Abstract
Tenofovir disoproxil fumarate (TDF) is associated with a risk of chronic kidney disease (CKD), especially in Asian populations. Data from the Thai national health insurance system was used to assess CKD incidence in patients receiving antiretroviral therapy in real-world practice. We analyzed data from patients who initiated one of the following first-line regimens: zidovudine + lamivudine + nevirapine (AZT + 3TC + NVP); zidovudine + lamivudine + efavirenz (AZT + 3TC + EFV); tenofovir + lamivudine + nevirapine (TDF + 3TC + NVP); tenofovir + lamivudine/emtricitabine + efavirenz (TDF + 3TC/FTC + EFV); and tenofovir +lamivudine +lopinavir/ritonavir (TDF + 3TC + LPV/r). CKD was defined as glomerular filtration rate <60 mL/min/1.73 m2 for >3 months, or a confirmed 2010 WHO diagnosis (ICD-10 code N183, N184, or N185). Death competing risk survival regression models were used. Among 27,313 participants, with a median age of 36.8 years and median follow-up of 2.3 years, 245 patients (0.9%) were diagnosed with CKD (incidence 3.2 per 1000 patient-years; 95% CI 2.8–3.6). Compared with patients receiving AZT + 3TC + NVP, the risk of CKD measured by adjusted sub-distribution hazard ratio (aSHR) was 6.5 (95% CI 3.9–11.1) in patients on TDF + 3TC + LPV/r, 3.8 (95% CI 2.3–6.0) in TDF + 3TC + NVP, and 1.6 (95% CI 1.2–2.3) in TDF + 3TC/FTC + EFV. Among patients receiving TDF, compared with those receiving TDF + 3TC/FTC + EFV, the aSHR was 4.0 (95% CI 2.3–6.8) in TDF + 3TC + LPV/r and 2.3 (95% CI 1.4–3.6) in TDF + 3TC + NVP. TDF was associated with an increased risk of CKD, especially when combined with LPV/r or NVP.
1. Introduction
The Global Burden of Disease estimated that 1.2 million people died from chronic kidney disease (CKD) in 2017 [1]. With an improvement in the access to antiretroviral therapy (ART), the causes of death in human immunodeficiency virus (HIV)-infected individuals are shifting from infectious diseases to non-communicable diseases, including several conditions associated with the use of some antiretroviral agents. CKD has emerged as an important problem in HIV-infected individuals [2,3,4]. A large meta-analysis of studies in HIV-infected adults from over 60 countries showed that the overall prevalence of CKD defined by an estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2 was 4.8% and 19.4% of patients with diabetes mellitus [5]. High levels of viral loads, low CD4 cell counts, diagnosis of acquired immunodeficiency syndrome (AIDS), low body weight, and the use of some antiretroviral agents, such as indinavir, tenofovir disoproxil fumarate (TDF), and ritonavir-boosted lopinavir (LPV/r), are well-documented risk factors for CKD in HIV-infected individuals [6,7,8,9]. Several studies have demonstrated an increased risk of CKD in patients receiving TDF, especially in combination with protease inhibitors, such as lopinavir/ritonavir. However, little is known about the actual burden of this problem in relation to first-line antiretroviral combinations used in adults within large treatment programs in the real world, especially in Asia where the bodyweight of adults with HIV tends to be lower than those in other high settings.
Since 2004, the Thailand National Health Security Office (NHSO) in charge of the National AIDS Program has provided free-of-charge health services and ART for HIV-infected individuals. Using data from adults registered with this program under the Universal Coverage Scheme (UCS) [10], we investigated the association between the incidence of CKD in adults with HIV and first-line ART regimens to help to inform clinicians when selecting a first-line regimen recommended by the 2019 World Health Organization guidelines [11].
2. Materials and Methods
2.1. Study Design and Participants
We performed a retrospective cohort study using data from the National AIDS Program, covering HIV-infected individuals who received ART. This program is under the Universal Coverage Scheme (UCS), which covers about three-quarters of the Thai population (~47 millions). Participants eligible for inclusion were as follows: (i) aged 18 years or older having registered for ART between October 2006 and September 2013 (Fiscal Years (FY) 2007 to 2013); (ii) received a first-line ART regimen recommended by the 2019 World Health Organization guideline [11]; and (iii) did not have, when they initiated treatment, chronic kidney disease (CKD) defined by the estimated glomerular filtration rate (eGFR) (estimated using the Chronic Kidney Disease Epidemiology Collaboration equation [12]) and ICD-10 code. We excluded participants who did not receive ART or had incomplete baseline or follow-up information.
