Probiotics for the Prevention of Antibiotic-Associated Diarrhea
Abstract
1. Introduction
2. History of Probiotics
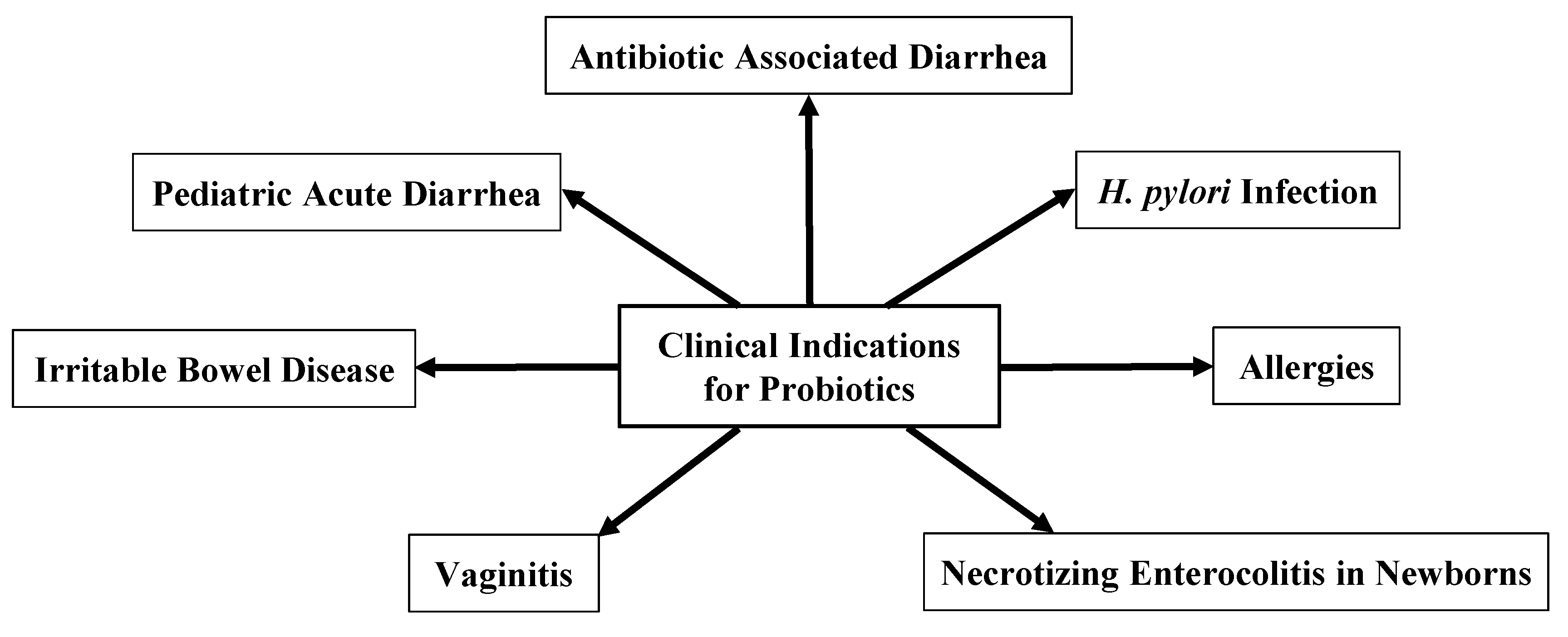
3. Clinical Uses for Probiotics
4. Antibiotic-Associated Diarrhea
- (i)
- Altering the diversity of gut bacteria: While antibiotics kill and target pathogens, they also impact the symbiotic bacteria integral to the gut microbiome. This decrease in bacterial diversity in the GI tract can drastically alter the immunological ecosystem. This puts the patient at greater risk of opportunistic infections and allows pathogens to competitively outcompete other bacteria [23,24,25].
