Current Concept of Quantitative Sensory Testing and Pressure Pain Threshold in Neck/Shoulder and Low Back Pain
Abstract
:1. Introduction
2. Quantitative Sensory Testing
3. Pressure Pain Threshold
3.1. Perceptions of Peripheral and Central Sensitization Can Be Quantified by PPT
3.2. PPT Analysis in Neck/Shoulder and Low Back Pain
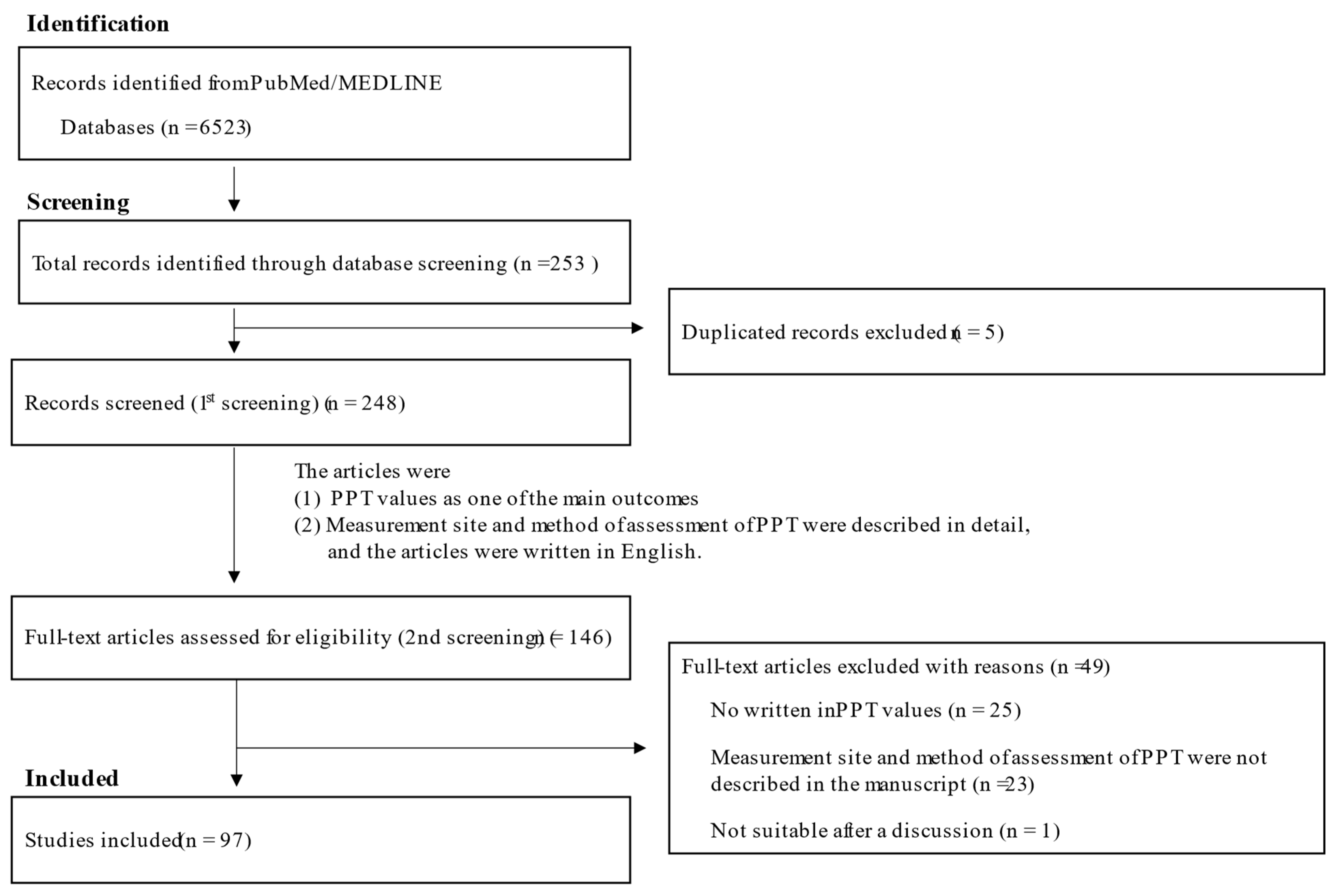
4. Systematic Review of PPT Values in Healthy Control Subjects and Patients with Neck/Shoulder and Low Back Pain
4.1. Methods of Literature Search and Inclusion Criteria
4.2. Study Selection
4.3. Quality Assessment and Risk of Bias Assessment
4.4. Neck/Shoulder Pain
4.5. Low Back Pain
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bergman, S. Management of musculoskeletal pain. Best Pract. Res. Clin. Rheumatol. 2007, 21, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Georgopoulos, V.; Akin-Akinyosoye, K.; Zhang, W.; McWilliams, D.F.; Hendrick, P.; Walsh, D.A. Quantitative sensory testing and predicting outcomes for musculoskeletal pain, disability, and negative affect: A systematic review and meta-analysis. Pain 2019, 160, 1920–1932. [Google Scholar] [CrossRef] [PubMed]
- Banic, B.; Petersen-Felix, S.; Andersen, O.K.; Radanov, B.P.; Villiger, P.M.; Arendt-Nielsen, L.; Curatolo, M. Evidence for spinal cord hypersensitivity in chronic pain after whiplash injury and in fibromyalgia. Pain 2004, 107, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Price, D.D.; Staud, R.; Robinson, M.E.; Mauderli, A.P.; Cannon, R.; Vierck, C.J. Enhanced temporal summation of second pain and its central modulation in fibromyalgia patients. Pain 2002, 99, 49–59. [Google Scholar] [CrossRef]
- Rolke, R.; Baron, R.; Maier, C.; Tölle, T.R.; Treede, R.D.; Beyer, A.; Binder, A.; Birbaumer, N.; Birklein, F.; Bötefür, I.C.; et al. Quantitative Sensory Testing in the German Research Network on Neuropathic Pain (DFNS): Standardized Protocol and Reference Values. Pain 2006, 123, 231–243. [Google Scholar] [CrossRef]
- Braun, M.; Bello, C.; Riva, T.; Hönemann, C.; Doll, D.; Urman, R.D.; Luedi, M.M. Quantitative Sensory Testing to Predict Postoperative Pain. Curr. Pain Headache Rep. 2021, 25, 3. [Google Scholar] [CrossRef]
- Treede, R.D. The role of quantitative sensory testing in the prediction of chronic pain. Pain 2019, 160 (Suppl. 1), S66–S69. [Google Scholar] [CrossRef]
- Timmerman, H.; Wilder-Smith, O.H.G.; Steegers, M.A.H.; Vissers, K.C.P.; Wolff, A.P. The Added Value of Bedside Examination and Screening QST to Improve Neuropathic Pain Identification in Patients with Chronic Pain. J. Pain Res. 2018, 11, 1307–1318. [Google Scholar] [CrossRef]
- Castien, R.F.; van der Wouden, J.C.; De Hertogh, W. Pressure pain thresholds over the cranio-cervical region in headache: A systematic review and meta-analysis. J. Headache Pain 2018, 19, 9. [Google Scholar] [CrossRef]
- Courtney, C.A.; Kavchak, A.E.; Lowry, C.D.; O’Hearn, M.A. Interpreting joint pain: Quantitative sensory testing in musculoskeletal management. J. Orthop. Sports Phys. Ther. 2010, 40, 818–825. [Google Scholar] [CrossRef]
- Uddin, Z.; MacDermid, J.C. Quantitative Sensory Testing in Chronic Musculoskeletal Pain. Pain Med. 2016, 17, 1694–1703. [Google Scholar] [CrossRef] [PubMed]
- Pavlaković, G.; Petzke, F. The role of quantitative sensory testing in the evaluation of musculoskeletal pain conditions. Curr. Rheumatol. Rep. 2010, 12, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Neziri, A.Y.; Scaramozzino, P.; Andersen, O.K.; Dickenson, A.H.; Arendt-Nielsen, L.; Curatolo, M. Reference values of mechanical and thermal pain tests in a pain-free population. Eur. J. Pain 2011, 15, 376–383. [Google Scholar] [CrossRef]
- Blankenburg, M.; Boekens, H.; Hechler, T.; Maier, C.; Krumova, E.; Scherens, A.; Magerl, W.; Aksu, F.; Zernikow, B. Reference Values for Quantitative Sensory Testing in Children and Adolescents: Developmental and Gender Differences of Somatosensory Perception. Pain 2010, 149, 76–88. [Google Scholar] [CrossRef]
- Edwards, R.R.; Fillingim, R.B. Age-associated differences in responses to noxious stimuli. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M180–M185. [Google Scholar] [CrossRef]
- Chesterton, L.S.; Barlas, P.; Foster, N.E.; Baxter, D.G.; Wright, C.C. Gender differences in pressure pain threshold in healthy humans. Pain 2003, 101, 259–266. [Google Scholar] [CrossRef]
- Andersen, S.; Petersen, M.W.; Svendsen, A.S.; Gazerani, P. Pressure pain thresholds assessed over temporalis, masseter, and frontalis muscles in healthy individuals, patients with tension-type headache, and those with migraine—A systematic review. Pain 2015, 156, 1409–1423. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Almeida, Y.; Fillingim, R.B. Can quantitative sensory testing move us closer to mechanism-based pain management? Pain Med. 2014, 15, 61–72. [Google Scholar] [CrossRef]
- Izumi, M.; Petersen, K.K.; Laursen, M.B.; Arendt-Nielsen, L.; Graven-Nielsen, T. Facilitated Temporal Summation of Pain Correlates with Clinical Pain Intensity after Hip Arthroplasty. Pain 2017, 158, 323–332. [Google Scholar] [CrossRef]
- Siao, P.; Cros, D.P. Quantitative sensory testing. Phys. Med. Rehabil. Clin. 2003, 14, 261–286. [Google Scholar] [CrossRef]
- Sangesland, A.; Støren, C.; Vaegter, H.B. Are preoperative experimental pain assessments correlated with clinical pain outcomes after surgery? A systematic review. Scand. J. Pain 2017, 15, 44–52. [Google Scholar] [CrossRef] [PubMed]
- van Helmond, N.; Aarts, H.M.; Timmerman, H.; Olesen, S.S.; Drewes, A.M.; Wilder-Smith, O.H.; Steegers, M.A.; Vissers, K.C. Is Preoperative Quantitative Sensory Testing Related to Persistent Postsurgical Pain? A Systematic Literature Review. Anesth. Analg. 2020, 131, 1146–1155. [Google Scholar] [CrossRef] [PubMed]
- Arant, K.R.; Katz, J.N.; Neogi, T. Quantitative sensory testing: Identifying pain characteristics in patients with osteoarthritis. Osteoarthr. Cartil. 2022, 30, 17–31. [Google Scholar] [CrossRef]
- Park, G.; Kim, C.W.; Park, S.B.; Kim, M.J.; Jang, S.H. Reliability and usefulness of the pressure pain threshold measurement in patients with myofascial pain. Ann. Rehabil. Med. 2011, 35, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Treede, R.D.; Rolke, R.; Andrews, K.; Magerl, W. Pain elicited by blunt pressure: Neurobiological basis and clinical relevance. Pain 2002, 98, 235–240. [Google Scholar] [CrossRef]
- Mutlu, E.K.; Ozdincler, A.R. Reliability and responsiveness of algometry for measuring pressure pain threshold in patients with knee osteoarthritis. J. Phys. Ther. Sci. 2015, 27, 1961–1965. [Google Scholar] [CrossRef] [PubMed]
- Woolf, C.J.; Thompson, S.W.N. The induction and maintenance of central sensitization is dependent on N-methyl-D-aspartic acid receptor activation; implications for the treatment of post-injury pain hypersensitivity states. Pain 1991, 44, 293–299. [Google Scholar] [CrossRef]
- Coronado, R.A.; Simon, C.B.; Valencia, C.; George, S.Z. Experimental pain responses support peripheral and central sensitization in patients with unilateral shoulder pain. Clin. J. Pain 2014, 30, 143–151. [Google Scholar] [CrossRef]
- Arjona Retamal, J.J.; Fernández Seijo, A.; Torres Cintas, J.D.; de-la-Llave-Rincón, A.I.; Caballero Bragado, A. Effects of Instrumental, Manipulative and Soft Tissue Approaches for the Suboccipital Region in Subjects with Chronic Mechanical Neck Pain. A Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2021, 18, 8636. [Google Scholar] [CrossRef]