2.2. Exposure
The exposure of interest was one of the first-line ART regimens: (i) zidovudine, lamivudine, and nevirapine (AZT + 3TC + NVP); (ii) zidovudine, lamivudine, and efavirenz (AZT + 3TC + EFV); (iii) tenofovir, lamivudine, and nevirapine (TDF + 3TC + NVP); (iv) tenofovir, lamivudine or emtricitabine, and efavirenz (TDF + 3TC/FTC + EFV); and (v) tenofovir, lamivudine, and lopinavir/ritonavir (TDF + 3TC + LPV/r) [10].
2.3. Outcomes
The outcome of interest was the diagnosis (confirmed within 3 months) of conditions defined by the 2010 WHO ICD-10 [13] as codes N183, N184, N185, i.e., CKD stage 3, 4, or 5 (kidney damage with moderately to severely decreased GFR [15–59 mL/min] or chronic uremia or end-stage kidney disease [13]); or eGFR < 60 mL/min/1.73 m2 for >3 months [14].
We used patients’ data from the date of ART initiation to last visit, switching of ART regimen, loss to follow up, end of study (30 September 2014), death, or confirmed diagnosis of CKD, whichever occurred first.
2.4. Variables
We extracted the following variables at time of ART initiation: sex, age, weight, height, serum creatinine levels, fasting plasma glucose, serum triglycerides, serum total cholesterol, absolute CD4 cell count, HIV-1 ribonucleic acid (RNA) load, and history of comorbidities, including type 2 diabetes, hypertension, ischemic heart disease, tubulo-interstitial nephritis, gout, urolithiasis, hepatitis B virus (HBV) infection, and hepatitis C virus (HCV) infection.
We considered that a patient was diagnosed with type 2 diabetes if either of the following records were available: confirmed fasting plasma glucose ≥126 mg/dL according to the 2013 American Diabetes Association criteria [15], or WHO diagnosis (ICD-10 code E11–E14) [13], or receipt of anti-diabetic drugs at least twice of medical services. Hyperlipidemia was defined by either total cholesterol ≥240 mg/dL or triglycerides ≥200 mg/dL [16]. Hypertension was defined by a confirmed WHO diagnosis (ICD-10 code I10–I15) or receipt of anti-hypertensive drugs. WHO ICD-10 was used to identify history of comorbidities: ischemic heart disease (codes I20–I25), tubulo-interstitial nephritis (codes N10–N12), gout (code M10), urolithiasis (codes N20–N23), HBV infection (codes B16, B170, B180 and B181), and HCV infection (codes B171 and B182) [17].
2.5. Statistical Analyses
The characteristics of participants in the study are presented as medians and interquartile ranges (IQR) for continuous variables, and as counts and percentages for categorical variables. The number of person-years of follow-up (PYFU) was calculated from the date of ART initiation to censoring date. For descriptive purposes, the overall CKD incidence rate was estimated by the number of newly diagnosed individuals divided by the total number of PYFU, and the 95% confidence interval (CI) was calculated using the quadratic approximation to the Poisson log-likelihood.
Regarding missing data, all models were adjusted for the availability of serum creatinine measurement records at baseline and during follow-up. We imputed missing data by multiple imputation with chained equations (MICE) based on logistic regression for variables with ≤20% missing data, and for time-updated absolute CD4 cell count values if data were missing for more than 9 months after the previous known value [18]. Baseline body mass index and time-updated HIV-1 RNA load were excluded from the main analysis because more than 20% of the values were missing.
A propensity score for each first-line ART regimen was developed to minimize the possible bias of the choice of ART by the physician in charge, and all models were stratified by propensity score. This score was generated using multinomial logistic regression based on a fiscal year of ART initiation, sex, age group, and history of comorbidities (HBV and HCV infection, hypertension, type 2 diabetes, ischemic heart disease, tubulo-interstitial nephritis, gout, and urolithiasis) (see Supplementary Materials Figure S1).
We estimated the cumulative incidence rate of CKD and corresponding 95% CI using a cumulative incidence function accounting for deaths without a prior CKD diagnosis as competing events [19].
The following variables potentially associated with risk of CKD were considered for analysis: fiscal year of ART initiation, sex, age categories (18–34, 35–44, 45–59, or ≥60 years), body mass index (<18.5, 18.5–22.9, 23.0–24.9, or ≥25 kg/m2), and history of comorbidities at treatment initiation, time updated absolute CD4 cell count (<200 or ≥200 cells/mm3), and HIV-1 RNA load (<1000 or ≥1000 copies/mL).
We analyzed the association between first-line ART regimens and risk of CKD using Fine and Gray competing risks survival regression [20,21]. Variables potentially associated with the risk of CKD (listed above) were tested in univariable models adjusting for potential confounders. We retained those associated at the p-value of 0.20 or less for multivariable analyses. We used a backward elimination approach regression analysis using the Wald chi-squared test starting to define the final model. The associations were expressed in adjusted sub-distribution hazard ratio (aSHR).
All analyses were performed using Stata software, version 15.1 (Stata Corp, College Station, TX, USA).