- (ii)
- Age of patient: While AAD can occur in any patient population, the pediatric population is particularly at risk. Since the infant microbiome is not fully developed, antibiotic use in this population can cause a longer, more drastic effect on the microbiome, including an increase in Proteobacteria and a decrease in the diversity of Actinobacteria [24].
- (iii)
- Spectrum of antibiotics: Antibiotic characteristics such as their mechanism of action, pharmacokinetics, and dosage can not only affect the targeted pathogen but can also lead to unintended consequences such as AAD. Broad-spectrum antibiotics such as clindamycin, which are particularly active against anaerobes, are associated with higher rates of AAD. On the other hand, narrow-spectrum antibiotics typically produce lower rates of AAD [26,27].
- (iv)
- Metabolic disturbances: The gut microbiome plays an important role in nutrition and metabolism. While most carbohydrates are absorbed in the small intestine, some carbohydrates are fermented by the bacteria and turned into short-chain fatty acids (SCFAs). When antibiotics kill and lyse these bacteria, excess amounts of non-absorbable carbohydrates remain in the gut. These non-absorbable carbohydrates pull in water by osmosis as they move towards the large intestine. This leads to the development of osmotic diarrhea [27].
- (v)
- Loss of colonization resistance: Colonization resistance is the ability of bacteria to prevent pathogenic microbes from invading. The gut microbiome regulates many metabolites including bile acids, carbohydrates, and amino acids. These metabolites help to defend against pathogens. One example is the regulation of Clostridium difficile through secondary bile acids. Secondary bile acids are produced by gut bacteria and inhibit C. difficile growth. Antibiotics destroy the gut microbiome, leading to the diminishment of secondary bile acids. This then allows C. difficile to flourish [27].
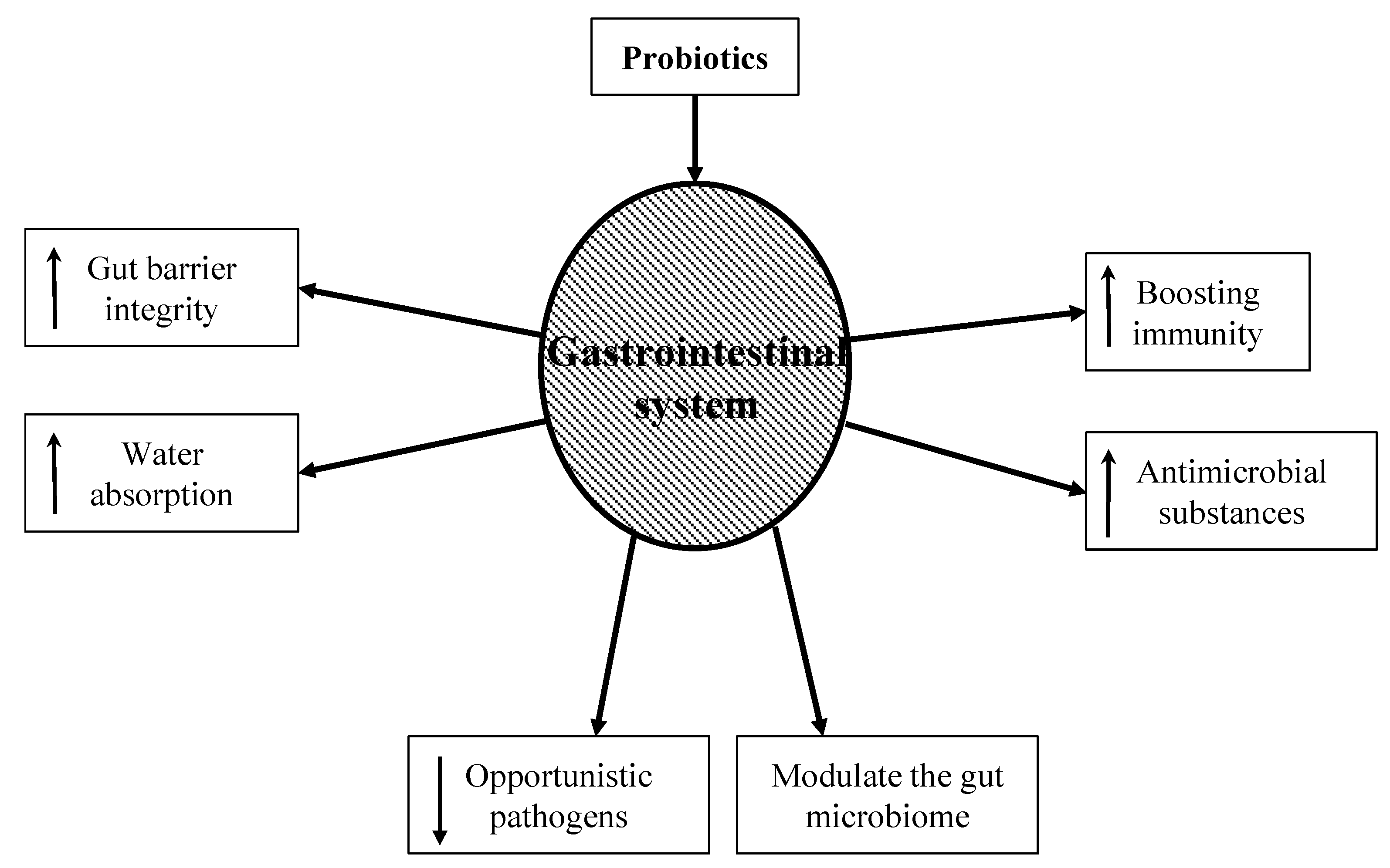
5. Probiotics in Preventing AAD
- (i)
