Geographical Disparities in Esophageal Cancer Incidence and Mortality in the United States
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Study Population
2.3. Statistical Analysis
2.4. Reporting Guidelines
3. Results
3.1. SEER Database
3.2. NCDB
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Cancer Research Fund. World Cancer Research Fund Oesophageal Cancer Statistics. Available online: https://www.wcrf.org/cancer-trends/oesophageal-cancer-statistics/ (accessed on 10 November 2022).
- American Cancer Society. American Cancer Society Key Statistics for Esophageal Cancer. Available online: https://www.cancer.org/content/dam/CRC/PDF/Public/8614.00.pdf (accessed on 10 November 2022).
- Uhlenhopp, D.J.; Then, E.O.; Sunkara, T.; Gaduputi, V. Epidemiology of esophageal cancer: Update in global trends, etiology and risk factors. Clin. J. Gastroenterol. 2020, 13, 1010–1021. [Google Scholar] [CrossRef] [PubMed]
- Kou, K.; Baade, P.D.; Gatton, M.; Cramb, S.M.; Sun, J.; Lu, Z.; Fu, Z.; Chu, J.; Xu, A.; Guo, X. Individual- and Area-Level Socioeconomic Inequalities in Esophageal Cancer Survival in Shandong Province, China: A Multilevel Analysis. Cancer Epidemiol. Biomark. Prev. 2019, 28, 1427–1434. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Liu, Y.; Peng, J.; Sun, C.; Rang, W. Trends of Esophageal Cancer Incidence and Mortality and Its Influencing Factors in China. Risk Manag. Healthc. Policy 2021, 14, 4809–4821. [Google Scholar] [CrossRef] [PubMed]
- Amorim, C.A.; De Souza, L.P.; Moreira, J.P.; Luiz, R.R.; De, V.C.A.J.; De Souza, H.S.P. Geographic distribution and time trends of esophageal cancer in Brazil from 2005 to 2015. Mol. Clin. Oncol. 2019, 10, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Zahnd, W.E.; James, A.S.; Jenkins, W.D.; Izadi, S.R.; Fogleman, A.J.; Steward, D.E.; Colditz, G.A.; Brard, L. Rural-Urban Differences in Cancer Incidence and Trends in the United States. Cancer Epidemiol. Biomark. Prev. 2018, 27, 1265–1274. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, C.; Wang, Q.; Li, Z.; Lin, J.; Wang, H. Differences in Stage of Cancer at Diagnosis, Treatment, and Survival by Race and Ethnicity Among Leading Cancer Types. JAMA Netw. Open 2020, 3, e202950. [Google Scholar] [CrossRef]
- Abbas, A.; Madison Hyer, J.; Pawlik, T.M. Race/Ethnicity and County-Level Social Vulnerability Impact Hospice Utilization Among Patients Undergoing Cancer Surgery. Ann. Surg. Oncol. 2021, 28, 1918–1926. [Google Scholar] [CrossRef]
- Salehi, O.; Vega, E.A.; Lathan, C.; James, D.; Kozyreva, O.; Alarcon, S.V.; Kutlu, O.C.; Herrick, B.; Conrad, C. Race, Age, Gender, and Insurance Status: A Comparative Analysis of Access to and Quality of Gastrointestinal Cancer Care. J. Gastrointest. Surg. 2021, 25, 2152–2162. [Google Scholar] [CrossRef]
- Okereke, I.C.; Westra, J.; Tyler, D.; Klimberg, S.; Jupiter, D.; Venkatesan, R.; Brooks, K.; Kuo, Y.F. Disparities in esophageal cancer care based on race: A National Cancer Database analysis. Dis. Esophagus 2022, 35, doab083. [Google Scholar] [CrossRef]
- Clark, J.M.; Boffa, D.J.; Meguid, R.A.; Brown, L.M.; Cooke, D.T. Regionalization of esophagectomy: Where are we now? J. Thorac. Thorac. Dis. 2019, 11 (Suppl. 12), S1633–S1642. [Google Scholar] [CrossRef]
- Gosain, R.; Ball, S.; Rana, N.; Groman, A.; Gage-Bouchard, E.; Dasari, A.; Mukherjee, S. Geographic and demographic features of neuroendocrine tumors in the United States of America: A population-based study. Cancer 2020, 126, 792–799. [Google Scholar] [CrossRef]
- Rana, N.; Gosain, R.; Lemini, R.; Wang, C.; Gabriel, E.; Mohammed, T.; Siromoni, B.; Mukherjee, S. Socio-Demographic Disparities in Gastric Adenocarcinoma: A Population-Based Study. Cancers 2020, 12, 157. [Google Scholar] [CrossRef]
- National Cancer Institute. About the SEER Program. Available online: https://seer.cancer.gov/about/ (accessed on 10 November 2022).