- Hernandez, J.V.L.; Calvo-Lobo, C.; Zugasti, A.M.; Fernandez-Carnero, J.; Beltran Alacreu, H. Effectiveness of Dry Needling with Percutaneous Electrical Nerve Stimulation of High Frequency Versus Low Frequency in Patients with Myofascial Neck Pain. Pain Physician 2021, 24, 135–143. [Google Scholar]
- Stieven, F.F.; Ferreira, G.E.; de Araújo, F.X.; Angellos, R.F.; Silva, M.F.; da Rosa, L.H.T. Immediate Effects of Dry Needling and Myofascial Release on Local and Widespread Pressure Pain Threshold in Individuals With Active Upper Trapezius Trigger Points: A Randomized Clinical Trial. J. Manip. Physiol. Ther. 2021, 44, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.K.; Dibai-Filho, A.V.; Soleira, G.; Machado, A.C.F.; Guirro, R.R.J. Reliability of pressure pain threshold on myofascial trigger points in the trapezius muscle of women with chronic neck pain. Rev. Assoc. Med. Bras. 2021, 67, 708–712. [Google Scholar] [CrossRef] [PubMed]
- Grimby-Ekman, A.; Ahlstrand, C.; Gerdle, B.; Larsson, B.; Sandén, H. Pain intensity and pressure pain thresholds after a light dynamic physical load in patients with chronic neck-shoulder pain. BMC Musculoskelet. Disord. 2020, 21, 266. [Google Scholar] [CrossRef] [PubMed]
- Heredia-Rizo, A.M.; Petersen, K.K.; Arendt-Nielsen, L.; Madeleine, P. Eccentric Training Changes the Pressure Pain and Stiffness Maps of the Upper Trapezius in Females with Chronic Neck-Shoulder Pain: A Preliminary Study. Pain Med. 2020, 21, 1936–1946. [Google Scholar] [CrossRef] [PubMed]
- Arias-Buría, J.L.; Franco-Hidalgo-Chacón, M.M.; Cleland, J.A.; Palacios-Ceña, M.; Fuensalida-Novo, S.; Fernández-de-Las-Peñas, C. Effects of Kinesio Taping on Post-Needling Induced Pain After Dry Needling of Active Trigger Point in Individuals With Mechanical Neck Pain. J. Manip. Physiol. Ther. 2020, 43, 32–42. [Google Scholar] [CrossRef]
- Shin, H.J.; Kim, S.H.; Hahm, S.C.; Cho, H.Y. Thermotherapy Plus Neck Stabilization Exercise for Chronic Nonspecific Neck Pain in Elderly: A Single-Blinded Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2020, 17, 5572. [Google Scholar] [CrossRef]
- Rodríguez-Huguet, M.; Rodríguez-Huguet, P.; Lomas-Vega, R.; Ibáñez-Vera, A.J.; Rodríguez-Almagro, D. Vacuum myofascial therapy device for non-specific neck pain. A single blind randomized clinical trial. Complement. Ther. Med. 2020, 52, 102449. [Google Scholar] [CrossRef]
- Alfawaz, S.; Lohman, E.; Alameri, M.; Daher, N.; Jaber, H. Effect of adding stretching to standardized procedures on cervical range of motion, pain, and disability in patients with non-specific mechanical neck pain: A randomized clinical trial. J. Bodyw. Mov. Ther. 2020, 24, 50–58. [Google Scholar] [CrossRef]
- Chatchawan, U.; Thongbuang, S.; Yamauchi, J. Characteristics and distributions of myofascial trigger points in individuals with chronic tension-type headaches. J. Phys. Ther. Sci. 2019, 31, 306–309. [Google Scholar] [CrossRef]
- Wang-Price, S.; Zafereo, J.; Brizzolara, K.; Mackin, B.; Lawson, L.; Seeger, D.; Lawson, S. Psychometric Properties of Pressure Pain Thresholds Measured in 2 Positions for Adults with and Without Neck-Shoulder Pain and Tenderness. J. Manip. Physiol. Ther. 2019, 42, 416–424. [Google Scholar] [CrossRef]
- Murray, M.; Lange, B.; Nørnberg, B.R.; Søgaard, K.; Sjøgaard, G. Self-administered physical exercise training as treatment of neck and shoulder pain among military helicopter pilots and crew: A randomized controlled trial. BMC Musculoskelet. Disord. 2017, 18, 147. [Google Scholar] [CrossRef] [PubMed]
- De Meulemeester, K.E.; Castelein, B.; Coppieters, I.; Barbe, T.; Cools, A.; Cagnie, B. Comparing Trigger Point Dry Needling and Manual Pressure Technique for the Management of Myofascial Neck/Shoulder Pain: A Randomized Clinical Trial. J. Manip. Physiol. Ther. 2017, 40, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Wassinger, C.A.; Rich, D.; Cameron, N.; Clark, S.; Davenport, S.; Lingelbach, M.; Smith, A.; Baxter, G.D.; Davidson, J. Cervical & thoracic manipulations: Acute effects upon pain pressure threshold and self-reported pain in experimentally induced shoulder pain. Man. Ther. 2016, 21, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Celenay, S.T.; Kaya, D.O.; Akbayrak, T. Cervical and scapulothoracic stabilization exercises with and without connective tissue massage for chronic mechanical neck pain: A prospective, randomised controlled trial. Man. Ther. 2016, 21, 144–150. [Google Scholar] [CrossRef]
- Pajediene, E.; Janusauskaite, J.; Samusyte, G.; Stasaitis, K.; Petrikonis, K.; Bileviciute-Ljungar, I. Patterns of acute whiplash-associated disorder in the Lithuanian population after road traffic accidents. J. Rehabil. Med. 2015, 47, 52–57. [Google Scholar] [CrossRef]
- Lopez-Lopez, A.; Alonso Perez, J.L.; González Gutierez, J.L.; La Touche, R.; Lerma Lara, S.; Izquierdo, H.; Fernández-Carnero, J. Mobilization versus manipulations versus sustain apophyseal natural glide techniques and interaction with psychological factors for patients with chronic neck pain: Randomized controlled trial. Eur. J. Phys. Rehabil. Med. 2015, 51, 121–132. [Google Scholar]
- Ge, H.Y.; Vangsgaard, S.; Omland, Ø.; Madeleine, P.; Arendt-Nielsen, L. Mechanistic experimental pain assessment in computer users with and without chronic musculoskeletal pain. BMC Musculoskelet. Disord. 2014, 15, 412. [Google Scholar] [CrossRef]
- Llamas-Ramos, R.; Pecos-Martín, D.; Gallego-Izquierdo, T.; Llamas-Ramos, I.; Plaza-Manzano, G.; Ortega-Santiago, R.; Cleland, J.; Fernández-de-Las-Peñas, C. Comparison of the short-term outcomes between trigger point dry needling and trigger point manual therapy for the management of chronic mechanical neck pain: A randomized clinical trial. J. Orthop. Sports Phys. Ther. 2014, 44, 852–861, Erratum in J. Orthop. Sports Phys. Ther. 2015, 45, 147. [Google Scholar] [CrossRef]
- Andersen, C.H.; Andersen, L.L.; Zebis, M.K.; Sjøgaard, G. Effect of scapular function training on chronic pain in the neck/shoulder region: A randomized controlled trial. J. Occup. Rehabil. 2014, 24, 316–324. [Google Scholar] [CrossRef]
- Casanova-Méndez, A.; Oliva-Pascual-Vaca, A.; Rodriguez-Blanco, C.; Heredia-Rizo, A.M.; Gogorza-Arroitaonandia, K.; Almazán-Campos, G. Comparative short-term effects of two thoracic spinal manipulation techniques in subjects with chronic mechanical neck pain: A randomized controlled trial. Man. Ther. 2014, 19, 331–337. [Google Scholar] [CrossRef]
- Cagnie, B.; Dewitte, V.; Coppieters, I.; Van Oosterwijck, J.; Cools, A.; Danneels, L. Effect of ischemic compression on trigger points in the neck and shoulder muscles in office workers: A cohort study. J. Manip. Physiol. Ther. 2013, 36, 482–489. [Google Scholar] [CrossRef]
- Yoo, I.G.; Yoo, W.G. The Effect of a New Neck Support Tying Method Using Thera-Band on Cervical ROM and Shoulder Muscle Pain after Overhead Work. J. Phys. Ther. Sci. 2013, 25, 843–844. [Google Scholar] [CrossRef] [PubMed]
- Lauche, R.; Materdey, S.; Cramer, H.; Haller, H.; Stange, R.; Dobos, G.; Rampp, T. Effectiveness of home-based cupping massage compared to progressive muscle relaxation in patients with chronic neck pain--a randomized controlled trial. PLoS ONE 2013, 8, e65378. [Google Scholar] [CrossRef]
- Fernández-Pérez, A.M.; Villaverde-Gutiérrez, C.; Mora-Sánchez, A.; Alonso-Blanco, C.; Sterling, M.; Fernández-de-Las-Peñas, C. Muscle trigger points, pressure pain threshold, and cervical range of motion in patients with high level of disability related to acute whiplash injury. J. Orthop. Sports Phys. Ther. 2012, 42, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Andersen, L.L.; Andersen, C.H.; Sundstrup, E.; Jakobsen, M.D.; Mortensen, O.S.; Zebis, M.K. Central adaptation of pain perception in response to rehabilitation of musculoskeletal pain: Randomized controlled trial. Pain Physician 2012, 15, 385–394. [Google Scholar] [PubMed]