- Boosting immunity: While the exact mechanism is still unknown, probiotic bacteria have been shown to boost the humoral immune response by increasing the numbers of IgM-, IgG-, and IgA-secreting cells. They also stimulate nonspecific immune responses such as activating macrophages [34].
- (ii)
- Increasing gut barrier integrity: The intestinal barrier is a heterogeneous system composed of a mucus layer, epithelium, and the underlying lamina propria. These create a physical barrier to gut microbes using multi-protein complexes called tight junctions. When the tight junctions are compromised, epithelium permeability increases, causing a leaky gut. A leaky gut is responsible for the development of many gastrointestinal conditions, such as irritable bowel syndrome, irritable bowel disease, and celiac disease. Probiotics can upregulate ZO-1 and occludin protein synthesis, thus protecting the integrity of the gut barrier [35].
- (iii)
- Producing antimicrobial substances: Probiotics produce a variety of substances that can be inhibitory to both gram-positive and gram-negative bacteria. These substances include hydrogen peroxide, bacteriocins, and organic acids. This can not only reduce the number of pathogenic bacteria but can also alter bacterial metabolism and limit toxin production [36,37].
- (iv)
- Modulating the gut microbiome: Probiotic use has been shown to re-equilibrate gut microbiome dysbiosis. Dysbiosis can occur when a patient is exposed to severe conditions such as prolonged antibiotic therapy, intense physical stress, and chronic illness. Probiotics metabolize complex carbohydrates and produce lactic acid and short-chain fatty acids. This reduces bacterial translocation, improves tight junction integrity, and stimulates mucin production [38].
- (v)
- Increasing water absorption: Aquaporins are water-channel membrane proteins expressed in many tissues with AQP1, 3, 4, and 8 mostly expressed in the colon. Pathogenic bacteria can disrupt these proteins, increase the water content in stool, and lead to dehydration. Probiotics have been shown to increase the expression of aquaporins and thus increase water absorption in the colon [39].