- American Cancer Society; American College of Surgeons. Available online: https://www.facs.org/quality-programs/cancer-programs/national-cancer-database/ (accessed on 10 November 2022).
- Delman, A.M.; Ammann, A.M.; Turner, K.M.; Vaysburg, D.M.; Van Haren, R.M. A narrative review of socioeconomic disparities in the treatment of esophageal cancer. J. Thorac. Dis. 2021, 13, 3801–3808. [Google Scholar] [CrossRef]
- Argyrakopoulou, G.; Dalamaga, M.; Spyrou, N.; Kokkinos, A. Gender Differences in Obesity-Related Cancers. Curr. Obes. Rep. 2021, 10, 100–115. [Google Scholar] [CrossRef]
- Nobel, T.B.; Livschitz, J.; Eljalby, M.; Janjigian, Y.Y.; Bains, M.S.; Adusumilli, P.S.; Jones, D.R.; Molena, D. Unique Considerations for Females Undergoing Esophagectomy. Ann. Surg. 2020, 272, 113–117. [Google Scholar] [CrossRef]
- Allen, J.E.; Desai, M.; Roumans, C.A.M.; Vennalaganti, S.; Vennalaganti, P.; Bansal, A.; Falk, G.; Lieberman, D.; Sampliner, R.; Thota, P.; et al. Low Risk of Progression of Barrett’s Esophagus to Neoplasia in Women. J. Clin. Gastroenterol. 2021, 55, 321–326. [Google Scholar] [CrossRef]
- Heo, J.W.; Kim, S.E.; Sung, M.K. Sex Differences in the Incidence of Obesity-Related Gastrointestinal Cancer. Int. J. Mol. Sci. 2021, 22, 1253. [Google Scholar] [CrossRef]
- Kaye, D.R.; Min, H.S.; Herrel, L.A.; Dupree, J.M.; Ellimoottil, C.; Miller, D.C. Costs of Cancer Care Across the Disease Continuum. Oncologist 2018, 23, 798–805. [Google Scholar] [CrossRef]
- Hall, I.J.; Tangka, F.K.L.; Sabatino, S.A.; Thompson, T.D.; Graubard, B.I.; Breen, N. Patterns and Trends in Cancer Screening in the United States. Prev. Chronic. Dis. 2018, 15, E97. [Google Scholar] [CrossRef]
- Osazuwa-Peters, N.; Simpson, M.C.; Rohde, R.L.; Challapalli, S.D.; Massa, S.T.; Adjei Boakye, E. Differences in Sociodemographic Correlates of Human Papillomavirus-Associated Cancer Survival in the United States. Cancer Control 2021, 28, 10732748211041894. [Google Scholar] [CrossRef]
- Chen, K.A.; Strassle, P.D.; Meyers, M.O. Socioeconomic factors in timing of esophagectomy and association with outcomes. J. Surg. Oncol. 2021, 124, 1014–1021. [Google Scholar] [CrossRef] [PubMed]
- Boffa, D.J.; Mallin, K.; Herrin, J.; Resio, B.; Salazar, M.C.; Palis, B.; Facktor, M.; McCabe, R.; Nelson, H.; Shulman, L.N. Survival After Cancer Treatment at Top-Ranked US Cancer Hospitals vs. Affiliates of Top-Ranked Cancer Hospitals. JAMA Netw. Open 2020, 3, e203942. [Google Scholar] [CrossRef]
- Brusselaers, N.; Mattsson, F.; Lagergren, J. Hospital and surgeon volume in relation to long-term survival after oesophagectomy: Systematic review and meta-analysis. Gut 2014, 63, 1393–1400. [Google Scholar] [CrossRef]
- Group, T.L. Complex Adult and Pediatric Surgery. Available online: https://ratings.leapfroggroup.org/measure/hospital/complex-adult-and-pediatric-surgery (accessed on 10 November 2022).