- Ge, H.Y.; Nie, H.; Madeleine, P.; Danneskiold-Samsøe, B.; Graven-Nielsen, T.; Arendt-Nielsen, L. Contribution of the local and referred pain from active myofascial trigger points in fibromyalgia syndrome. Pain 2009, 147, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Gerdle, B.; Lemming, D.; Kristiansen, J.; Larsson, B.; Peolsson, M.; Rosendal, L. Biochemical alterations in the trapezius muscle of patients with chronic whiplash associated disorders (WAD)—A microdialysis study. Eur. J. Pain 2008, 12, 82–93. [Google Scholar] [CrossRef]
- Lemming, D.; Sörensen, J.; Graven-Nielsen, T.; Lauber, R.; Arendt-Nielsen, L.; Gerdle, B. Managing chronic whiplash associated pain with a combination of low-dose opioid (remifentanil) and NMDA-antagonist (ketamine). Eur. J. Pain 2007, 11, 719–732. [Google Scholar] [CrossRef]
- Ylinen, J.; Nykänen, M.; Kautiainen, H.; Häkkinen, A. Evaluation of repeatability of pressure algometry on the neck muscles for clinical use. Man. Ther. 2007, 12, 192–197. [Google Scholar] [CrossRef]
- Ylinen, J.; Häkkinen, A.; Nykänen, M.; Kautiainen, H.; Takala, E.P. Neck muscle training in the treatment of chronic neck pain: A three-year follow-up study. Eura. Medicophys. 2007, 43, 161–169. [Google Scholar]
- Ojala, T.; Arokoski, J.P.; Partanen, J. The effect of small doses of botulinum toxin a on neck-shoulder myofascial pain syndrome: A double-blind, randomized, and controlled crossover trial. Clin. J. Pain 2006, 22, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Ylinen, J.; Takala, E.P.; Kautiainen, H.; Nykänen, M.; Häkkinen, A.; Pohjolainen, T.; Karppi, S.L.; Airaksinen, O. Effect of long-term neck muscle training on pressure pain threshold: A randomized controlled trial. Eur. J. Pain 2005, 9, 673–681. [Google Scholar] [CrossRef] [PubMed]
- Nabeta, T.; Kawakita, K. Relief of chronic neck and shoulder pain by manual acupuncture to tender points--a sham-controlled randomized trial. Complement. Ther. Med. 2002, 10, 217–222. [Google Scholar] [CrossRef]
- Waling, K.; Sundelin, G.; Ahlgren, C.; Järvholm, B. Perceived pain before and after three exercise programs--a controlled clinical trial of women with work-related trapezius myalgia. Pain 2000, 85, 201–207. [Google Scholar] [CrossRef]
- Taimela, S.; Takala, E.P.; Asklöf, T.; Seppälä, K.; Parviainen, S. Active treatment of chronic neck pain: A prospective randomized intervention. Spine 2000, 25, 1021–1027. [Google Scholar] [CrossRef] [PubMed]
- Otto, A.; Emery, K.; Côté, J.N. Sex differences in perceptual responses to experimental pain before and after an experimental fatiguing arm task. Biol. Sex Differ. 2019, 10, 39. [Google Scholar] [CrossRef] [PubMed]
- Sacramento, L.S.; Camargo, P.R.; Siqueira-Júnior, A.L.; Ferreira, J.P.; Salvini, T.F.; Alburquerque-Sendín, F. Presence of Latent Myofascial Trigger Points and Determination of Pressure Pain Thresholds of the Shoulder Girdle in Healthy Children and Young Adults: A Cross-sectional Study. J. Manip. Physiol. Ther. 2017, 40, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Wytrążek, M.; Huber, J.; Lipiec, J.; Kulczyk, A. Evaluation of palpation, pressure algometry, and electromyography for monitoring trigger points in young participants. J. Manip. Physiol. Ther. 2015, 38, 232–243. [Google Scholar] [CrossRef]
- Shin, S.J.; An, D.H.; Oh, J.S.; Yoo, W.G. Changes in pressure pain in the upper trapezius muscle, cervical range of motion, and the cervical flexion-relaxation ratio after overhead work. Ind. Health 2012, 50, 509–515. [Google Scholar] [CrossRef]
- Binderup, A.T.; Arendt-Nielsen, L.; Madeleine, P. Pressure pain sensitivity maps of the neck-shoulder and the low back regions in men and women. BMC Musculoskelet. Disord. 2010, 11, 234. [Google Scholar] [CrossRef]
- Saíz-Llamosas, J.R.; Fernández-Pérez, A.M.; Fajardo-Rodríguez, M.F.; Pilat, A.; Valenza-Demet, G.; Fernández-de-Las-Peñas, C. Changes in neck mobility and pressure pain threshold levels following a cervical myofascial induction technique in pain-free healthy subjects. J. Manip. Physiol. Ther. 2009, 32, 352–357. [Google Scholar] [CrossRef] [PubMed]
- Fernández-de-Las-Peñas, C.; Alonso-Blanco, C.; Cleland, J.A.; Rodríguez-Blanco, C.; Alburquerque-Sendín, F. Changes in pressure pain thresholds over C5-C6 zygapophyseal joint after a cervicothoracic junction manipulation in healthy subjects. J. Manip. Physiol. Ther. 2008, 31, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Ge, H.Y.; Madeleine, P.; Cairns, B.E.; Arendt-Nielsen, L. Hypoalgesia in the referred pain areas after bilateral injections of hypertonic saline into the trapezius muscles of men and women: A potential experimental model of gender-specific differences. Clin. J. Pain 2006, 22, 37–44. [Google Scholar] [CrossRef]
- Nie, H.; Kawczynski, A.; Madeleine, P.; Arendt-Nielsen, L. Delayed onset muscle soreness in neck/shoulder muscles. Eur. J. Pain 2005, 9, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Marcuzzi, A.; Wrigley, P.J.; Dean, C.M.; Graham, P.L.; Hush, J.M. From acute to persistent low back pain: A longitudinal investigation of somatosensory changes using quantitative sensory testing-an exploratory study. Pain Rep. 2018, 3, e641. [Google Scholar] [CrossRef]
- Dias, L.V.; Cordeiro, M.A.; Schmidt de Sales, R.; Dos Santos, M.M.B.R.; Korelo, R.I.G.; Vojciechowski, A.S.; de Mace do, A.C.B. Immediate analgesic effect of transcutaneous electrical nerve stimulation (TENS) and interferential current (IFC) on chronic low back pain: Randomised placebo-controlled trial. J. Bodyw. Mov. Ther. 2021, 27, 181–190. [Google Scholar] [CrossRef]
- Nim, C.G.; O’Neill, S.; Geltoft, A.G.; Jensen, L.K.; Schiøttz-Christensen, B.; Kawchuk, G.N. A cross-sectional analysis of persistent low back pain, using correlations between lumbar stiffness, pressure pain threshold, and heat pain threshold. Chiropr. Man. Ther. 2021, 29, 34. [Google Scholar] [CrossRef] [PubMed]
- Leemans, L.; Elma, Ö.; Nijs, J.; Wideman, T.H.; Siffain, C.; den Bandt, H.; Van Laere, S.; Beckwée, D. Transcutaneous electrical nerve stimulation and heat to reduce pain in a chronic low back pain population: A randomized controlled clinical trial. Braz. J. Phys. Ther. 2021, 25, 86–96. [Google Scholar] [CrossRef]
- Nim, C.G.; Kawchuk, G.N.; Schiøttz-Christensen, B.; O’Neill, S. The effect on clinical outcomes when targeting spinal manipulation at stiffness or pain sensitivity: A randomized trial. Sci. Rep. 2020, 10, 14615. [Google Scholar] [CrossRef]
- Volpato, M.P.; Breda, I.C.A.; de Carvalho, R.C.; de Castro Moura, C.; Ferreira, L.L.; Silva, M.L.; Silva, J.R.T. Single Cupping Thearpy Session Improves Pain, Sleep, and Disability in Patients with Nonspecific Chronic Low Back Pain. J. Acupunct. Meridian Stud. 2020, 13, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Wang-Price, S.; Zafereo, J.; Couch, Z.; Brizzolara, K.; Heins, T.; Smith, L. Short-term effects of two deep dry needling techniques on pressure pain thresholds and electromyographic amplitude of the lumbosacral multifidus in patients with low back pain-a randomized clinical trial. J. Man. Manip. Ther. 2020, 28, 254–265. [Google Scholar] [CrossRef] [PubMed]
- Vaegter, H.B.; Petersen, K.K.; Sjodsholm, L.V.; Schou, P.; Andersen, M.B.; Graven-Nielsen, T. Impaired exercise-induced hypoalgesia in individuals reporting an increase in low back pain during acute exercise. Eur. J. Pain 2021, 25, 1053–1063. [Google Scholar] [CrossRef] [PubMed]