- (vi)
- Decreasing opportunistic pathogens: Probiotics decrease the number of pathogenic bacteria by producing inhibitory substances such as bacteriocins, blocking adhesion sites on the intestinal epithelial surfaces, and competing for nutrients. These mechanisms are important for prophylaxis and the treatment of infections. The ability of probiotics to co-aggregate can lead to a protective barrier that prevents pathogenic bacteria to colonize the epithelium [40].
6. Randomized Controlled Trials (RCT)
7. Probiotics for Reducing C. difficile-Associated Diarrhea
8. Considerations for Probiotics Use
9. Safety of Probiotics
10. Practicality and the Current Clinical Practice of Using Probiotics in AAD
11. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- van den Nieuwboer, M.; Van De Burgwal, L.H.; Claassen, E. A quantitative key-opinion-leader analysis of innovation barriers in probiotic research and development: Valorisation and improving the tech transfer cycle. PharmaNutrition 2015, 4, 9–18. [Google Scholar] [CrossRef]
- Gasbarrini, G.; Bonvicini, F.; Gramenzi, A. Probiotics History. J. Clin. Gastroenterol. 2016, 50 (Suppl. 2), S116–S119. [Google Scholar] [CrossRef] [PubMed]
- Lilly, D.M.; Stillwell, R.H. Probiotics: Growth-Promoting Factors Produced by Microorganisms. Science 1965, 147, 747–748. [Google Scholar] [CrossRef] [PubMed]
- McFarland, L.V. From yaks to yogurt: The history, development, and current use of probiotics. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2015, 60 (Suppl. 2), S85–S90. [Google Scholar] [CrossRef]
- Martin, R.; Langella, P. Emerging Health Concepts in the Probiotics Field: Streamlining the Definitions. Front. Microbiol. 2019, 10, 1047. [Google Scholar] [CrossRef] [PubMed]
- Tamime, A.Y. Fermented milks: A historical food with modern applications—A review. Eur. J. Clin. Nutr. 2002, 56 (Suppl. 4), S2–S15. [Google Scholar] [CrossRef] [PubMed]
- Barnett, J.A. A history of research on yeasts 2: Louis Pasteur and his contemporaries, 1850–1880. Yeast 2000, 16, 755–771. [Google Scholar] [CrossRef]
- Metchnikoff, E. Lactic acid as inhibiting intestinal putrefaction. In The Prolongation of Life. Optimistic Studies; G.P. Putnam’s Sons: New York, NY, USA, 1908; Chapter V; pp. 161–183. [Google Scholar]
- Tissier, H. Treatment of intestinal infections using bacterial flora of the intestine. Crit Rev. Soc. Biol. 1906, 60, 359–361. [Google Scholar]
- FAO/WHO. Guidelines for the Evaluation of Probiotics in Food; Joint FAO/WHO Working Group: London, ON, Canada, 2002.
- Blander, J.M.; Longman, R.S.; Iliev, I.D.; Sonnenberg, G.F.; Artis, D. Regulation of inflammation by microbiota interactions with the host. Nat. Immunol. 2017, 18, 851–860. [Google Scholar] [CrossRef]
- Savidge, T.C. Epigenetic Regulation of Enteric Neurotransmission by Gut Bacteria. Front. Cell. Neurosci. 2015, 9, 503. [Google Scholar] [CrossRef]
