLNs in rectal cancer published. However, to the best of our knowledge, no bibliometric analysis in this field has been carried out and published. In this study, we performed a bibliometric analysis to describe the literature related to LLNs in rectal cancer from 1994 (the first article published) to 2022 to reveal the current status and trends in this field. Cooperation networks were constructed to investigate the cooperation relationships among countries, institutions and authors. Co-citation analysis was conducted to investigate the journals, authors and literature that have made outstanding contributions in this field. Keyword co-occurrence analysis was performed to investigate the research focus.
4. Discussion
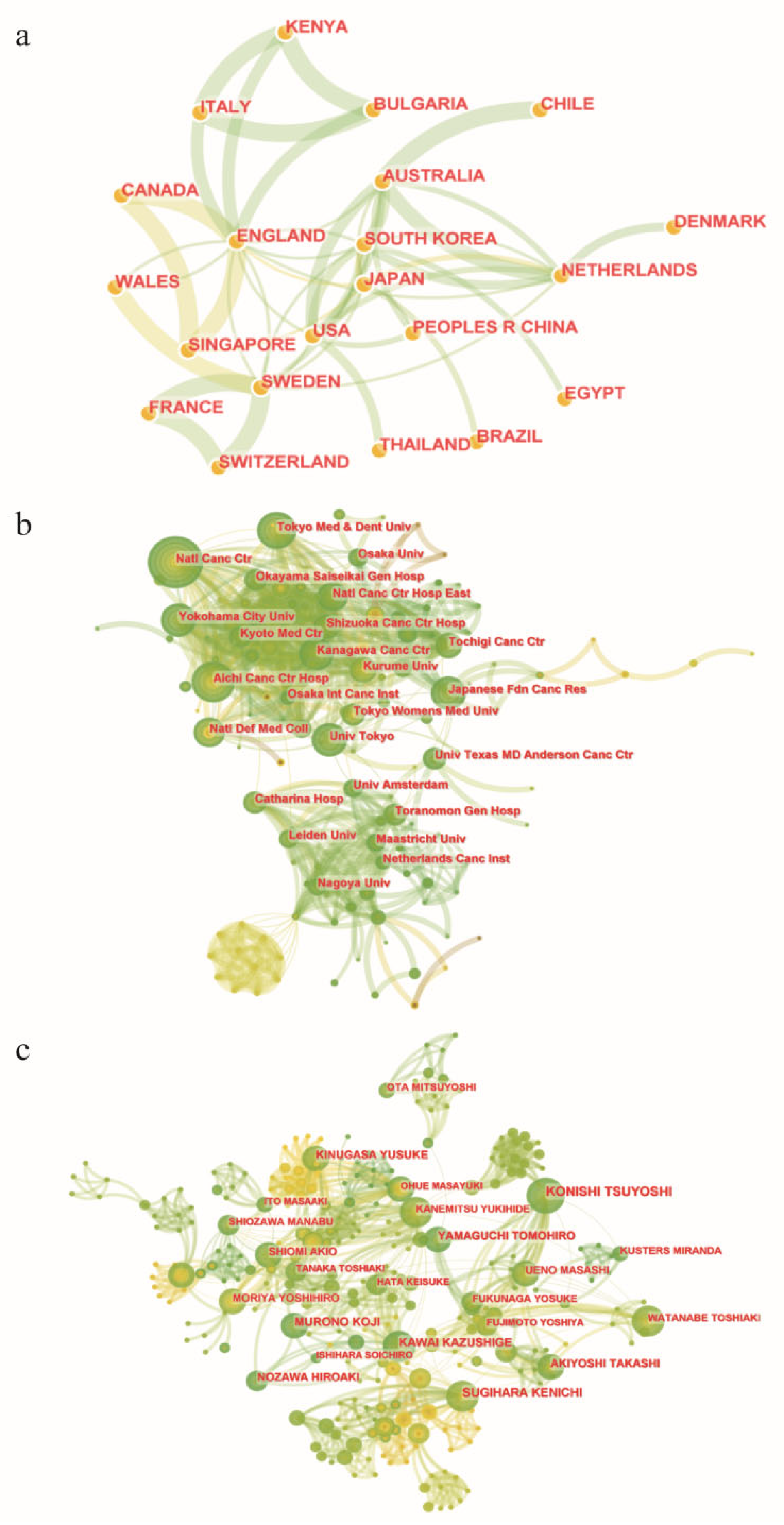
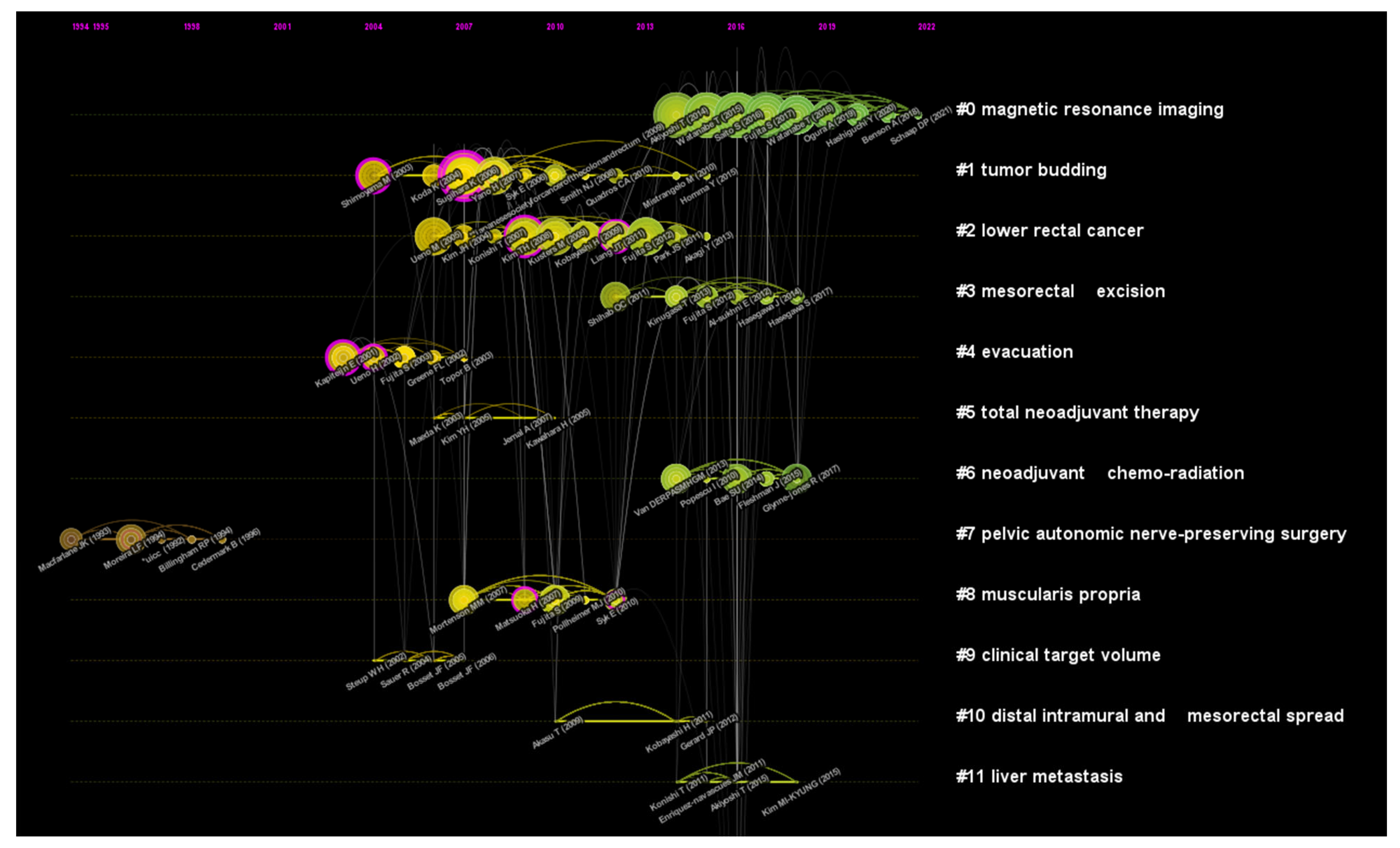
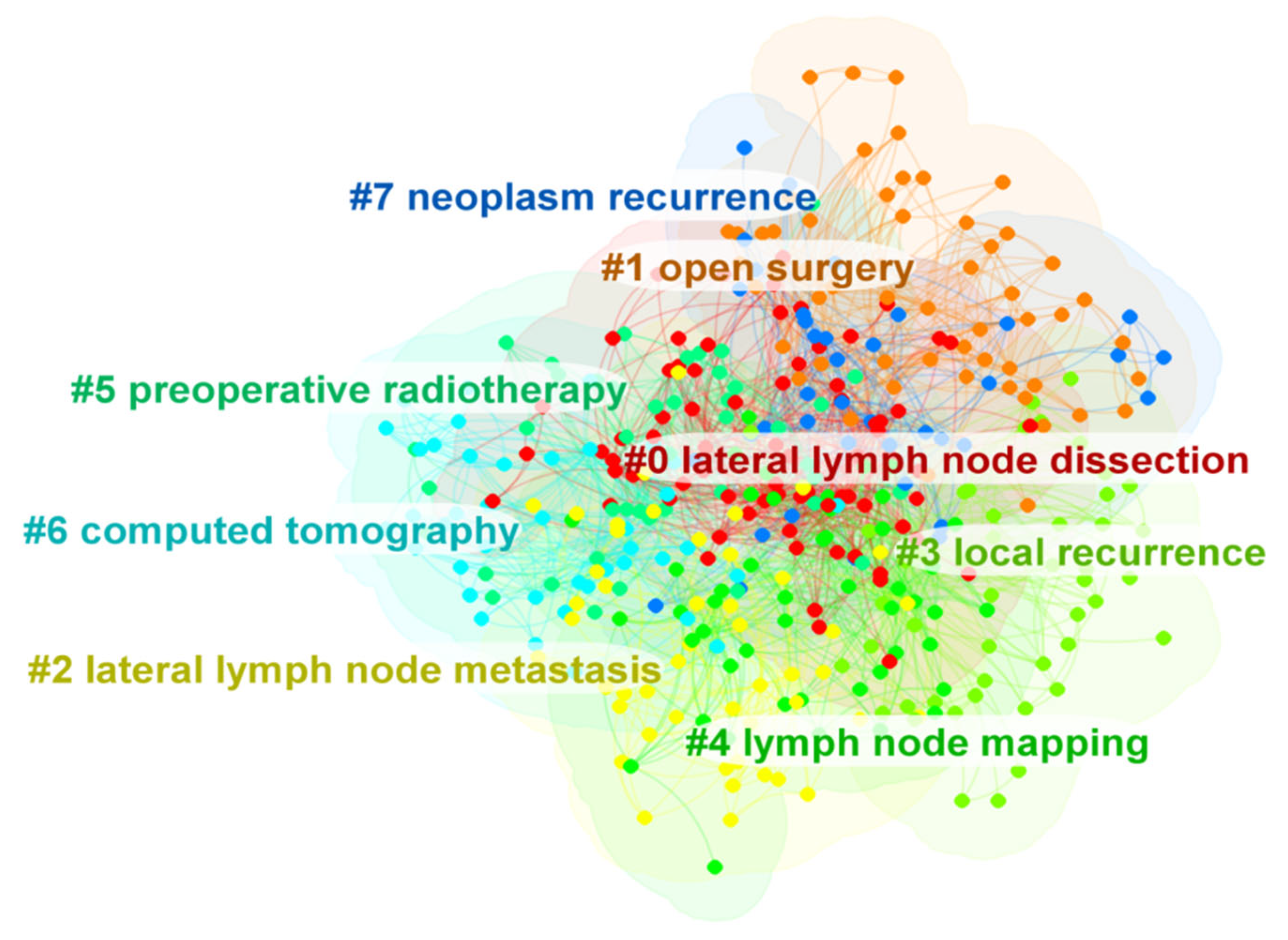
To the best of our knowledge, this is the first bibliometric analysis to describe the literature related to LLNs in rectal cancer. Due to the rapid evolution of research on LLNs in rectal cancer, it may be challenging for researchers and colorectal surgeons to identify the most influential studies and the hot topics in this field. Therefore, we conducted this bibliometric analysis using CiteSpace. Japan has the largest number of published articles. All the top 10 most productive institutions are from Japan except Sichuan University. Konishi Tsuyoshi is the author with the largest number of published articles. The International Journal of Colorectal Disease has published the most papers in this field, but Diseases of the Colon and Rectum was the most frequently co-cited journal. Shin Fujita was the most frequently co-cited author. The JCOG0212 trial published in Annals of Surgery was the most cited article. Preoperative chemoradiotherapy, multicenter, lateral lymph node dissection and metastasis are the recent hot keywords, and LLND had the highest burst strength.