- Fagundes Loss, J.; de Souza da Silva, L.; Ferreira Miranda, I.; Groisman, S.; Santiago Wagner Neto, E.; Souza, C.; Tarragô Candotti, C. Immediate effects of a lumbar spine manipulation on pain sensitivity and postural control in individuals with nonspecific low back pain: A randomized controlled trial. Chiropr. Man. Ther. 2020, 28, 25. [Google Scholar] [CrossRef] [PubMed]
- Plaza-Manzano, G.; Cancela-Cilleruelo, I.; Fernández-de-Las-Peñas, C.; Cleland, J.A.; Arias-Buría, J.L.; Thoomes-de-Graaf, M.; Ortega-Santiago, R. Effects of Adding a Neurodynamic Mobilization to Motor Control Training in Patients with Lumbar Radiculopathy Due to Disc Herniation: A Randomized Clinical Trial. Am. J. Phys. Med. Rehabil. 2020, 99, 124–132. [Google Scholar] [CrossRef]
- Moreira, R.F.C.; Moriguchi, C.S.; Carnaz, L.; Foltran, F.A.; Silva, L.C.C.B.; Coury, H.J.C.G. Effects of a workplace exercise program on physical capacity and lower back symptoms in hospital nursing assistants: A randomized controlled trial. Int. Arch. Occup. Environ. Health 2021, 94, 275–284. [Google Scholar] [CrossRef]
- Bond, B.M.; Kinslow, C.D.; Yoder, A.W.; Liu, W. Effect of spinal manipulative therapy on mechanical pain sensitivity in patients with chronic nonspecific low back pain: A pilot randomized, controlled trial. J. Man. Manip. Ther. 2020, 28, 15–27. [Google Scholar] [CrossRef]
- Chapman, K.B.; van Roosendaal, B.K.; Yousef, T.A.; Vissers, K.C.; van Helmond, N. Dorsal Root Ganglion Stimulation Normalizes Measures of Pain Processing in Patients with Chronic Low-Back Pain: A Prospective Pilot Study using Quantitative Sensory Testing. Pain Pract. 2021, 21, 568–577. [Google Scholar] [CrossRef]
- Aspinall, S.L.; Jacques, A.; Leboeuf-Yde, C.; Etherington, S.J.; Walker, B.F. Pressure pain threshold and temporal summation in adults with episodic and persistent low back pain trajectories: A secondary analysis at baseline and after lumbar manipulation or sham. Chiropr. Man. Ther. 2020, 28, 36. [Google Scholar] [CrossRef]
- Aspinall, S.L.; Leboeuf-Yde, C.; Etherington, S.J.; Walker, B.F. Changes in pressure pain threshold and temporal summation in rapid responders and non-rapid responders after lumbar spinal manipulation and sham: A secondary analysis in adults with low back pain. Musculoskelet. Sci. Pract. 2020, 47, 102137. [Google Scholar] [CrossRef]
- Yildiz, S.H.; Ulaşli, A.M.; Özdemir Erdoğan, M.; Dikici, Ö.; Arikan Terzi, E.S.; Dündar, Ü.; Solak, M. Assessment of Pain Sensitivity in Patients with Chronic Low Back Pain and Association With HTR2A Gene Polymorphism. Arch. Rheumatol. 2016, 32, 3–9. [Google Scholar] [CrossRef]
- Imamura, M.; Chen, J.; Matsubayashi, S.R.; Targino, R.A.; Alfieri, F.M.; Bueno, D.K.; Hsing, W.T. Changes in pressure pain threshold in patients with chronic nonspecific low back pain. Spine 2013, 38, 2098–2107. [Google Scholar] [CrossRef] [PubMed]
- Aspinall, S.L.; Jacques, A.; Leboeuf-Yde, C.; Etherington, S.J.; Walker, B.F. No difference in pressure pain threshold and temporal summation after lumbar spinal manipulation compared to sham: A randomised controlled trial in adults with low back pain. Musculoskelet. Sci. Pract. 2019, 43, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Neziri, A.Y.; Curatolo, M.; Limacher, A.; Nüesch, E.; Radanov, B.; Andersen, O.K.; Arendt-Nielsen, L.; Jüni, P. Ranking of parameters of pain hypersensitivity according to their discriminative ability in chronic low back pain. Pain 2012, 153, 2083–2091. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, R.C.; Parisi, J.R.; Prado, W.A.; de Araújo, J.E.; Silva, A.M.; Silva, J.R.T.; Silva, M.L. Single or Multiple Electroacupuncture Sessions in Nonspecific Low Back Pain: Are We Low-Responders to Electroacupuncture? J. Acupunct. Meridian Stud. 2018, 11, 54–61. [Google Scholar] [CrossRef]
- Bodes Pardo, G.; Lluch Girbés, E.; Roussel, N.A.; Gallego Izquierdo, T.; Jiménez Penick, V.; Pecos Martín, D. Pain Neurophysiology Education and Therapeutic Exercise for Patients with Chronic Low Back Pain: A Single-Blind Randomized Controlled Trial. Arch. Phys. Med. Rehabil. 2018, 99, 338–347. [Google Scholar] [CrossRef]
- O’Neill, S.; Kjær, P.; Graven-Nielsen, T.; Manniche, C.; Arendt-Nielsen, L. Low pressure pain thresholds are associated with, but does not predispose for, low back pain. Eur. Spine J. 2011, 20, 2120–2125. [Google Scholar] [CrossRef]
- Joseph, L.H.; Hancharoenkul, B.; Sitilertpisan, P.; Pirunsan, U.; Paungmali, A. Effects of Massage as a Combination Therapy with Lumbopelvic Stability Exercises as Compared to Standard Massage Therapy in Low Back Pain: A Randomized Cross-Over Study. Int. J. Ther. Massage Bodyw. 2018, 11, 16–22. [Google Scholar]
- Koppenhaver, S.L.; Walker, M.J.; Rettig, C.; Davis, J.; Nelson, C.; Su, J.; Fernández-de-Las-Peñas, C.; Hebert, J.J. The association between dry needling-induced twitch response and change in pain and muscle function in patients with low back pain: A quasi-experimental study. Physiotherapy 2017, 103, 131–137. [Google Scholar] [CrossRef]
- Farasyn, A.; Lassat, B. Pressure pain thresholds in patients with chronic nonspecific low back pain. J. Bodyw. Mov. Ther. 2016, 20, 224–234. [Google Scholar] [CrossRef]
- Paungmali, A.; Joseph, L.H.; Sitilertpisan, P.; Pirunsan, U.; Uthaikhup, S. Lumbopelvic Core Stabilization Exercise and Pain Modulation Among Individuals with Chronic Nonspecific Low Back Pain. Pain Pract. 2017, 17, 1008–1014. [Google Scholar] [CrossRef]
- Mohanty, P.P.; Pattnaik, M. Effect of stretching of piriformis and iliopsoas in coccydynia. J. Bodyw. Mov. Ther. 2017, 21, 743–746. [Google Scholar] [CrossRef] [PubMed]
- Falla, D.; Gizzi, L.; Tschapek, M.; Erlenwein, J.; Petzke, F. Reduced task-induced variations in the distribution of activity across back muscle regions in individuals with low back pain. Pain 2014, 155, 944–953. [Google Scholar] [CrossRef] [PubMed]
- Imamura, M.; Alfieri, F.M.; Filippo, T.R.; Battistella, L.R. Pressure pain thresholds in patients with chronic nonspecific low back pain. J. Back Musculoskelet. Rehabil. 2016, 29, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Balaguier, R.; Madeleine, P.; Vuillerme, N. Is One Trial Sufficient to Obtain Excellent Pressure Pain Threshold Reliability in the Low Back of Asymptomatic Individuals? A Test-Retest Study. PLoS ONE 2016, 11, e0160866. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, J.; Yamagata, M.; Ogata, S.; Shimizu, K.; Ikeda, Y.; Takahashi, K. Relationship between low-back pain, muscle spasm and pressure pain thresholds in patients with lumbar disc herniation. Eur. Spine J. 2006, 15, 41–47. [Google Scholar] [CrossRef]
- Weinkauf, B.; Deising, S.; Obreja, O.; Hoheisel, U.; Mense, S.; Schmelz, M.; Rukwied, R. Comparison of nerve growth factor-induced sensitization pattern in lumbar and tibial muscle and fascia. Muscle Nerve 2015, 52, 265–272. [Google Scholar] [CrossRef]
- Calvo-Lobo, C.; Diez-Vega, I.; Martínez-Pascual, B.; Fernández-Martínez, S.; de la Cueva-Reguera, M.; Garrosa-Martín, G.; Rodríguez-Sanz, D. Tensiomyography, sonoelastography, and mechanosensitivity differences between active, latent, and control low back myofascial trigger points: A cross-sectional study. Medicine 2017, 96, e6287. [Google Scholar] [CrossRef]
- de Oliveira, R.F.; Liebano, R.E.; Costa Lda, C.; Rissato, L.L.; Costa, L.O. Immediate effects of region-specific and non-region-specific spinal manipulative therapy in patients with chronic low back pain: A randomized controlled trial. Phys. Ther. 2013, 93, 748–756. [Google Scholar] [CrossRef]