- Magnusdottir, S.; Ravcheev, D.; de Crecy-Lagard, V.; Thiele, I. Systematic genome assessment of B-vitamin biosynthesis suggests co-operation among gut microbes. Front. Genet. 2015, 6, 148. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Brady, A.; Jones, C.; Song, Y.; Darton, T.C.; Blohmke, C.J.; Pollard, A.J.; Magder, L.S.; Fasano, A.; Sztein, M.B.; et al. Compositional and Functional Differences in the Human Gut Microbiome Correlate with Clinical Outcome following Infection with Wild-Type Salmonella enterica Serovar Typhi. mBio 2018, 9, e00686-18. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, T.; Sequoia, J. Probiotics for Gastrointestinal Conditions: A Summary of the Evidence. Am. Fam. Physician 2017, 96, 170–178. [Google Scholar] [PubMed]
- Goderska, K.; Agudo Pena, S.; Alarcon, T. Helicobacter pylori treatment: Antibiotics or probiotics. Appl. Microbiol. Biotechnol. 2018, 102, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Losurdo, G.; Cubisino, R.; Barone, M.; Principi, M.; Leandro, G.; Ierardi, E.; Di Leo, A. Probiotic monotherapy and Helicobacter pylori eradication: A systematic review with pooled-data analysis. World J. Gastroenterol. 2018, 24, 139–149. [Google Scholar] [CrossRef]
- Islam, S.U. Clinical Uses of Probiotics. Medicine 2016, 95, e2658. [Google Scholar] [CrossRef]
- Shenoy, A.; Gottlieb, A. Probiotics for oral and vulvovaginal candidiasis: A review. Dermatol. Ther. 2019, 32, e12970. [Google Scholar] [CrossRef]
- Sniffen, J.C.; McFarland, L.V.; Evans, C.T.; Goldstein, E.J.C. Choosing an appropriate probiotic product for your patient: An evidence-based practical guide. PLoS ONE 2018, 13, e0209205. [Google Scholar] [CrossRef]
- Suez, J.; Zmora, N.; Segal, E.; Elinav, E. The pros, cons, and many unknowns of probiotics. Nat. Med. 2019, 25, 716–729. [Google Scholar] [CrossRef]
- McFarland, L.V. Diarrhoea associated with antibiotic use. BMJ 2007, 335, 54–55. [Google Scholar] [CrossRef]
- Milner, E.; Stevens, B.; An, M.; Lam, V.; Ainsworth, M.; Dihle, P.; Stearns, J.; Dombrowski, A.; Rego, D.; Segars, K. Utilizing Probiotics for the Prevention and Treatment of Gastrointestinal Diseases. Front. Microbiol. 2021, 12, 689958. [Google Scholar] [CrossRef] [PubMed]
- Ianiro, G.; Tilg, H.; Gasbarrini, A. Antibiotics as deep modulators of gut microbiota: Between good and evil. Gut 2016, 65, 1906–1915. [Google Scholar] [CrossRef] [PubMed]
- Mekonnen, S.A.; Merenstein, D.; Fraser, C.M.; Marco, M.L. Molecular mechanisms of probiotic prevention of antibiotic-associated diarrhea. Curr. Opin. Biotechnol. 2020, 61, 226–234. [Google Scholar] [CrossRef] [PubMed]
- McFarland, L.V.; Surawicz, C.M.; Stamm, W.E. Risk factors for Clostridium difficile carriage and C. difficile-associated diarrhea in a cohort of hospitalized patients. J. Infect. Dis. 1990, 162, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Silverman, M.A.; Konnikova, L.; Gerber, J.S. Impact of Antibiotics on Necrotizing Enterocolitis and Antibiotic-Associated Diarrhea. Gastroenterol. Clin. N. Am. 2017, 46, 61–76. [Google Scholar] [CrossRef]
- McFarland, L.V. Epidemiology, risk factors and treatments for antibiotic-associated diarrhea. Dig. Dis. 1998, 16, 292–307. [Google Scholar] [CrossRef]
- Goossens, H.; Ferech, M.; Vander Stichele, R.; Elseviers, M. Outpatient antibiotic use in Europe and association with resistance: A cross-national database study. Lancet 2005, 365, 579–587. [Google Scholar] [CrossRef]
- Hayes, S.R.; Vargas, A.J. Probiotics for the Prevention of Pediatric Antibiotic-Associated Diarrhea. Explore 2016, 12, 463–466. [Google Scholar] [CrossRef]