- Jiang, R.; Liu, Y.; Ward, K.C.; Force, S.D.; Pickens, A.; Sancheti, M.S.; Javidfar, J.; Fernandez, F.G.; Khullar, O.V. Excess Cost and Predictive Factors of Esophagectomy Complications in the SEER-Medicare Database. Ann. Thorac. Surg. 2018, 106, 1484–1491. [Google Scholar] [CrossRef] [PubMed]
- Fischer, C.; Lingsma, H.; Klazinga, N.; Hardwick, R.; Cromwell, D.; Steyerberg, E.; Groene, O. Volume-outcome revisited: The effect of hospital and surgeon volumes on multiple outcome measures in oesophago-gastric cancer surgery. PLoS ONE 2017, 12, e0183955. [Google Scholar] [CrossRef] [PubMed]
- Arnold, B.N.; Chiu, A.S.; Hoag, J.R.; Kim, C.H.; Salazar, M.C.; Blasberg, J.D.; Boffa, D.J. Spontaneous regionalization of esophageal cancer surgery: An analysis of the National Cancer Database. J. Thorac. Dis. 2018, 10, 1721–1731. [Google Scholar] [CrossRef]

| Characteristics | All 1 (N = 49,421) | Rural 1 (N = 6199) | Urban 1 (N = 43,222) | p Value 2 |
|---|---|---|---|---|
| Age | 65.4 (11.5) | 65.1 (11.2) | 65.4 (11.5) | 0.08 |
| Sex | <0.001 | |||
| Male | 39,367 (78.6%) | 5088 (82.1%) | 33,737 (78.1%) | |
| Female | 10,730 (21.4%) | 1111 (17.9%) | 9485 (21.9%) | |
| Race | <0.001 | |||
| non-Hispanic White | 37,564 (75%) | 5356 (86.4%) | 32,067 (74.2%) | |
| non-Hispanic Black | 6751 (13.5%) | 549 (8.9%) | 6193 (14.3%) | |
| Hispanic | 3078 (6.1%) | 172 (2.8%) | 2893 (6.7%) | |
| Other | 2704 (5.4%) | 122 (2%) | 2069 (4.8%) | |
| Marital Status | <0.001 | |||
| Single | 22,037 (44%) | 2448 (39.5%) | 19,285 (44.6%) | |
| Married | 28,060 (56%) | 3751 (60.5%) | 23,937 (55.4%) | |
| Insurance | <0.001 | |||
| Uninsured | 786 (1.6%) | 142 (2.3%) | 643 (1.5%) | |
| Insured | 20,639 (41.2%) | 2760 (44.5%) | 17,836 (41.3%) | |
| Unknown | 28,672 (57.2%) | 3297 (53.2%) | 24,743 (57.2%) | |
| Histology | <0.001 | |||
| Adenocarcinoma | 28,675 (57.2%) | 3977 (64.2%) | 24,584 (56.9%) | |
| Squamous Cell Carcinoma | 21,422 (42.8%) | 2222 (35.8%) | 18,638 (43.1%) | |