- Alfieri, F.M.; Lima, A.R.S.; Battistella, L.R.; Silva, N.C.O.V.E. Superficial temperature and pain tolerance in patients with chronic low back pain. J. Bodyw. Mov. Ther. 2019, 23, 583–587. [Google Scholar] [CrossRef]
- Zheng, Z.; Wang, J.; Gao, Q.; Hou, J.; Ma, L.; Jiang, C.; Chen, G. Therapeutic evaluation of lumbar tender point deep massage for chronic non-specific low back pain. J. Tradit. Chin. Med. 2012, 32, 534–537. [Google Scholar] [CrossRef]
- McSweeney, T.P.; Thomson, O.P.; Johnston, R. The immediate effects of sigmoid colon manipulation on pressure pain thresholds in the lumbar spine. J. Bodyw. Mov. Ther. 2012, 16, 416–423. [Google Scholar] [CrossRef]
- O’Neill, S.; Larsen, J.B.; Nim, C.; Arendt-Nielsen, L. Topographic mapping of pain sensitivity of the lower back-a comparison of healthy controls and patients with chronic non-specific low back pain. Scand. J. Pain. 2019, 19, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Wang, X.; Zhang, J.; Wang, Y. Changes in pressure pain thresholds and Basal electromyographic activity after instrument-assisted spinal manipulative therapy in asymptomatic participants: A randomized, controlled trial. J. Manip. Physiol. Ther. 2012, 35, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Binderup, A.T.; Holtermann, A.; Søgaard, K.; Madeleine, P. Pressure pain sensitivity maps, self-reported musculoskeletal disorders and sickness absence among cleaners. Int. Arch. Occup. Environ. Health 2011, 84, 647–654. [Google Scholar] [CrossRef] [PubMed]
- McPhee, M.E.; Graven-Nielsen, T. Recurrent low back pain patients demonstrate facilitated pronociceptive mechanisms when in pain, and impaired antinociceptive mechanisms with and without pain. Pain 2019, 160, 2866–2876. [Google Scholar] [CrossRef]
- Farasyn, A.; Meeusen, R. The influence of non-specific low back pain on pressure pain thresholds and disability. Eur. J. Pain 2005, 9, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Żywień, U.; Barczyk-Pawelec, K.; Sipko, T. Associated Risk Factors with Low Back Pain in White-Collar Workers-A Cross-Sectional Study. J. Clin. Med. 2022, 11, 1275. [Google Scholar] [CrossRef]
- Mailloux, C.; Beaulieu, L.D.; Wideman, T.H.; Massé-Alarie, H. Within-session test-retest reliability of pressure pain threshold and mechanical temporal summation in healthy subjects. PLoS ONE 2021, 16, e0245278. [Google Scholar] [CrossRef]
- Petersson, M.; Abbott, A. Lumbar interspinous pressure pain threshold values for healthy young men and women and the effect of prolonged fully flexed lumbar sitting posture: An observational study. World J. Orthop. 2020, 11, 158–166. [Google Scholar] [CrossRef]
- Kołcz, A.; Jenaszek, K. Assessment of pressure pain threshold at the cervical and lumbar spine region in the group of professionally active nurses: A cross-sectional study. J. Occup. Health 2020, 62, e12108. [Google Scholar] [CrossRef]
- Nothnagel, H.; Puta, C.; Lehmann, T.; Baumbach, P.; Menard, M.B.; Gabriel, B.; Gabriel, H.H.W.; Weiss, T.; Musial, F. How stable are quantitative sensory testing measurements over time? Report on 10-week reliability and agreement of results in healthy volunteers. J. Pain Res. 2017, 10, 2067–2078. [Google Scholar] [CrossRef] [PubMed]
- Selva-Sarzo, F.; Fernández-Carnero, S.; Sillevis, R.; Hernández-Garcés, H.; Benitez-Martinez, J.C.; Cuenca-Zaldívar, J.N. The Direct Effect of Magnetic Tape® on Pain and Lower-Extremity Blood Flow in Subjects with Low-Back Pain: A Randomized Clinical Trial. Sensors 2021, 21, 6517. [Google Scholar] [CrossRef] [PubMed]
| QST Type | Sensation/Modulation | Stimulus Modalities |
|---|---|---|
| Thermal | Warm | Warm detection threshold (WDT) |
| Cold | Cold detection threshold (CDT) | |
| Pain | Heat pain threshold (HPT) | |
| Cold pain threshold (CPT) | ||
| Suprathreshold heat pain intensity (STHPI) | ||
| Mechanical | Vibration | Vibration detection threshold (VDT) |
| Pain | Pressure pain threshold (PPT) | |
| Suprathreshold pressure pain intensity (STPPI) | ||
| Pressure pain tolerance (PPTol) | ||
| Electrical | Pain | Electrical pain threshold (EPT) |
| Electrical pain tolerance (EPTol) | ||
| Dynamic | Wind-up | Temporal summation (TS) |
| Excitability of spinal cord neurons | ||
| Diffuse noxious inhibitory controls (DNIC) | Conditioned pain modulation (CPM) |
| Authors | Object of Study/Subject | Areas of PPT Examination | Device (Units) | PPT Values in Patients/People with Neck and Shoulder Pain; Mean (±SD) | PPT Values in Healthy Volunteers/Control; Mean (±SD) | |
|---|---|---|---|---|---|---|
| Arjona Retamal JJ et al. | 2021 | Chronic neck pain | Upper trapezius | Digital algometer (kg/cm2) (FPX 25, Wagner Ins, Greenwich, CT) | Upper trapezius: 1.35–1.56 | none |
| Leon Hernández JV et al. | 2021 | Chronic neck pain | Upper trapezius | Digital algometer (kg/cm2) (Wagner Ins, greenwich, CT, USA) | Uppe tripezius: 4.11–4.14 | none |
| Stieven FF et al. | 2021 | Chronic neck pain | Upper trapezius | Digital algometer (kgf) (FPX 25, Wagner Ins, Greenwich, CT) | Upper trapezius: 1.35–1.46 | none |
| Oliveira AK et al. | 2021 | Chronic neck pain | Upper trapezius | Algometer (kg/cm2) (PTR-300 model, Instrutherm, São Paulo, SP, Brazil) | Upper trapezius: 1.44–1.76 | none |
| Grimby-Ekman A et al. | 2020 | Chronic neck-shoulder pain | Upper trapezius | Distal algometer (kpa) (Somedic AB, Farsta, Sweden) | Upper trapezius: 376–411 | Upper trapezius: 335–436 |
| Heredia-Rizo AM et al. | 2020 | Neck-shoulder pain | Upper trapezius | Electronic pressure algometer (kpa) (Somedic AB, Horby, Sweden) | Upper trapezius: 226.1 ± 103.2 (177.8–274.4) | Upper trapezius: 282.2 ± 109.4 |
| Arias-Buria JL et al. | 2020 | Mechanical neck pain | Triger point area of shoulder | Mechanical algometer (kpa) (Pain Diagnosis and Treatment Inc., New York, NY, USA) | Triger point area of shoulder: 145.4–148.9 | none |
| Shin HJ et al. | 2020 | Chronic neck pain | Upper trapezius Splenius capitis Levator scapulae | Digital algometer (kg) (Somedic AB, Farsta, Sweden) | Upper trapezius: 2.41–2.56 Splenius capitis: 2.56–2.90 Levator scapulae: 2.07–2.38 | none |
| Rodríguez-Huguet M et al. | 2020 | Mechanical neck pain | Upper trapezius Suboccipital | Algometer (N/cm2) (Wagner Ins, Greenwich, CT, USA) | Upper trapezius: 1.46 Suboccipital: 1.20–1.22 | none |
| Alfawaz SS et al. | 2020 | Non-specific mechanical neck pain | Upper trapezius | Handheld algometer (N/cm2) (Force five™, Wagner Ins, Greenwich, CT, USA) | Upper trapezius: 4.0–4.9 | none |
| Chatchawan U et al. | 2019 | Chronic tension-type headache (CTTH) Episodic tension-type headaches (ETTH) | Head, neck, shoulder and upper back. | Manual algometer (kg/cm2) (Force Dial FDK/FDN Series Mechanical Force Gage; Wagner Ins, Greenwich, CT, USA). | Head, neck, shoulder and upper back: 0.7–1.2 | none |
| Wang-Price S et al. | 2018 | Neck-shoulder pain | Middle deltoid Levator scapulae Upper trapezius | Handheld computerized pressure algometer (kpa) (Medoc Ltd., Ramat Yishai, Israel) | Middle deltoid: 194.7–228.6 Levator scapulae: 244.1–246.5 Upper trapezius: 167.6–204.1 | Middle deltoid: 248.9–309.2 Levator scapulae: 313.7–322.0 Upper trapezius: 229.3–234.6 |
| Murray M et al. | 2017 | Neck-shoulder pain among military helicopter pilots and crew | Trapezius Upper neck extensors | Handheld electronic pressure algometer (kpa) (Type II Algometer, Somedic Production AB, Sweden) | Trapezius: 405–434 Upper neck extensors: 334–347 | none |