- Mills, J.P.; Rao, K.; Young, V.B. Probiotics for prevention of Clostridium difficile infection. Curr. Opin. Gastroenterol. 2018, 34, 3–10. [Google Scholar] [CrossRef]
- Hempel, S.; Newberry, S.J.; Maher, A.R.; Wang, Z.; Miles, J.N.; Shanman, R.; Johnsen, B.; Shekelle, P.G. Probiotics for the prevention and treatment of antibiotic-associated diarrhea: A systematic review and meta-analysis. JAMA 2012, 307, 1959–1969. [Google Scholar] [CrossRef]
- Wieers, G.; Belkhir, L.; Enaud, R.; Leclercq, S.; Philippart de Foy, J.M.; Dequenne, I.; de Timary, P.; Cani, P.D. How Probiotics Affect the Microbiota. Front. Cell. Infect. Microbiol. 2019, 9, 454. [Google Scholar] [CrossRef]
- Isolauri, E.; Sutas, Y.; Kankaanpaa, P.; Arvilommi, H.; Salminen, S. Probiotics: Effects on immunity. Am. J. Clin. Nutr. 2001, 73, 444S–450S. [Google Scholar] [CrossRef] [PubMed]
- La Fata, G.; Weber, P.; Mohajeri, M.H. Probiotics and the Gut Immune System: Indirect Regulation. Probiotics Antimicrob. Proteins 2018, 10, 11–21. [Google Scholar] [CrossRef]
- Atassi, F.; Servin, A.L. Individual and co-operative roles of lactic acid and hydrogen peroxide in the killing activity of enteric strain Lactobacillus johnsonii NCC933 and vaginal strain Lactobacillus gasseri KS120.1 against enteric, uropathogenic and vaginosis-associated pathogens. FEMS Microbiol. Lett. 2010, 304, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Zavisic, G.; Petricevic, S.; Radulovic, Z.; Begovic, J.; Golic, N.; Topisirovic, L.; Strahinic, I. Probiotic features of two oral Lactobacillus isolates. Braz. J. Microbiol. [Publ. Braz. Soc. Microbiol.] 2012, 43, 418–428. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.; DuPont, H.L. New approaches for bacteriotherapy: Prebiotics, new-generation probiotics, and synbiotics. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2015, 60 (Suppl. 2), S108–S121. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhu, B.; Xu, J.; Liu, Y.; Qiu, E.; Li, Z.; He, Y.; Zhou, H.; Bai, Y.; Zhi, F. Bacteroides fragilis Protects Against Antibiotic-Associated Diarrhea in Rats by Modulating Intestinal Defenses. Front. Immunol. 2018, 9, 1040. [Google Scholar] [CrossRef]
- Rolfe, R.D. The role of probiotic cultures in the control of gastrointestinal health. J. Nutr. 2000, 130, 396S–402S. [Google Scholar] [CrossRef]
- Goldenberg, J.Z.; Lytvyn, L.; Steurich, J.; Parkin, P.; Mahant, S.; Johnston, B.C. Probiotics for the prevention of pediatric antibiotic-associated diarrhea. Cochrane Database Syst. Rev. 2015, 12, CD004827. [Google Scholar] [CrossRef] [PubMed]
- Blaabjerg, S.; Artzi, D.M.; Aabenhus, R. Probiotics for the Prevention of Antibiotic-Associated Diarrhea in Outpatients-A Systematic Review and Meta-Analysis. Antibiotics 2017, 6, 21. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.; Scott, K.; Klaenhammer, T.R.; Quigley, E.; Sanders, M.E. Probiotic nomenclature matters. Gut Microbes 2016, 7, 1–2. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chatterjee, S.; Kar, P.; Das, T.; Ray, S.; Gangulyt, S.; Rajendiran, C.; Mitra, M. Randomised placebo-controlled double blind multicentric trial on efficacy and safety of Lactobacillus acidophilus LA-5 and Bifidobacterium BB-12 for prevention of antibiotic-associated diarrhoea. J. Assoc. Physicians India 2013, 61, 708–712. [Google Scholar] [PubMed]