| Grade | <0.001 | |||
| I/II | 19,950 (39.8%) | 2535 (40.9%) | 17,109 (39.6%) | |
| III/IV | 21,052 (42%) | 2710 (43.7%) | 18,071 (41.8%) | |
| Unknown | 9095 (18.2%) | 954 (15.4%) | 8042 (18.6%) | |
| Stage | 0.1 | |||
| Localized | 13,436 (26.8%) | 1710 (27.6%) | 11,509 (26.6%) | |
| Regional | 17,193 (34.3%) | 2132 (34.4%) | 14,840 (34.3%) | |
| Distant | 19,468 (38.9%) | 2357 (38%) | 16,873 (39%) | |
| Dead | 0.9 | |||
| Yes | 44,048 (87.9%) | 5441 (87.8%) | 37,956 (87.8%) | |
| No | 6049 (12.1%) | 758 (12.2%) | 5266 (12.2%) |
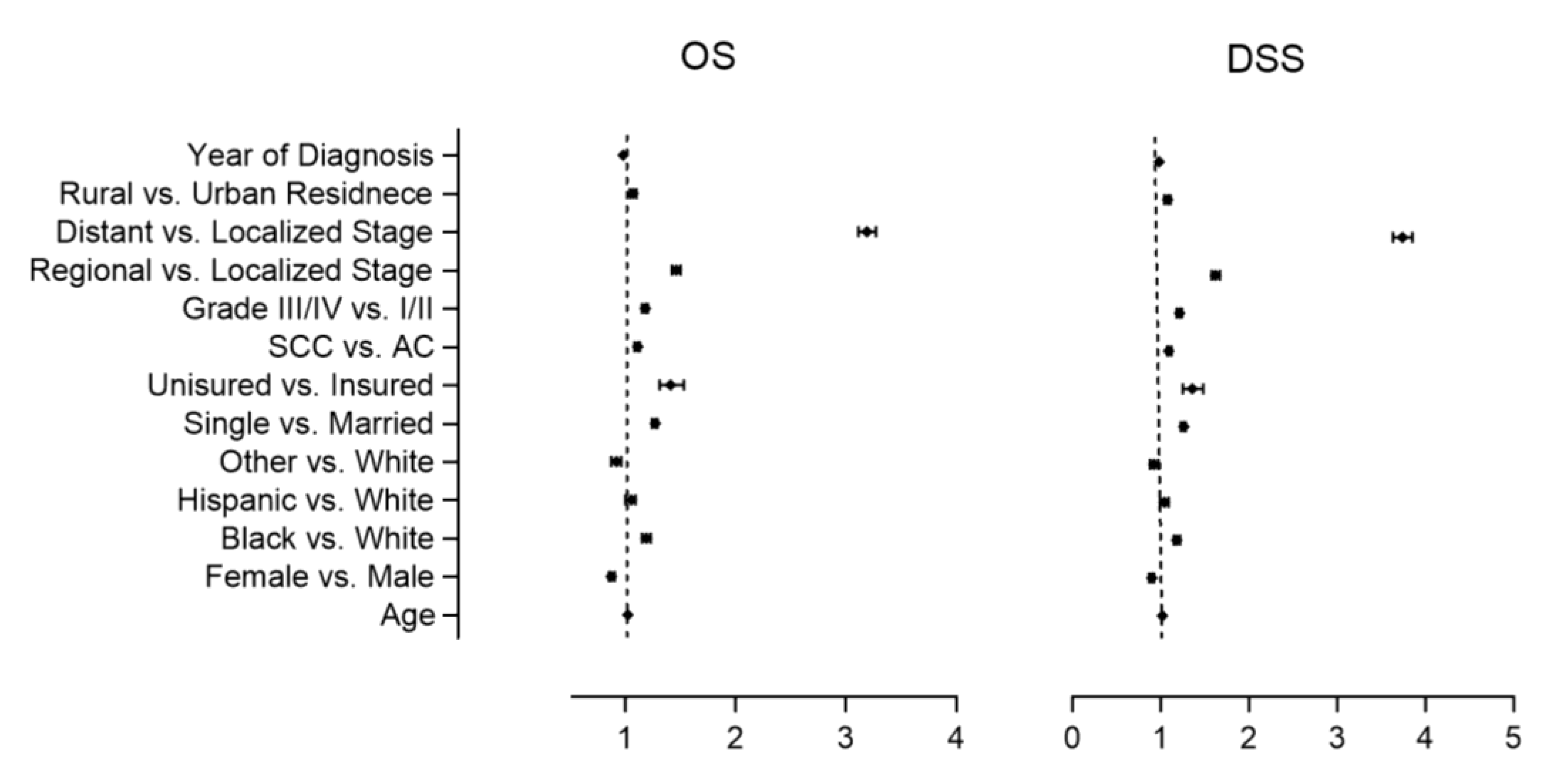
| Variables | Overall Survival HR (95% CI) | p Value 1 | Disease Specific Survival HR (95% CI) | p Value 1 |
|---|---|---|---|---|
| Age | 1.01 | <0.001 | 1.01 | <0.001 |
| (1.01–1.01) | (1.01–1.01) | |||
| Sex | ||||
| Female vs. Male | 1.01 | 0.3 | 1.01 | 0.4 |
| (0.99–1.04) | (0.99–1.04) | |||
| Race | ||||
| non-Hispanic Black vs. non-Hispanic White | 1.37 | <0.001 | 1.37 | <0.001 |
| (1.33–1.40) | (1.33–1.41) | |||
| Hispanic vs. non-Hispanic White | 1.04 | 0.05 | 1.04 | 0.07 |
| (1.00–1.08) | (1.00–1.08) | |||
| Other vs. non-Hispanic White | 0.97 | 0.2 | 0.98 | 0.4 |
| (0.93–1.02) | (0.93–1.03) | |||
| Marital Status | ||||
| Single vs. Married | 1.27 | <0.001 | 1.26 | <0.001 |
| (1.25–1.30) | (1.23–1.28) | |||
| Insurance status | ||||
| Uninsured vs. Insured | 1.43 | <0.001 | 1.44 | <0.001 |
| (1.32–1.54) | (1.32–1.56) | |||
| Histology | ||||
| Squamous Cell Carcinoma vs. Adenocarcinoma | 1.3 | <0.001 | 1.28 | <0.001 |
| (1.28–1.33) | (1.25–1.31) | |||
| Grade | ||||
| III/IV vs. I/II | 1.29 | <0.001 | 1.35 | <0.001 |
| (1.27–1.32) | (1.32–1.38) | |||
| Stage | ||||
| Regional vs. Localized | 1.32 | <0.001 | 1.48 | <0.001 |
| (1.29–1.36) | (1.44–1.52) | |||
| Distant vs. Localized | 2.76 | <0.001 | 3.28 | <0.001 |
| (2.70–2.83) | (3.19–3.37) | |||
| Residence | ||||
| Rural vs. Urban | 1.01 | 0.5 | 1.01 | 0.5 |
| (0.98–1.04) | (0.98–1.04) | |||