| De Meulemeester KE et al. | 2017 | Myofascial neck/shoulder pain | Upper and middle trapezius, levator scapulae, infraspinatus and supraspinatus | Wagner FPX Digital Algometer (kg/cm2) | Upper and middle trapezius, levator scapulae, infraspinatus and supraspinatus: 16.20–20.63 | none |
| Wassinger CA et al. | 2016 | Shoulder pain. | Shoulder | Electronic pressure algometer (kg/cm2) (Wagner Ins, Greenwich, CT, USA) | Shoulder: 5.67–5.73 | none |
| Toprak Celenay S et al. | 2016 | Neck pain | C7 and acromion at the middle point of the upper trapezius muscle | Digital algometer (kg/cm2) (JTech Medical Industries, ZEVEX Company) | Trapezius: 7.05–7.74 | none |
| Pajediene E et al. | 2015 | Whiplash associated pain | Upper part of the body | Hand-held pressure algometer (kg/cm2) (Pain TestTM Algometer, Wagner Force dial FDK 20) | Upper part of the body: 2.87 ± 1.21 | Upper part of the body: 3.71 ± 1.69 |
| Lopez-Lopez A et al. | 2015 | Chronic neck pain | C2 spinous process | Digital algometer (N/cm2) (FDX 25, Wagner Ins, Greenwich, CT, USA) | C2 spinous: 1.49–1.70 | none |
| Ge HY et al. | 2014 | Computer users with or without pain in the neck-shoulder and forearmregions | Most painful or dominant side of the neck-shoulder region | Digital algometer (kpa) (Somedic AB, Hörby, Sweden) | Most painful or dominant side of the neck-shoulder region: 203.4–308.6 | Most painful or dominant side of the neck-shoulder region: 238.7–337.1 |
| Llamas-Ramos R et al. | 2014 | Chronic neck pain | C7 spinous process | Mechanical algometer (kpa) (Pain Dignosis and Treatment Inc., New York, NY, USA) | C7 spinous process: 188.1 ± 49.4 | none |
| Andersen CH et al. | 2014 | Chronic neck/shoulder pain | Upper trapezius Lower trapezius | Pressure algometer (kpa) (Algometer Type 2, Somedic, Hörby, Sweden) | Upper trapezius: 277–303 Lower trapezius: 308–383 | none |
| Casanova-Méndez A et al. | 2014 | Chronic neck pain | Upper tarapezius C4 spinous process T4 spinous process | Analog pressure algometer (kg/cm2) (Baseline®, FEI Inc., White Plains, NY, USA) | C4 spinous process: 1.96–2.01 T4 spinous process: 3.35–3.70 Upper tripezius: 2.79–3.46 | none |
| Cagnie B et al. | 2013 | Office workers with mild neck and shoulder complaints | Triger point in Levator scapula/Upper Trapezius/Splenius cervicis | Electronic algometer (N) (compuFET; Hoggan Health Industries, Inc., West Jordan, UT, USA) | Triger point in Levator scapula/Upper Trapezius/Splenius cervicis: 16.2–24.5 | none |
| Yoo IG et al. | 2013 | Neck-shoulder pain | Upper trapezius Middle trapezius | Dolorimeter pressure algometer (lb) (Fabrication Enterprises, White Plains, NY, USA) | Upper trapezius: 7.2 ± 1.8 Middle trapezius: 5.8 ± 1.4 | Upper trapezius: 6.3 ± 2.0 Middle trapezius: 5.0 ± 1.2 |
| Lauche R et al. | 2013 | Chronic neck pain | Levator scapula Trapezius upper Semispinalis capitis | Digital algometer (kpa) (Somedic AB, Hörby, Sweden) | Levator scapula: 273.4–343.6 Trapezius upper: 229.0–273.7 Semispinalis capitis: 178.9–219.5 | none |
| Casanova-Méndez A et al. | 2013 | Chronic neck pain | C4 and T4 spinous process Trapezius | Analogue pressure algometer (kg/cm2) (Baseline, FEI Inc., White Plains, NY, USA) | C4 spinous process: 1.96–2.01 T4 spinous process: 3.35–3.70 Trapezius: 2.79–3.46 | none |
| Fernández-Pérez AM et al. | 2012 | Whiplash associated pain | Articular pillar of the C5–6 zygapophyseal joints | Electronic algometer (kpa) (Somedic AB, Sweden) | Articular pillar of the C5–6 zygapophyseal joints: 139.8–158.0 | Articular pillar of the C5–6 zygapophyseal joints: 205.4–212.7 |
| Andersen LL et al. | 2012 | Neck-shoulder pain | Upper Trapezius | Electronic pressure algometer (kpa) (Wagner Ins, greenwich, CT, USA) | Upper trapezius: 219–260 | none |
| Andersen LL et al. | 2012 | Neck/shoulder pain | Upper trapezius | Electronic pressure algometer (kpa) (Wagner Ins, Greenwich, CT, USA) | Trapezius: 219–260 | none |
| Ge HY et al. | 2009 | Fibromyalgia syndrome | Upper trapezius | Electronic algometer (kpa) (Somedic AB, Sweden) | Trapezius: 151–156 | Trapezius: 151–156 |
| Gerdle B et al. | 2008 | Whiplash associateddisorders | Trapezius | Electronic pressure algometer (kpa) (Somedic Algometer type 2, Sollentuna, Sweden) | Trapezius, low cervical and spraspinatus: 95–130 | Trapezius, low cervical and spraspinatus: 230–300 |
| Lemming D et al. | 2007 | Whiplash associated pain | Infraspinatus | Electronic algometer (kpa) (Somedic AB, Sweden) | Infraspinatus: 224.6–270.5 | none |
| Ylinen J et al. | 2007 | Chronic neck pain | Splenius capitis Trapezius Levator scapulae | Hand-held digital pressure algometer (N/cm2) (Force fiveTM, Wagner Instruments, Box 1217, Greenwich, CT 06836, USA) | Splenius capitis: 38.9–39.6 Trapezius: 38.3–40.3 Levator scapulae: 60.2–60.7 | none |
| Ylinen J et al. | 2007 | Chronic neck pain | Strenum Trapezius middle Lavator scapulae Trapezius upper | Handheld algometer (N/cm2) (Force five™, Wagner Ins, Greenwich, CT, USA) | Strenum: 31–34 Trapezius middle: 28–31 Lavator scapulae: 43–51 Trapezius upper: 27–31 | none |
| Ojala T et al. | 2006 | Neck-Shoulder Myofascial Pain Syndrome | Trigger point in neck and shoulder | Dolorimeter (kg/cm2) (Pain Diagnostic, Fisher). | Trigger point in neck and shoulder: 5.1–5.3 | none |
| Ylinen J et al. | 2005 | Chronic neck pain | Sternum Trapezius middle Levator scapulae Trapezius upper | Handheld electronic pressure algometer (N/cm2) (Force five™, Wagner Ins, Greenwich, CT, USA) | Strenum: 31–36 Trapezius middle: 28–38 Lavator scapulae: 45–59 Trapezius upper: 28–38 | none |
| Nabeta T et al. | 2002 | Neck/shoulder pain | Neck Shoulder | Pressure algometer (kg/cm2) (Yufu-Seiki, F P Meter, with probe of 10 mm) | Neck: 1.6–1.7 Shoulder: 2.0–2.4 | none |
| Waling K, Sundelin G et al. | 2000 | Work-related trapezius myalgia | Trapezius upper/middle/lower | Somedicw pressure algometer (kpa) (Somedic Production AB, Sollentuna, Sweden). | Trapezius upper/middle/lower: 194–253 | none |
| Taimela S et al. | 2000 | Chronic neck pain | Trapezius Levator Scapulae | Mechanical force gauge (N/cm2) | Trapezius: 25.9–29.6 Levator Scapulae: 40.9–41.7 | none |
| Otto A et al. | 2019 | Healthy volunteer | Deltoid Upper trapezius | Pressure algometer (kpa) (Somedic AB, Farsta, Sweden, probe size of 1 cm2 surface area) | none | Deltoid: 304.42 ± 103.42 Upper trapezius: 77.3 ± 126.90 |
| Sacramento LS et al. | 2017 | Healthy Children and Young Adults | Upper Trapezius Supraspinatus Infraspinatus Levator Scapulae Deltoid | Digital pressure algometer (kg/cm2) (OE-220, ITO Physiotherapy and Rehabilitation, Ito, Japan) | none | Children: Upper Trapezius: 1.4 ± 0.6 Supraspinatus: 2.0 ± 0.7 Infraspinatus: 2.2 ± 0.7 Deltoid: 2.1 ± 0.8 C5–6 joint: 1.3 ± 0.5 Adults: Upper Trapezius: 2.5 ± 0.9 Supraspinatus: 3.5 ± 1.3 Infraspinatus: 3.8 ± 1.3 Deltoid: 2.7 ± 1.1 C5–6 joint: 2.1 ± 0.7 |
| Wytrążek M et al. | 2014 | Healthy volunteerwithout triger points | Trapezius (upper part) n = 13 Sternocleido-mastoid n = 12 Deltoid (middle part) n = 48 Infraspinatus n = 13 | Algometer (kg/cm2) (Force Dial FDK/FDN Series Push Pull Force Gage; Wagner Instruments, Riverside, CT, USA) | none | Trapezius (upper part): 8.38–9.5 Sternocleido-mastoid: 5.92–6.33 Deltoid (middle part): 7.85–8.56 Infraspinatus: 8.32–10 |
| Shin SJ et al. | 2012 | Healthy worker | Upper trapezius | Dolorimeter (Lb) (Fabrication Enterprises, White Plains, NY, USA) | none | Upper trapezius: 7.3–8.8 |
| Binderup AT et al. | 2010 | Healthy volunteer | Upper trapezius Middle trapezius Lower trapezius Spinal processes | Hand-held algometer (kpa) (Somedic® Algometer type 2, Sweden) | none | Upper trapezius: 295.2 ± 95.9 Middle trapezius: 347.5 ± 103.5 Lower trapezius: 373.0 ± 121.1 Spinal processes: 369.6 ± 116.5 |