Rectal cancer is one of the most common malignant tumors with high morbidity and mortality [
1,
2]. Currently, there is still controversy regarding the treatment of LLNs between Western and Eastern colorectal surgeons. In Western countries, nCRT plus TME is recommended to treat patients with clinical stage II/III rectal cancer or suspicious LLNM [
5,
6]. LLND was not recommended because it causes more postoperative complications and does not provide additional oncological benefits [
16,
17]. Conversely, Japanese colorectal surgeons tend to consider LLNs as regional lymph nodes, and the latest JSCCR guideline recommended prophylactic LLND for rectal cancer patients [
13]. Therefore, many studies related to LLNs or LLND have been conducted in Japan.
Japan has made outstanding contributions to this field. This bibliometric analysis showed that Japan has the largest number of published articles, and 9 of the top 10 most productive institutions were from Japan. Furthermore, all top 10 productive authors and the top 5 co-cited authors were Japanese. Of note, our team (Sichuan University), as the only non-Japanese institution of the top 10 productive institutions, has started focusing on this field [
18,
19,
20,
21,
22,
23]. In summary, although some institutions of other countries began to appear, Japanese institutions and authors dominated the field of LLNs in rectal cancer.
It is difficult to decide who contributed most to this field. Konishi Tsuyoshi was the most productive author, and Shin Fujita was the most frequently co-cited author. When considering both the number of publications and citations, Kenichi Sugihara made a great contribution to this field. However, as the third co-cited author, Takashi Akiyoshi published two highly cited articles, both included in the top 10 co-cited references.
The most influential reference must be the JCOG0212 study [
24]. It is a multicenter randomized controlled trial (RCT) in Japan showing that the noninferiority of TME to TME plus LLND could not be confirmed and LLND could significantly reduce LR by 5.2%. This study had a significant impact on the development of guidelines. Although there was no significant difference in overall survival (OS) or relapse-free survival (RFS) between the two groups, patients might benefit from LLND from the perspective of local control. However, this trial only included patients without clinical LLNM, and these patients did not receive nCRT, which limits the generalization of this finding. In addition, our previous meta-analysis, which compared nCRT + TME with nCRT + TME + LLND, also revealed that LLND could significantly reduce local lateral recurrence (LLR) [
18]. A recent propensity score matching study showed that clinical LLNM patients had poor OS and DFS, even after LLND, which means that rectal cancer patients who received LLND might gain better long-term survival [
25]. However, the decision to perform LLND should be made carefully due to postoperative complications. Therefore, it is important to determine the LLND indications. A previous study revealed that LLNs with a short-axis diameter ≥8 mm before nCRT were an independent risk factor for pathologically positive LLNs [
26]. Another study further reported that young age and a short distance from the anal verge were independent risk factors [
27]. The Lateral Node Study Consortium of Japan found that a short-axis LLN size of more than 7 mm on primary magnetic resonance imaging (MRI) led to a high LLR [
28]. In addition, there was no LLR at 3 years in patients with good shrinkage of LLNs after nCRT (the short axis decreased from more than 7 mm on the primary MRI to less than 4 mm on restaging MRI). However, patients with LLNs whose short axes were still greater than 4 mm on restaging MRI had a high LLR rate (52.3% at 5 years) [
29]. Therefore, they recommended restaging MRI when deciding whether to perform LLND and pointed out that LLND might be omitted for the above patients with good shrinkage. Further prospective studies are needed in this field.
Actually, LLND is always a hot topic in this field. Keyword burst analysis, which can reflect research hotspots in academic fields, demonstrated that LLND had the highest burst strength (6.96), starting from 2020. Of note, LLND began to appear in 1994, which means that it has been studied from a very early time and has become a hotspot in recent years. We believe part of the reason for this phenomenon may be the publication of JCOG0212.
Apart from LLND, the cluster analysis showed that open surgery, lateral lymph node metastasis, local recurrence, lymph node mapping, preoperative radiotherapy, computed tomography and neoplasm recurrence were also hot topics in this field. Overall, researchers have mainly focused on which intervention (nCRT or LLND or nCRT + LLND) can reduce the LR of patients with LARC. We also observed that colorectal surgeons have begun to focus on the improvement of LLND surgical techniques [
21,
30,
31,
32,
33,
34]. However, high-quality studies (RCTs) are still lacking.
This study has several limitations. First, this bibliometric analysis only included the WOS core database. Second, non-English studies were excluded. Third, some studies unrelated to the topic were manually removed. All of the abovementioned factors might lead to selection bias.