3. Results
3.1. Study Population and Follow-Up
Of 152,664 HIV-infected adults, 100,081 were excluded from the study because their first-line ART regimens were not recommended by WHO guidelines, in addition to 25,270 patients because of insufficient data. Thus, a total of 27,313 patients were included in this analysis, and 15,389 (56.3%) were males.
Over the 8-year study period, of these 27,313 patients, 817 (3.0%) were lost to follow-up, 5840 (21.4%) switched to a second-line ART regimen, and 1844 (6.8%) died (Figure 1). The median duration of follow-up for the first-line ARV drug regiment was 2.3 years (IQR 1.5–3.6), accounting for a total 76,168 PYFU.
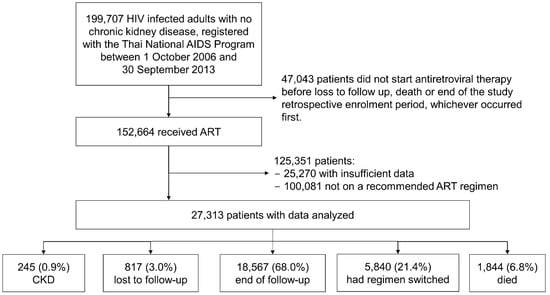
Figure 1.
Patients’ disposition: reasons for patient selection for retrospective participation in the study analyses. Abbreviations: AIDS, acquired immunodeficiency syndrome; ART, antiretroviral therapy; CKD, chronic kidney disease; HIV, human immunodeficiency virus.
At baseline, the median age was 36.8 years (IQR, 30.8–43.3), body mass index 20.4 kg/m2 (IQR, 18.6–22.6), and absolute CD4 cell count 146 cells/mm3 (IQR, 49–244). The most common comorbidities were hypertension (3.9%), HBV infection (3.8%), tubulo-interstitial nephritis (1.9%), and DM (1.9%). Table 1 shows the distribution of baseline characteristics overall and by ART regimen.

Table 1.
Baseline characteristics of HIV-infected adults who received the recommended first-line antiretroviral therapy regimens.
3.2. Incidence of CKD
During the total 76,168 PYFU, of 27,313 adults, 245 (0.9%) were diagnosed with CKD, leading to a cumulative incidence of 3.2 per 1000 PYFU (95% CI 2.8–3.6). The incidence rate of CKD was lower during the first 2 years of follow-up compared to the incidence thereafter (see Supplementary Materials Table S1).
The incidence rate of CKD was higher in adults who received a TDF containing ART regimen (5.2 per 1000 PYFU; 95% CI 4.4–6.3) compared to those receiving a non-TDF containing ART regimen (2.3 per 1000 PYFU; 95% CI 2.0–2.8) (Table 2).

Table 2.
Incidence per 1000 person-years of follow-up (PYFU) of chronic kidney disease according to the baseline characteristics among 27,313 HIV-infected adults who received one of the first-line antiretroviral therapy regimens followed for a median 2.3 years in the study.
The estimated cumulative incidence of CKD at 8 years of follow-up was 1.9% (95% CI 1.4–2.3). It was 1.5% (95% CI 0.1–2.2) in patients on AZT + 3TC + NVP, 2.8% (95% CI 1.5–4.9) on TDF + 3TC/FTC + EFV, 4.0% (95% CI 2.6–5.9) on TDF + 3TC + NVP, 1.3% (95% CI 0.9–2.0) on AZT + 3TC + EFV, and 5.1% (95% CI 3.0–8.1) on TDF + 3TC + LPV/r.
3.3. Association between First-Line Antiretroviral Treatment Regimen and Chronic Kidney Disease
In univariable analyses, the risk of CKD diagnosis was associated with older age at baseline, history of type 2 diabetes, hypertension, tubule-interstitial nephritis, gout and urolithiasis at baseline, and time-updated absolute CD4 cell count <200 cells/mm3 (all p ≤ 0.05, Table 3).

Table 3.
Factors associated with the risk of chronic kidney disease in HIV-infected adults who received currently recommended first-line antiretroviral therapy regimens (number of patients 27,313, except if otherwise specified).
In the final model adjusting for sex, age, history of type 2 diabetes, hypertension gout, and urolithiasis at baseline, time-update absolute CD4 cell count <200 cells/mm3, availability of previous serum creatinine measurement and propensity score stratification, and compared to AZT + 3TC + NVP, the following three regimens were associated with a higher risk of CKD: TDF + 3TC + LPV/r (aSHR 6.5, 95% CI 3.9–11.1), TDF + 3TC + NVP (aSHR 3.8, 95% CI 2.3–6.0), and TDF + 3TC/FTC + EFV (aSHR 1.6, 95% CI 1.2–2.3) (Table 3). Among patients receiving TDF, compared to TDF + 3TC/FTC + EFV, the risk was higher on TDF + 3TC + LPV/r (aSHR 4.0, CI 2.3–6.8) or TDF + 3TC + NVP (aSHR 2.3, CI 1.4–3.6). The estimated cumulative incidence rates by ART regimen are presented in Figure 2.