| Saíz-Llamosas JR et al. | 2009 | Healthy volunteer | C5-C6 zygapophyseal joints | Electronic algometer (kpa) (Somedic AB, Sweden) | none | C5-C6 zygapophyseal joints: 175.4–185.5 |
| Fernández-de-Las-Peñas C et al. | 2008 | Healthy volunteer | C5-C6 zygapophyseal joints | Electronic algometer (kpa) (Somedic AB, Sweden) | none | C5-C6 zygapophyseal joints: 308.4–334.5 |
| Ge HY et al. | 2006 | Healthy volunteer | Trapezius Posterolateral neck | Pressure algometer (kpa) (Somedic Algometer type 2, Sollentuna, Sweden) | none | Trapezius: 320–430 Posterolateral neck: 420–445 |
| Ge HY et al. | 2005 | Healthy volunteer | Trapezius Posterolateral neck | Pressure algometer (kpa) (Somedicw Algometer type 2, Sollentuna, Sweden) | none | Trapezius: 440–550 Posterolateral neck: 365–405 |
| Nie H et al. | 2005 | Healthy volunteer | Cervical muscle: processus transversus C5 Cervical myotendinous spot: processus transversus C7 Upper trapezius: middle point of processus spinosus C7 and acromion Levator scapulae: 2 cm superior to the angulus superior scapulae Angulus superior scapulae 1 cm medial to the acromioclavicular joint Supraspinatus: 3 cm superior to the middle of spina scapulae Infraspinatus: 3 cm distal to the middle of spina scapulae Middle trapezius: middle point of processus spinosus and medial border of spina scapulae Lower trapezius | Electronic pressure algometer (kpa) (Somedic Algometer type 2, Sweden) | none | Neck/shoulder: Average 322 |
| Authors | Object of Study/Subject | Areas of PPT Examination | Device (Units) | PPT Values in Patients/People with Low Back Pain; Mean (±SD) | PPT Values in Healthy Volunteers/Control; Mean (±SD) | |
|---|---|---|---|---|---|---|
| Zywien U et al. | 2022 | White-collar workers | Lumbar | FDIX RS232 algometer from Wagner (N/cm2) | Lumbar: 32.93–64.09 | Lumbar: 33.10–64.15 |
| Selva-Sarzo F et al. | 2021 | Chronic LBP | Lumbar | Wagner Force Dial FDK 20 algometer (kgf) | Lumbar: 3.68–6.83 | none |
| Dias LV et al. | 2021 | Chronic LBP | Bilaterally 5 cm from the spinal process of L3 and L5 | Pressure algometer (kgf) (EMG System® of Brazil). | L3: 3.5–5.2 L5: 3.6–5.3 | none |
| Nim CG et al. | 2021 | Non-specific LBP | L1 segment L2 segment L3 segment L4 segment L5 segment | Pressure algometer (kpa) (Model 2, Somedic, Sweden) | L1 segment: 522 ± 244 L2 segment: 482 ± 228 L3 segment: 472 ± 225 L4 segment: 455 ± 225 L5 segment: 445 ± 229 | none |
| Mailloux et al. | 2021 | Healthy volunteers | Lumbar erector spinae (LES) 2–3 cm laterally to L4/L5 S1 spinous process | Handheld digital algometer (kpa) (1-cm2 probe–FPIX, Wagner Instruments, Greenwich, CT, USA) | none | LES: 547.2–559.7 S1 spinous process: 517.2–536.7 |
| Leemans L et al. | 2020 | Chronic LBP | 2 cm lateral to the L3 spinous process 2 cm lateral to the L5 spinous process Near the poste-rior superior iliac spines (PSIS) | Digital pres-sure algometer (kgf) (Wagner Force Ten) | Lower back: 6.7–7.0 | none |
| Nim CG et al. | 2020 | Non-specific LBP | Lumbar | Pressure algometer (kpa) (Model 2, Somedic, Sweden) | Lumbar (with pain): 488.73 ± 330.95 Lumbar (with stiffness): 436.6 ± 364.9 | none |
| Volpato MP et al. | 2020 | Chronic non-specific LBP | BL23 (Shenshu) BL24 (Qihaishu) BL25 (Dachangshu) | Pressure algometer (unknown) (EMG 830C, EMG System, São José dos Campos, Brazil) | BL23 (Shenshu): 7025–7844 BL24 (Qihaishu): 7258–7285 BL25 (Dachangshu): 6445–7173 | none |
| Wang-Price S et al. | 2020 | LBP | Lumbar | Hand-held computerized pressure algometer (kpa) (Medoc Ltd., Ramat Yishai, Israel) | Lumbar: 383.4 ± 185.5 | none |
| Vaegter HB et al. | 2020 | LBP | Erector spinae muscle | Manual pressure algometry (kpa) (Somedic Sales AB) | Erector spinae muscle: 450–586 | none |
| Fagundes Loss J et al. | 2020 | LBP | Spinal Process/Erector | 10 kgf analogic pressure algometer (kgf) (Wagner Instruments, Greenwich, CT-USA) | Spinal Process: 6.1–7.0 Erector: 6.8–7.2 | none |
| Plaza-Manzano G et al. | 2020 | Lumbar radiculopathy | Common peroneal Tibialis | Mechanical pressure algometer (kg/cm2) (Pain Diagnosis and Treatment Inc., New York) | Common peroneal: 2.1–2.3 Tibialis: 3.2–3.4 | none |
| Moreira RFC et al. | 2020 | Hospital nursing assistants with LBP | Dorsal longissimus | Hand-held algometer (kgf/cm2) (Pain Diagnostic Treatment, New York, USA) | Dorsal longissimus: 5.52–6.55 | none |
| Bond BM et al. | 2020 | Non-specific LBP | Lumbar Paraspinal Musculature | Digital algometer (kg/cm2) (Wagner Instruments, Greenwich, Connecticut) | Lumbar Paraspinal Musculature: 3.36–3.39 | none |
| Chapman KB et al. | 2020 | Chronic LBP | The most painful side of the back | Pressure algometer (N/cm2) (Wagner Instruments, Greenwich, CT, USA) | The most painful side of the back: 28.7 ± 4.1 | none |
| Aspinall SL et al. | 2020 | Chronic LBP | Lumber | Digital pressure algometer (kg/cm2) (FPIX 50, Wagner Instruments, Connecticut, USA) | Lumber: 4.1–4.4 | Lumber: 5.5 ± 4.1 |
| Aspinall SL et al. | 2020 | LBP | Lumber | Digital pressure algometer (kg/cm2) (FPIX 50, Wagner Instruments, Connecticut, USA) | Lumber: 4.14–4.30 | none |
| Petersson M et al. | 2020 | Healthy volunteers | Spinous processes from L1 to L5 (L1-L2, L2-L3, L3-L4 and L4-L5). | Pressure algometer (kpa) (SOMEDIC Electronics brand, Solna, Sweden) | none | Spinous processes L1-L2: 353–405 L2-L3: 319–361 L3-L4: 299–370 L4-L5: 306–321 |
| Aspinall SL et al. | 2019 | LBP | Lumber | Digital pressure algometer (kg/cm2) (FPIX 50, Wagner Instruments, Connecticut, USA) | Lumber: 5.3 ± 3.3 | none |
| Alfieri FM et al. | 2019 | Non-specific LBP | ParavertebralL4–5 | J Tech algometer (lb) (Salt Lake City, UT, USA) | Paravertebral: 8.1–8.3 L4–5: 8.3 ± 3.6 | Paravertebral: 12.9–13.1 L4–5: 12.8 ± 5.6 |
| McPhee ME et al. | 2019 | Recurrent LBP | L1 and L5 (3.5 cm lateral to the L1 and L5 spinous processes) | Rubbertipped handheld pressure algometer (kpa) (Somedic, Norra Mellby, Sweden) | L1: 405 L5: 415 | L1: 550 L5: 560 |
| Kołcz A et al. | 2019 | Professionally active nurses | Erector spinae muscle | AlgoMed FPIX 50 (Medoc, Yishai, Israel) | none | Erector spinae muscle: 30 ± 1.8 |
| Marcuzzi A et al. | 2018 | Acute LBP | Back | Pressure algometer (kpa) (FDK40; Wagner Instrument, Greenwich, CT) | Back: 2.6–2.7 | Back: 2.2 ± 0.3 |
| de Carvalho RC et al. | 2018 | LBP | 2 cm lateral to the L1, 3, and 5 spinous process | Pressure algometer (unknown) (EMG 830C, EMG System, São José dos Campos, Brazil) | 2 cm lateral to the L1, 3, and 5 spinous process: 5.29–5.68 | none |
| Bodes Pardo G et al. | 2018 | Chronic LBP | Spinal process of L3 | Fisher algometer (kg/cm2) (Force Dial model FDK 40) | Spinal process of L3: 2.8–3.0 | none |
| Joseph LH et al. | 2018 | Elite female weight lifters with Chronic non-specific LBP | Lumbar region | Pressure algometer (kpa) (Algometer type II, Somedic SenseLab AB, Sweden) | Lumbar region: 472.28–440.64 | none |
| O’Neill S et al. | 2018 | Non-specific LBP | Back-Lumbar | Pressure algometer (kpa) (Somedic model 2, 1 cm2 probe, Hørby, Sweden), | Back-Lumbar: 322 ± 196 | Back-Lumbar: 398 ± 194 |
| Yildiz SH et al. | 2017 | Chronic LBP | L1 Paravertebral level L3 Paravertebral L5 Paravertebral | Manual algometer (unknown) | L1 Paravertebral: 6.7–7.3 L3 Paravertebral: 6.8–7.3 L5 Paravertebral: 6.7–7.2 | L1 Paravertebral: 7.6–9.5 L3 Paravertebral: 7.4–9.4 L5 Paravertebral: 7.6–9.5 |