- Glanville, J.; King, S.; Guarner, F.; Hill, C.; Sanders, M.E. A review of the systematic review process and its applicability for use in evaluating evidence for health claims on probiotic foods in the European Union. Nutr. J. 2015, 14, 16. [Google Scholar] [CrossRef]
- de Vrese, M.; Kristen, H.; Rautenberg, P.; Laue, C.; Schrezenmeir, J. Probiotic lactobacilli and bifidobacteria in a fermented milk product with added fruit preparation reduce antibiotic associated diarrhea and Helicobacter pylori activity. J. Dairy Res. 2011, 78, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Tankanow, R.M.; Ross, M.B.; Ertel, I.J.; Dickinson, D.G.; McCormick, L.S.; Garfinkel, J.F. A double-blind, placebo-controlled study of the efficacy of Lactinex in the prophylaxis of amoxicillin-induced diarrhea. DICP Ann. Pharmacother. 1990, 24, 382–384. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.N.; Kim, N.; Lee, S.H.; Park, Y.S.; Hwang, J.H.; Kim, J.W.; Jeong, S.H.; Lee, D.H.; Kim, J.S.; Jung, H.C.; et al. The effects of probiotics on PPI-triple therapy for Helicobacter pylori eradication. Helicobacter 2008, 13, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Vanderhoof, J.A.; Whitney, D.B.; Antonson, D.L.; Hanner, T.L.; Lupo, J.V.; Young, R.J. Lactobacillus GG in the prevention of antibiotic-associated diarrhea in children. J. Pediatr. 1999, 135, 564–568. [Google Scholar] [CrossRef]
- Arvola, T.; Laiho, K.; Torkkeli, S.; Mykkanen, H.; Salminen, S.; Maunula, L.; Isolauri, E. Prophylactic Lactobacillus GG reduces antibiotic-associated diarrhea in children with respiratory infections: A randomized study. Pediatrics 1999, 104, e64. [Google Scholar] [CrossRef]
- Thomas, M.R.; Litin, S.C.; Osmon, D.R.; Corr, A.P.; Weaver, A.L.; Lohse, C.M. Lack of effect of Lactobacillus GG on antibiotic-associated diarrhea: A randomized, placebo-controlled trial. Mayo Clin. Proc. 2001, 76, 883–889. [Google Scholar] [CrossRef]
- Urbancsek, H.; Kazar, T.; Mezes, I.; Neumann, K. Results of a double-blind, randomized study to evaluate the efficacy and safety of Antibiophilus in patients with radiation-induced diarrhoea. Eur. J. Gastroenterol. Hepatol. 2001, 13, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Ojetti, V.; Bruno, G.; Ainora, M.E.; Gigante, G.; Rizzo, G.; Roccarina, D.; Gasbarrini, A. Impact of Lactobacillus reuteri Supplementation on Anti-Helicobacter pylori Levofloxacin-Based Second-Line Therapy. Gastroenterol. Res. Pract. 2012, 2012, 740381. [Google Scholar] [CrossRef] [PubMed]
- Park, S.K.; Park, D.I.; Choi, J.S.; Kang, M.S.; Park, J.H.; Kim, H.J.; Cho, Y.K.; Sohn, C.I.; Jeon, W.K.; Kim, B.I. The effect of probiotics on Helicobacter pylori eradication. Hepato-Gastroenterology 2007, 54, 2032–2036. [Google Scholar]
- Erdeve, O.; Tiras, U.; Dallar, Y. The probiotic effect of Saccharomyces boulardii in a pediatric age group. J. Trop. Pediatrics 2004, 50, 234–236. [Google Scholar] [CrossRef] [PubMed]
- Duman, D.G.; Bor, S.; Ozutemiz, O.; Sahin, T.; Oguz, D.; Istan, F.; Vural, T.; Sandkci, M.; Isksal, F.; Simsek, I.; et al. Efficacy and safety of Saccharomyces boulardii in prevention of antibiotic-associated diarrhoea due to Helicobacterpylori eradication. Eur. J. Gastroenterol. Hepatol. 2005, 17, 1357–1361. [Google Scholar] [CrossRef]