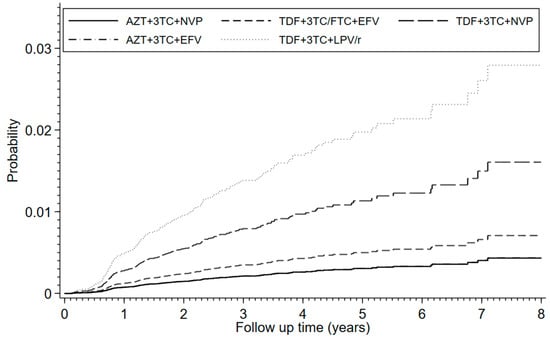
Figure 2.
Cumulative incidence function of chronic kidney disease, accounting for deaths without prior CKD diagnosis as competing events. Analyses were adjusted for sex, age, history of type 2 diabetes, hypertension, gout, urolithiasis, serum creatinine levels, and propensity score stratification. The follow-up time was from ART initiation until last visit, switching of ART regimen, loss to follow up, end of study, death or confirmed diagnosis of CKD, whichever occurred first. The overall cumulative incidence rates were: 0.0036 (95% CI 0.0030–0.0044) at 1 year of follow-up, 0.0071 (0.0061–0.0083) at 2 years, 0.0101 (0.0088–0.0116) at 3 years, 0.0120 (0.0104–0.0138) at 4 years, 0.0137 (0.0117–0.0158) at 5 years, 0.0146 (0.0124–0.0170) at 6 years, 0.0174 (0.0140–0.0213) at 7 years, and 0.0185 (0.0146–0.0230) at 8 years.
4. Discussion
Using data from 27,313 HIV-infected adults on ART over a median follow up of 2.3 years (76,168 PYFU), the estimated incidence rate of CKD was 3.2 per 1000 PYFU. Factors independently associated with CKD diagnosis were older age, type 2 diabetes, gout and urolithiasis, which are known risk factors in the general population [22,23,24,25], receipt of TDF-containing regimens, and absolute CD4 cell count <200 cells/mm3, both being factors related to HIV infection.
The Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) study in Europe, the USA, and Australia reported a CKD incidence rate of 1.8 per 1000 PYFU (95% CI 1.6–2.0) in HIV-positive adults [26], lower than in our study. However, in our study, data on atazanavir and abacavir were too scarce to be analyzed and compared to D:A:D study Two studies in Asia reported higher CKD incidence rates than in our study. The HIV Netherlands Australia Thailand Research Collaboration (HIV-NAT) reported an overall CKD incidence rate of 10.4 per 1000 PYFU, but no breakdown by ART regimen was provided, limiting the possibility to compare [27]. The AIDS Clinical Center of National Center for Global Health and Medicine (NCGM) in Japan reported an overall CKD incidence rate of 20.6 per 1000 PYFU (95% CI 17.6–24.2), probably due to a high percentage of patients (83%) on ritonavir-boosted protease inhibitors [28].
In our population, older age, T2DM, and hypertension were associated with the risk of CKD, as in the general population [28,29,30,31]. HBV and HCV co-infections were not associated with the risk of CKD in our study, although an association with HBV was found in other studies [32,33] and with HCV [8].
Two studies in Africa reported that the risk of CKD preferably occurred during the first 3 years following HIV diagnosis and in the case of low CD4 cell counts [34,35]. In the INSIGHT Strategic Timing of AntiRetroviral Treatment (START) trial, ART-naïve patients with CD4 cell counts ≤500 cells/μL had a higher prevalence of CKD than those with higher CD4 cell counts [29]. We also found an association with lower CD4 cell counts. An increased HIV-1 RNA load has also been reported as a significant risk factor [27], but we could not analyze this association due to insufficient baseline HIV-1 RNA load data.
We found that TDF exposure increased the risk of CKD, as in the EuroSIDA Study Group [36], the U.S. veterans study [37], a prospective cohort from France [38], the Canadian HIV Observational Cohort study [8], and the D:A:D study [26]. However, the NCGM study found no increase in the incidence of CKD among patients on TDF (despite a median observation duration of 5.1 years), but a higher drop in eGFR with longer exposure to TDF [28]. Interestingly, a retrospective study in Kenya [39] raised the question of a potential risk of CKD associated with NVP when in combination with TDF. To our knowledge, only one other study, also in Thailand, showed that patients on TDF + 3TC + NVP had a higher risk of renal impairment compared to patients on TDF + 3TC + EFV [40]. It is unclear whether this could be explained by confounding factors that were not taken into account.