| Year of diagnosis | 1.01 | <0.001 | 0.98 | <0.001 |
| (1.01–1.01) | (0.98–0.98) |
| Variables | Overall Survival HR (95% CI) | p Value 1 | Disease Specific Survival HR (95% CI) | p Value 1 |
|---|---|---|---|---|
| Age | 1.02 | <0.001 | 1.02 | <0.001 |
| (1.02–1.02) | (1.02–1.02) | |||
| Sex | ||||
| Female vs. Male | 0.87 | <0.001 | 0.9 | <0.001 |
| (0.85–0.90) | (0.87–0.92) | |||
| Race | ||||
| non-Hispanic Black vs. non-Hispanic White | 1.19 | <0.001 | 1.18 | <0.001 |
| (1.15–1.23) | (1.15–1.22) | |||
| Hispanic vs. non-Hispanic White | 1.05 | 0.02 | 1.04 | 0.08 |
| (1.00–1.09) | (0.99–1.09) | |||
| Other vs. non-Hispanic White | 0.92 | <0.001 | 0.92 | 0.003 |
| (0.87–0.96) | (0.88–0.97) | |||
| Marital Status | ||||
| Single vs. Married | 1.27 | <0.001 | 1.26 | <0.001 |
| (1.24–1.29) | (1.23–1.28) | |||
| Insurance status | ||||
| Uninsured vs. Insured | 1.41 | <0.001 | 1.36 | <0.001 |
| (1.31–1.53) | (1.25–1.48) | |||
| Histology | ||||
| Squamous Cell Carcinoma vs. Adenocarcinoma | 1.11 | <0.001 | 1.09 | <0.001 |
| (1.08–1.13) | (1.07–1.12) | |||
| Grade | ||||
| III/IV vs. I/II | 1.18 | <0.001 | 1.21 | <0.001 |
| (1.15–1.20) | (1.18–1.24) | |||
| Stage | ||||
| Regional vs. Localized | 1.46 | <0.001 | 1.62 | <0.001 |
| (1.42–1.50) | (1.58–1.67) | |||
| Distant vs. Localized | 3.19 | <0.001 | 3.74 | <0.001 |
| (3.11–3.27) | (3.63–3.85) | |||
| Residence | ||||
| Rural vs. Urban | 1.07 | <0.001 | 1.08 | <0.001 |
| (1.04–1.10) | (1.04–1.11) | |||
| Year of diagnosis | 0.98 | <0.001 | 0.98 | <0.001 |
| (0.98–0.98) | (0.98–0.98) |
| Characteristics | All 1 (N = 72,226) | Rural 1 (N = 12,930) | Urban 1 (N = 59,296) | p Value 2 |
|---|---|---|---|---|
| Median Income | <0.001 | |||
| ≤USD 50,353 | 30,103 (43.1%) | 8677 (72.5%) | 20,516 (36.9%) | |
| ≥USD 50,354 | 39,760 (56.9%) | 3296 (27.5%) | 35,105 (63.1%) | |
| Insurance | <0.001 | |||
| Uninsured | 2675 (3.6%) | 494 (3.8%) | 2122 (3.6%) | |
| Private | 27,154 (36.2%) | 4158 (32.2%) | 21,904 (36.9%) | |