| Shane LG et al. | 2017 | LBP | L3, L4, and L5 paraspinal muscles | Digital pressurealgometer (N/cm2) (Wagner Force Ten FDX, Wagner Instruments, Greenwich, CT) | L3, L4, and L5 paraspinal muscles: 6.32–6.59 | none |
| Paungmali A et al. | 2017 | Chronic non-specific LBP | Lumbar region | Pressure algometer (kpa) (Algometer type II; Somedic Production AB, Sollentuna, Sweden) | Lumbar region: 509.8 ± 133.3 | none |
| Mohanty PP et al. | 2017 | Coccydynia | Coccygeal region | Modified syringe algometer (unknown) | Coccygeal region: 2.2–2.5 | none |
| Calvo-Lobo C et al. | 2017 | Myofascial pain syndrome | Lumbar erector spinae muscles | Manual mechanical algometer (kg/cm2) (FDK/FDN, Wagner Instruments, 1217 Greenwich, CT 06836) | N = 20 (Active trigger points): 2.97 ± 0.82 N = 20 (Latent trigger points): 3.56 ± 0.77 | N = 20 (Controls points): 4.49 ± 0.90 |
| Nothnagel H et al. | 2017 | Healthy volunteers | Lumbar paraspinal | Pressure gauge device (kpa) (FDN200, Wagner Instruments, Greenwich, CT, USA) | none | Lumbar paraspinal: 589.68–628.32 |
| Farasyn A et al. | 2016 | Chronic non-specific LBP | Erector spinae T8 Erector spinae T10 Erector spinae L1 Erector spinae L3 Gluteus maximus pars superior Gluteus maximus pars inferior | Electric pressure algometer (kg/cm2) (MYOMETER, Penny & Giles, U.K.), | Erector spinae T8: 3.96 ± 1.30 Erector spinae T10: 3.73 ± 1.10 Erector spinae L1: 3.71 ± 1.20 Erector spinae L3: 5.29 ± 1.27 Gluteus maximus pars superior: 3.73 ± 1.17 Gluteus maximus pars inferior: 3.84 ± 0.94 | Erector spinae T8: 7.03 ± 1.50 Erector spinae T10: 7.77 ± 1.31 Erector spinae L1: 8.69 ± 1.66 Erector spinae L3: 9.86 ± 1.41 Gluteus maximus pars superior: 9.10 ± 1.83Gluteus maximus pars inferior: 8.81 ± 2.01 |
| Imamura M et al. | 2016 | Chronic non-specific LBP | Gluteus medius middle portion Gluteus medius posterior portion Gluteus minimus Gluteus maximus Piriformis Quadratus Lumborum Iliopsoas Ligaments T12-L1/ L1-L2/ L2-L3/L3-L4/L4-L5 L5-S1/S1-S2/S2-S3 | Pressure algometer (kg/cm2) (Pain Diagnostics, Great Neck, NY). | Gluteus medius middle portion: 5.08 ± 2.12 Gluteus medius posterior portion: 4.92 ± 1.95 Gluteus minimus: 5.36 ± 2.20 Gluteus maximus: 5.20 ± 2.25 Piriformis: 5.70 ± 2.60 Quadratus Lumborum: 4.61 ± 1.85 Iliopsoas: 3.97 ± 1.78 Ligaments T12-L1: 5.09 ± 2.63 L1-L2: 4.97 ± 2.71 L2-L3: 4.96 ± 2.73 L3-L4: 4.93 ± 2.58 L4-L5: 4.74 ± 2.22 L5-S1: 4.83 ± 2.54 S1-S2: 5.00 ± 2.91 S2-S3: 5.25 ± 2.91 | none |
| Balaguier R et al. | 2016 | Healthy volunteers | Lumbar spinal processes L1-L5 | Somedic Algometer (kpa) (Type 2, Sollentuna, Sweden) | none | Lumbar spinal processes L1: 593.4–654.6 L2: 616.0–664.1 L3: 573.4–638.6 L4: 560.9–657.6 L5: 607.5–653.9 |
| Weinkauf B et al. | 2015 | Healthy volunteers | Lumbar | Homogeneous pressure (kpa) (Wagner Instruments, USA) | none | Lumbar: 703 ± 24 |
| Falla D et al. | 2014 | Chronic non-specific LBP | 8 locations at lumbar | Electronic algometer (kpa) (Somedic Production, Stockholm, Sweden) | 8 locations at lumbar: 268.0 ± 165.9 | 8 locations at lumbar: 320.1 ± 162.1 |
| Imamura M et al. | 2013 | Chronic LBP | Suprainterspinous ligaments situated between T12–L/L1–L2/L2–L3/L3–L4/L4–L5/L5–S1/S1–S2/S2–S3 | Pressure algometer (kg/cm2) (Pain Diagnostics, Great Neck, NY) | Suprainterspinous ligaments situated between T12–L: 5.06 ± 2.47 L1–L2: 4.84 ± 2.23 L2–L3: 4.64 ± 2.05 L3–L4: 4.83 ± 2.19 L4–L5: 4.65 ± 1.74 L5–S1: 5.40 ± 3.20 S1–S2: 5.77 ± 3.55 S2–S3: 5.71 ± 3.51 | Suprainterspinous ligaments situated between T12–L: 7.16 ± 2.53 L1–L2: 7.29 ± 2.21 L2–L3: 7.49 ± 2.01 L3–L4: 7.46 ± 2.57 L4–L5: 8.10 ± 2.46 L5–S1: 8.38 ± 2.40 S1–S2: 8.89 ± 2.63 S2–S3: 8.75 ± 2.18 |
| de Oliveira RF et al. | 2013 | Chronic non-specific LBP | L3/L5 spinous process | Pressure algometer (N) (Kratos model DDK, Kratos Ltd., São Paulo, Brazil) | L3/L5 spinous process: 48.90–49.63 | none |
| Neziri AY et al. | 2012 | Chronic LBP | Site of most severe pain at low back Nonpainful site at low back | Electronic pressure algometer (kpa) (Somedic, Hörby, Sweden) | Site of most severe pain at low back: 168 ± 113 Nonpainful site at low back: 249 ± 132 | Site of most severe pain at low back: 352 ± 131 Nonpainful site at low back: 352 ± 131 |
| Zheng Z et al. | 2012 | Chronic non-specific LBP | Lumbar tender point | Model OE-220, made in Ito ultrashort wave corporation of Japan (kg/cm2) | Lumbar tender point: 3.7–3.8 | none |
| McSweeney TP et al. | 2012 | Healthy volunteers | Paravertebral soft tissue at L1 | Handheld manual digital pressure algometer (N) (Wagner FPX 25) | none | Paravertebral soft tissue at L1: 53.7–60.1 |
| Yu X et al. | 2012 | Healthy volunteers | L5-S1 zygapophyseal joints | Mechanical pressure algometer (kg/cm2) (Wagner, Greenwich, CT) | none | L5-S1 zygapophyseal joints: 4.87–5.01 |
| O’Neill S et al. | 2011 | Chronic LBP | Spinous process of L4 | Pressure algometer (kpa) (Model 2, Somedic, Sweden) | Spinous process of L4: 677 | Spinous process of L4: 755 |
| Binderup AT et al. | 2011 | Healthy volunteers (cleaners) | Spinal processes L1–5 Low back Erector Spinae | Hand-held pressure algometer (kpa) (Somedic Algometer type 2, Sweden) | none | Spinal processes L1–5: 427.9 ± 204.7 Erector Spinae: 409.8 ± 194.5 |
| Hirayama J et al. | 2006 | Lumbar disc herniation | 2 cm lateral to the L1, 3 and 5 spinous process 5 cm lateral to the L1 and 3 spinous process | Electronic pressure algometer (kpa) (Somedic, Farsta, Sweden) | 2 cm lateral to the L1: 451.4 ± 214.8 L3: 348.5 ± 179.6 L5: 267.7 ± 105.1 5 cm lateral to the L1: 325.6 ± 133.0 L3: 297.1 ± 157.2 | 2 cm lateral to the L1: 438.1–441.3 L3: 418.5–424.5 L5: 395.5–401.3 5 cm lateral to the L1: 331.8–362.1 L3: 314.0–335.3 |
| Farasyn A et al. | 2005 | Subacute non-specific LBP | Erector spinae mass T6, T10, L1, L3 and L5 Gluteus maximus Gluteus medius Tensor fasciae latae (TFL). | Mechanical Fischer pressure algometer (kg/cm2) (Pain Diagnostics and Thermography, Great Neck, NY, USA) | Erector spinae mass T6: 6.7 ± 1.8 T10: 6.6 ± 1.1 L1: 6.4 ± 1.2 L3: 5.3 ± 14 L5: 7.2 ± 1.6 Gluteus maximus: 6.4 ± 1.6 Gluteus medius: 6.1 ± 1.6 TFL: 6.3 ± 1.5 | Erector spinae mass T6: 7.6 ± 1.1 T10: 7.4 ± 1.1 L1: 7.4 ± 1.2 L3: 7.7 ± 1.7 L5: 9.5 ± 1.2 Gluteus maximus: 8.0 ± 1.5 Gluteus medius: 7.2 ± 1.5 TFL: 7.1 ± 1.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suzuki, H.; Tahara, S.; Mitsuda, M.; Izumi, H.; Ikeda, S.; Seki, K.; Nishida, N.; Funaba, M.; Imajo, Y.; Yukata, K.; et al. Current Concept of Quantitative Sensory Testing and Pressure Pain Threshold in Neck/Shoulder and Low Back Pain. Healthcare 2022, 10, 1485. https://doi.org/10.3390/healthcare10081485
Suzuki H, Tahara S, Mitsuda M, Izumi H, Ikeda S, Seki K, Nishida N, Funaba M, Imajo Y, Yukata K, et al. Current Concept of Quantitative Sensory Testing and Pressure Pain Threshold in Neck/Shoulder and Low Back Pain. Healthcare. 2022; 10(8):1485. https://doi.org/10.3390/healthcare10081485
Chicago/Turabian StyleSuzuki, Hidenori, Shu Tahara, Mao Mitsuda, Hironori Izumi, Satoshi Ikeda, Kazushige Seki, Norihiro Nishida, Masahiro Funaba, Yasuaki Imajo, Kiminori Yukata, and et al. 2022. "Current Concept of Quantitative Sensory Testing and Pressure Pain Threshold in Neck/Shoulder and Low Back Pain" Healthcare 10, no. 8: 1485. https://doi.org/10.3390/healthcare10081485
APA StyleSuzuki, H., Tahara, S., Mitsuda, M., Izumi, H., Ikeda, S., Seki, K., Nishida, N., Funaba, M., Imajo, Y., Yukata, K., & Sakai, T. (2022). Current Concept of Quantitative Sensory Testing and Pressure Pain Threshold in Neck/Shoulder and Low Back Pain. Healthcare, 10(8), 1485. https://doi.org/10.3390/healthcare10081485