As for the use of protease inhibitor, the Canadian HIV Observational Cohort study [8] and the D:A:D study [26] reported that the exposure to LPV/r was significantly associated with an increased risk of CKD, as in our study. In HIV-positive adults with kidney dysfunction, plasma TDF concentration in peripheral blood mononuclear cells has been shown to be significantly higher among patients receiving LPV/r compared with those receiving an NNRTI (NVP or EFV) [41]. A recent study from the USA National Historical Cohort of HIV-infected Veterans reported that an incidence rate of CKD in patients on EFV + FTC + TDF of 39.3 per 1000 PYFU (95% CI 34.0–45.3), significantly lower when compared to ritonavir-boosted PI (atazanavir, LPV, or darunavir) +FTC + TDF (66.1 per 1000 PYFU, 95% CI 55.7–77.9) (HR 0.6, 95% CI 0.5–0.7) [42].
In our study, attending physicians switched the regimens of 5840 (21.4%) patients due to virologic failure or side effects. The choice of the new regimen was guided by HIV-1 genotypic resistance test results, adherence, potential drug–drug interactions, relevant comorbidities, drug availability in the national program, and national guidelines. The cost of laboratory exams and drugs were not supported by the patients but by The National AIDS Program.
There were several limitations in our study. The NAP database was primarily designed to facilitate the reimbursement of costs incurred by hospitals for the delivery of HIV-related medical services, and risk factors for CKD were not systematically recorded. This study was a retrospective analysis of data collected in a real-world setting and the choice of an ART regimen was not randomized, but made by attending physicians based on baseline characteristics and guidelines. The use of a propensity score may partly correct this bias, but cannot totally eliminate it. Another limitation is that some variables, such as laboratory results at ART initiation, were not recorded in the database (cholesterol, triglycerides, and HIV-1 RNA load) and could not be fully assessed in our analysis. Nevertheless, this national database provides a unique source of information reflecting the actual CKD burden in people living with HIV.
5. Conclusions
Based on the analysis of a large national dataset from HIV-infected patients treated in the real world, the risk of CKD was relatively low overall and mostly concentrated in patients receiving TDF with a higher incidence in those also on NVP or LPV/r. The results of our study support the current recommendation of dose adjustment in patients with kidney function impairment.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/healthcare10081490/s1. Figure S1: Distribution of the study population according to the probability of each patient being assigned to a specific first-line antiretroviral regimen given their characteristics (propensity score). These graphs show the distribution of propensity score for each drug combination. The propensity score was calculated using multinomial (polytomous) logistic regression based on fiscal year, sex, age group, and history of comorbidities (hepatitis B and hepatitis C infection, hypertension, type 2 diabetes mellitus, ischemic heart disease, tubulo-interstitial nephritis, gout, and urolithiasis) at antiretroviral therapy initiation. Abbreviations: AZT, zidovudine; 3TC, lamivudine; TDF, tenofovir; FTC, emtricitabine; NVP, nevirapine; EFV, efavirenz; LPV/r, ritonavir-boosted lopinavir; Table S1: Incidence and cumulative incidence rate of chronic kidney disease in HIV-infected patients according to the duration of follow-up.
Author Contributions
Conceptualization, N.P., K.N., G.J., C.B., S.B., A.T., N.K., P.T. and R.C.; methodology, N.P., K.N., G.J. and A.T.; software, N.P.; validation, N.P., K.N. and G.J.; formal analysis, N.P., K.N., N.S. and J.Y.M.; investigation, N.P., K.N. and G.J.; resources, N.P.; data curation, N.P.; writing—original draft preparation, N.P., K.N., G.J. and T.R.C.; writing—review and editing, N.P., K.N. and G.J.; visualization, N.P., K.N. and G.J.; supervision, G.J., K.N. and N.K.; project administration, K.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The Ethical Committee of the Faculty of Medicine, Chiang Mai University, Thailand waived the requirement for patient consent and approved the design of the work on 18 March 2014 (114/2014, Research ID: COM-2557-02140). The work was performed in accordance with the Declaration of Helsinki.
Informed Consent Statement
Patient consent was waived due to this was a retrospective analysis using anonymized records collected for quality and financial purposes by the insurance system managed by the National Health Security Office (NHSO) in Thailand.
Data Availability Statement
The data will not be shared because of patient confidentiality.