| Government | 43,199 (57.6%) | 7961 (61.6%) | 33,924 (57.2%) | |
| Unknown | 1916 (2.6%) | 317 (2.5%) | 1346 (2.3%) | |
| Distance traveled for care (miles) | 34.2 (112.9) | 67.1 (130.1) | 27.0 (107.4) | <0.001 |
| Treatment facility | <0.001 | |||
| Community | 34,255 (45.7%) | 7415 (57.3%) | 26,042 (43.9%) | |
| Academic/Integrated | 39,730 (53.0%) | 5358 (41.4%) | 32,503 (54.8%) | |
| Unknown | 959 (1.3%) | 157 (1.2%) | 751 (1.3%) | |
| Time from diagnosis to treatment start (days) | 36.5 (35.8) | 36.8 (43.1) | 36.5 (33.9) | 0.1 |
| Time from diagnosis to chemotherapy (days) | 40.8 (36.2) | 41.6 (47.0) | 40.6 (33.3) | 0.03 |
| Type of chemotherapy | <0.001 | |||
| No chemotherapy | 20,486 (27.3%) | 3293 (25.5%) | 16,291 (27.5%) | |
| Single-agent chemotherapy | 3872 (5.2%) | 701 (5.4%) | 3052 (5.1%) | |
| Multiagent chemotherapy | 38,436 (51.3%) | 7000 (54.1%) | 30,229 (51.0%) | |
| Unknown Chemotherapy | 4151 (5.5%) | 589 (4.6%) | 3265 (5.5%) | |
| Unknown if chemotherapy received | 7999 (10.7%) | 1347 (10.4%) | 6459 (10.9%) | |
| Radiation sequence | <0.001 | |||
| No radiation | 59,793 (79.8%) | 10,088 (78.0%) | 47,606 (80.3%) | |
| Radiation before surgery | 11,652 (15.5%) | 2182 (16.9%) | 8947 (15.1%) | |
| Radiation after surgery | 2200 (2.9%) | 428 (3.3%) | 1703 (2.9%) | |
| Radiation before and after surgery | 97 (0.1%) | 19 (0.1%) | 75 (0.1%) | |
| Intraoperative radiation | 3 (<0.1%) | 1 (<0.1%) | 2 (<0.1%) | |
| Intraoperative radiation with other therapy | 4 (<0.1%) | 2 (<0.1%) | 2 (<0.1%) | |
| Unknown sequence | 1195 (1.6%) | 210 (1.6%) | 961 (1.6%) | |
| Time from diagnosis to surgery (days) | 98.3 (68.7) | 99.1 (65.3) | 98.2 (69.4) | 0.1 |
| Number of regional lymph nodes examined | 0.4 | |||
| < 15 | 62,607 (83.5%) | 10,809 (83.6%) | 49,843 (84.1%) | |
| ≥ 15 | 7770 (10.4%) | 1340 (10.4%) | 5998 (10.1%) | |
| Unknown | 4567 (6.1%) | 781 (6.0%) | 3455 (5.8%) | |
| Positive surgical margins | <0.001 | |||