Acknowledgments
We would like to thank the National Health Security Office (NHSO), Thailand for providing the data. This research was supported by Faculty of Medicine, Chiang Mai University.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bikbov, B.; Purcell, C.A.; Levey, A.S.; Smith, M.; Abdoli, A.; Abebe, M.; Adebayo, O.M.; Afarideh, M.; Agarwal, S.K.; Agudelo-Botero, M.; et al. Global, regional, and national burden of chronic kidney disease, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Lond. Engl. 2020, 395, 709–733. [Google Scholar] [CrossRef] [Green Version]
- Calza, L.; Sachs, M.; Colangeli, V.; Borderi, M.; Granozzi, B.; Malosso, P.; Comai, G.; Corradetti, V.; La Manna, G.; Viale, P. Prevalence of chronic kidney disease among HIV-1-infected patients receiving a combination antiretroviral therapy. Clin. Exp. Nephrol. 2019, 23, 1272–1279. [Google Scholar] [CrossRef] [PubMed]
- Vassalotti, J.A.; Winston, J.A. CKD in HIV-infected patients: From the new plague to chronic care management. Am. J. Kidney Dis. 2015, 65, 823–825. [Google Scholar] [CrossRef] [PubMed]
- Winston, J.A. HIV and CKD epidemiology. Adv. Chronic Kidney Dis. 2010, 17, 19–25. [Google Scholar] [CrossRef]
- Ekrikpo, U.E.; Kengne, A.P.; Bello, A.K.; Effa, E.E.; Noubiap, J.J.; Salako, B.L.; Rayner, B.L.; Remuzzi, G.; Okpechi, I.G. Chronic kidney disease in the global adult HIV-infected population: A systematic review and meta-analysis. PLoS ONE 2018, 13, e0195443. [Google Scholar] [CrossRef] [Green Version]
- Fernando, S.K.; Finkelstein, F.O.; Moore, B.; Weissman, S. Prevalence of chronic kidney disease in an urban HIV infected population. Am. J. Med. Sci. 2008, 335, 89–94. [Google Scholar] [CrossRef]
- Liegeon, G.; Harrison, L.; Nechba, A.; Halue, G.; Banchongkit, S.; Nilmanat, A.; Yutthakasemsunt, N.; Pathipvanich, P.; Thongpaen, S.; Lertkoonalak, R.; et al. Long term renal function in Asian HIV-1 infected adults receiving tenofovir disoproxil fumarate without protease inhibitors. J. Infect. 2019, 79, 454–461. [Google Scholar] [CrossRef]
- Rossi, C.; Raboud, J.; Walmsley, S.; Cooper, C.; Antoniou, T.; Burchell, A.N.; Hull, M.; Chia, J.; Hogg, R.S.; Moodie, E.E.M.; et al. Hepatitis C co-infection is associated with an increased risk of incident chronic kidney disease in HIV-infected patients initiating combination antiretroviral therapy. BMC Infect. Dis. 2017, 17, 246. [Google Scholar] [CrossRef]
- Swanepoel, C.R.; Atta, M.G.; D’Agati, V.D.; Estrella, M.M.; Fogo, A.B.; Naicker, S.; Post, F.A.; Wearne, N.; Winkler, C.A.; Cheung, M.; et al. Kidney disease in the setting of HIV infection: Conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2018, 93, 545–559. [Google Scholar] [CrossRef] [Green Version]
- Chaivooth, S.; Bhakeecheep, S.; Ruxrungtham, K.; Teeraananchai, S.; Kerr, S.; Teeraratkul, A.; Sirinirund, P.; Ongwandee, S.; Avihingsanon, A.; Benjarattanaporn, P.; et al. The challenges of ending AIDS in Asia: Outcomes of the Thai National AIDS Universal Coverage Programme, 2000–2014. J. Virus Erad. 2017, 3, 192–199. [Google Scholar] [CrossRef]
- World Health Organization. Update of Recommendations on First- and Second-Line Antiretroviral Regimens. Available online: https://www.who.int/hiv/pub/arv/arv-update-2019-policy/en/ (accessed on 20 January 2019).
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F.; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. The International Classification of Diseases and Related Health Problems 10th Revision, Version for 2010. Available online: http://apps.who.int/classifications/icd10/browse/2010/en (accessed on 20 January 2019).
- Levin, A.S.; Bilous, R.W.; Coresh, J. Chapter 1: Definition and classification of CKD. Kidney Int. Suppl. 2013, 3, 19–62. [Google Scholar] [CrossRef] [Green Version]
- American Diabetes Association Standards of Medical Care in Diabetes—2013. Diabetes Care 2013, 36, S11–S66. [CrossRef] [PubMed] [Green Version]
- Jellinger, P.S.; Smith, D.A.; Mehta, A.E.; Ganda, O.; Handelsman, Y.; Rodbard, H.W.; Shepherd, M.D.; Seibel, J.A. AACE task force for management of dyslipidemia and prevention of atherosclerosis American association of clinical endocrinolo-gists’ guidelines for management of dyslipidemia and prevention of atherosclerosis. Endocr. Pract. 2012, 18, 1–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Writing Group Members; Roger, V.L.; Go, A.S.; Lloyd-Jones, D.M.; Benjamin, E.J.; Berry, J.D.; Borden, W.B.; Bravata, D.M.; Dai, S.; Ford, E.S.; et al. Heart disease and stroke statistics—2012 update: A report from the American heart association. Circulation 2012, 125, e2–e220. [Google Scholar] [CrossRef] [PubMed]
- Royston, P.; White, I.R. Multiple imputation by chained equation (MICE): Implementation in stata. J. Stat. Softw. 2011, 45. [Google Scholar] [CrossRef] [Green Version]
- Loader, C. Local Regression and Likelihood; Statistics and Computing; Springer: New York, NY, USA, 1999; ISBN 978-0-387-98775-0. [Google Scholar]
- Lim, H.J.; Zhang, X.; Dyck, R.; Osgood, N. Methods of competing risks analysis of end-stage renal disease and mortality among people with diabetes. BMC Med. Res. Methodol. 2010, 10, 97. [Google Scholar] [CrossRef] [Green Version]
- Fine, J.P.; Ray, M. A proportional hazards model for the subdistribution of a competing risk. J. Am. Stat. Assoc. 1999, 94, 496–509. [Google Scholar] [CrossRef]
- Hsieh, M.-H.; Lu, P.-L.; Kuo, M.-C.; Lin, W.-R.; Lin, C.-Y.; Lai, C.-C.; Tsai, J.-J.; Chen, T.-C.; Hwang, S.-J.; Chen, Y.-H. Prevalence of and associated factors with chronic kidney disease in human immunodeficiency virus-infected patients in Taiwan. J. Microbiol. Immunol. Infect. 2015, 48, 256–262. [Google Scholar] [CrossRef] [Green Version]
- Aekplakorn, W.; Bunnag, P.; Woodward, M.; Sritara, P.; Cheepudomwit, S.; Yamwong, S.; Yipintsoi, T.; Rajatanavin, R. A risk score for predicting incident diabetes in the Thai population. Diabetes Care 2006, 29, 1872–1877. [Google Scholar] [CrossRef] [Green Version]
- Jiamjarasrangsi, W.; Aekplakorn, W. Incidence and predictors of type 2 diabetes among professional and office workers in Bangkok, Thailand. J. Med. Assoc. Thai. 2005, 88, 1896–1904. [Google Scholar] [PubMed]
- Jiamjarasrangsi, W.; Lohsoonthorn, V.; Lertmaharit, S.; Sangwatanaroj, S. Incidence and predictors of abnormal fasting plasma glucose among the university hospital employees in Thailand. Diabetes Res. Clin. Pract. 2008, 79, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Mocroft, A.; Lundgren, J.D.; Ross, M.; Fux, C.A.; Reiss, P.; Moranne, O.; Morlat, P.; Monforte, A.D.; Kirk, O.; Ryom, L. Cumulative and current exposure to potentially nephrotoxic antiretrovirals and development of chronic kidney disease in HIV-positive individuals with a normal baseline estimated glomerular filtration rate: A prospective international cohort study. Lancet HIV 2015, 3, e23–e32. [Google Scholar] [CrossRef]
- Pongpirul, W.; Pongpirul, K.; Ananworanich, J.; Klinbuayaem, V.; Avihingsanon, A.; Prasithsirikul, W. Chronic kidney disease incidence and survival of Thai HIV-infected patients. AIDS Lond. Engl. 2018, 32, 393–398. [Google Scholar] [CrossRef]
- Suzuki, S.; Nishijima, T.; Kawasaki, Y.; Kurosawa, T.; Mutoh, Y.; Kikuchi, Y.; Gatanaga, H.; Oka, S. Effect of tenofovir disoproxil fumarate on incidence of chronic kidney disease and rate of estimated glomerular filtration rate decrement in HIV-1–Infected treatment-Naïve Asian patients: Results from 12-year observational cohort. AIDS Patient Care STDs 2017, 31, 105–112. [Google Scholar] [CrossRef]
- Achhra, A.C.; Mocroft, A.; Ross, M.J.; Ryom, L.; Lucas, G.M.; Furrer, H.; Neuhaus, J.; Somboonwit, C.; Kelly, M.; Gatell, J.M.; et al. Kidney disease in antiretroviral-naïve HIV-positive adults with high CD4 counts: Prevalence and predictors of kidney disease at enrolment in the INSIGHT Strategic Timing of AntiRetroviral Treatment (START) trial. HIV Med. 2015, 16, 55–63. [Google Scholar] [CrossRef] [Green Version]