| No | 21,110 (28.2%) | 3748 (29.0%) | 16,281 (27.5%) | |
| Yes | 1660 (2.2%) | 304 (2.4%) | 1294 (2.2%) | |
| Unknown | 51,174 (69.6%) | 8878 (68.7%) | 41,721 (70.4%) | |
| Duration of inpatient hospital stay | 11.2 (13.3) | 11.7 (13.3) | 11.1 (13.2) | <0.001 |
| 30 day readmission | 0.2 | |||
| Unplanned | 1656 (2.2%) | 266 (2.1%) | 1310 (2.2%) | |
| Planned or not readmitted | 73,288 (97.8%) | 12,664 (97.9%) | 57,986 (97.8%) | |
| 30 day mortality | 0.2 | |||
| Alive | 21,466 (89.6%) | 3779 (89.2%) | 16,536 (89.3%) | |
| Dead | 662 (2.8%) | 135 (3.2%) | 506 (2.7%) | |
| No surgery or < 30 day follow up | 1837 (7.7%) | 321 (7.6%) | 1465 (7.9%) | |
| 90 day mortality | 0.02 | |||
| Alive | 20,603 (86.0%) | 3596 (84.9%) | 15,888 (85.8%) | |
| Dead | 1417 (5.9%) | 294 (6.9%) | 1082 (5.8%) | |
| No surgery or <90 day follow up | 1945 (8.1%) | 345 (8.1%) | 1537 (8.3%) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vedire, Y.; Rana, N.; Groman, A.; Siromoni, B.; Yendamuri, S.; Mukherjee, S. Geographical Disparities in Esophageal Cancer Incidence and Mortality in the United States. Healthcare 2023, 11, 685. https://doi.org/10.3390/healthcare11050685
Vedire Y, Rana N, Groman A, Siromoni B, Yendamuri S, Mukherjee S. Geographical Disparities in Esophageal Cancer Incidence and Mortality in the United States. Healthcare. 2023; 11(5):685. https://doi.org/10.3390/healthcare11050685
Chicago/Turabian StyleVedire, Yeshwanth, Navpreet Rana, Adrienne Groman, Beas Siromoni, Sai Yendamuri, and Sarbajit Mukherjee. 2023. "Geographical Disparities in Esophageal Cancer Incidence and Mortality in the United States" Healthcare 11, no. 5: 685. https://doi.org/10.3390/healthcare11050685
APA StyleVedire, Y., Rana, N., Groman, A., Siromoni, B., Yendamuri, S., & Mukherjee, S. (2023). Geographical Disparities in Esophageal Cancer Incidence and Mortality in the United States. Healthcare, 11(5), 685. https://doi.org/10.3390/healthcare11050685