- Lapadula, G.; Bernasconi, D.P.; Casari, S.; Maggiolo, F.; Cauda, R.; Di Pietro, M.; Ladisa, N.; Sighinolfi, L.; Zoppo, S.D.; Sabbatini, F.; et al. Risk of chronic kidney disease among patients developing mild renal impairment during Tenofovir-Containing antiretroviral treatment. PLoS ONE 2016, 11, e0162320. [Google Scholar] [CrossRef] [Green Version]
- Kalyesubula, R.; Nankabirwa, J.I.; Ssinabulya, I.; Siddharthan, T.; Kayima, J.; Nakibuuka, J.; Salata, R.A.; Mondo, C.; Kamya, M.R.; Hricik, D. Kidney disease in Uganda: A community based study. BMC Nephrol. 2017, 18, 116. [Google Scholar] [CrossRef] [Green Version]
- Si, J.; Yu, C.; Guo, Y.; Bian, Z.; Qin, C.; Yang, L.; Chen, Y.; Yin, L.; Li, H.; Lan, J.; et al. Chronic hepatitis B virus infection and risk of chronic kidney disease: A population-based prospective cohort study of 0.5 million Chinese adults. BMC Med. 2018, 16, 93. [Google Scholar] [CrossRef] [Green Version]
- Fabrizi, F.; Cerutti, R.; Ridruejo, E. Hepatitis B virus infection as a risk factor for chronic kidney disease. Expert Rev. Clin. Pharmacol. 2019, 12, 867–874. [Google Scholar] [CrossRef]
- Rwegerera, G.; Bayani, M.; Taolo, E.; Habte, D. The prevalence of chronic kidney disease and associated factors among patients admitted at princess marina hospital, Gaborone, Botswana. Niger. J. Clin. Pract. 2017, 20, 313–319. [Google Scholar] [CrossRef] [PubMed]
- de Waal, R.; Cohen, K.; Fox, M.P.; Stinson, K.; Maartens, G.; Boulle, A.; Igumbor, E.; Davies, M.-A. Changes in estimated glomerular filtration rate over time in South African HIV-1-infected patients receiving tenofovir: A retrospective cohort study. J. Int. AIDS Soc. 2017, 20, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mocroft, A.; Kirk, O.; Gatell, J.; Reiss, P.; Gargalianos, P.; Zilmer, K.; Beniowski, M.; Viard, J.-P.; Staszewski, S.; Lundgren, J. Chronic renal failure among HIV-1-Infected patients. AIDS Lond. Engl. 2007, 21, 1119–1127. [Google Scholar] [CrossRef] [PubMed]
- Scherzer, R.; Gandhi, M.; Estrella, M.M.; Tien, P.C.; Deeks, S.; Grunfeld, C.; Peralta, C.A.; Shlipak, M.G. A chronic kidney disease risk score to determine tenofovir safety in a prospective cohort of HIV-positive male veterans. AIDS 2014, 28, 1289–1295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flandre, P.; Pugliese, P.; Cuzin, L.; Bagnis, C.I.; Tack, I.; Cabié, A.; Poizot-Martin, I.; Katlama, C.; Brunet-François, C.; Yazdanpanah, Y.; et al. Risk factors of chronic kidney disease in HIV-infected patients. Clin. J. Am. Soc. Nephrol. 2011, 6, 1700–1707. [Google Scholar] [CrossRef] [Green Version]
- Ambetsa, M.O.; Makori, J.O.; Osanjo, G.O.; Oluka, M.; Maitai, C.K.; Guantai, A.N.; McClelland, S.; Okalebo, F.A. Incidence and Risk Factors of Renal Dysfunction in Patients on Nevirapine-Based Regimens at a Referral Hospital in Kenya. Afr. J. Pharmacol. Ther. 2015, 4, 48–58. [Google Scholar]
- Manosuthi, W.; Mankatitham, W.; Lueangniyomkul, A.; Prasithsirikul, W.; Tantanathip, P.; Suntisuklappon, B.; Narkksoksung, A.; Nilkamhang, S.; Sungkanuparph, S. Renal impairment after switching from stavudine/lamivudine to tenofovir/lamivudine in NNRTI-Based antiretroviral regimens. AIDS Res. Ther. 2010, 7, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cressey, T.R.; Avihingsanon, A.; Halue, G.; Leenasirimakul, P.; Sukrakanchana, P.-O.; Tawon, Y.; Jaisieng, N.; Jourdain, G.; Podany, A.T.; Fletcher, C.V.; et al. Plasma and intracellular pharmacokinetics of tenofovir disoproxil fumarate 300 mg every 48 hours vs 150 mg once daily in HIV-Infected adults with moderate renal function impairment. Clin. Infect. Dis. Publ. Infect. Dis. Soc. Am. 2015, 61, 633–639. [Google Scholar] [CrossRef] [Green Version]
- LaFleur, J.; Bress, A.P.; Esker, S.; Knippenberg, K.; Crook, J.; Nyman, H.; Bedimo, R.; Tebas, P.; Rosenblatt, L. Brief report: Tenofovir-Associated nephrotoxicity among a US national historical cohort of HIV-Infected veterans: Risk modification by concomitant antiretrovirals. J. Acquir. Immune Defic. Syndr. 2018, 77, 325–330. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).