- Cindoruk, M.; Erkan, G.; Karakan, T.; Dursun, A.; Unal, S. Efficacy and safety of Saccharomyces boulardii in the 14-day triple anti-Helicobacter pylori therapy: A prospective randomized placebo-controlled double-blind study. Helicobacter 2007, 12, 309–316. [Google Scholar] [CrossRef]
- Zojaji, H.; Ghobakhlou, M.; Rajabalinia, H.; Ataei, E.; Jahani Sherafat, S.; Moghimi-Dehkordi, B.; Bahreiny, R. The efficacy and safety of adding the probiotic Saccharomyces boulardiito standard triple therapy for eradication of H.pylori: A randomized controlled trial. Gastroenterol. Hepatol. Bed Bench 2013, 6, S99–S104. [Google Scholar]
- McFarland, L.V.; Evans, C.T.; Goldstein, E.J.C. Strain-Specificity and Disease-Specificity of Probiotic Efficacy: A Systematic Review and Meta-Analysis. Front. Med. 2018, 5, 124. [Google Scholar] [CrossRef]
- Sun, X.; Hirota, S.A. The roles of host and pathogen factors and the innate immune response in the pathogenesis of Clostridium difficile infection. Mol. Immunol. 2015, 63, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Barbut, F.; Petit, J.C. Epidemiology of Clostridium difficile-associated infections. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2001, 7, 405–410. [Google Scholar] [CrossRef]
- Brown, E.; Talbot, G.H.; Axelrod, P.; Provencher, M.; Hoegg, C. Risk factors for Clostridium difficile toxin-associated diarrhea. Infect. Control Hosp. Epidemiol. Off. J. Soc. Hosp. Epidemiol. Am. 1990, 11, 283–290. [Google Scholar] [CrossRef]
- Pakyz, A.L.; Jawahar, R.; Wang, Q.; Harpe, S.E. Medication risk factors associated with healthcare-associated Clostridium difficile infection: A multilevel model case-control study among 64 US academic medical centres. J. Antimicrob. Chemother. 2014, 69, 1127–1131. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.A.; Khanafer, N.; Daneman, N.; Fisman, D.N. Meta-analysis of antibiotics and the risk of community-associated Clostridium difficile infection. Antimicrob. Agents Chemother. 2013, 57, 2326–2332. [Google Scholar] [CrossRef] [PubMed]
- Mayfield, J.L.; Leet, T.; Miller, J.; Mundy, L.M. Environmental control to reduce transmission of Clostridium difficile. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2000, 31, 995–1000. [Google Scholar] [CrossRef] [PubMed]
- Carroll, K.C.; Bartlett, J.G. Biology of Clostridium difficile: Implications for epidemiology and diagnosis. Annu. Rev. Microbiol. 2011, 65, 501–521. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Reid, G.; Gadir, A.A.; Dhir, R. Probiotics: Reiterating What They Are and What They Are Not. Front. Microbiol. 2019, 10, 424. [Google Scholar] [CrossRef]
- Sanders, M.E.; Akkermans, L.M.; Haller, D.; Hammerman, C.; Heimbach, J.T.; Hörmannsperger, G.; Huys, G. Safety assessment of probiotics for human use. Gut Microbes 2010, 1, 164–185. [Google Scholar] [CrossRef]
- Doron, S.; Snydman, D.R. Risk and safety of probiotics. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2015, 60 (Suppl. 2), S129–S134. [Google Scholar] [CrossRef]
- Bassetti, S.; Frei, R.; Zimmerli, W. Fungemia with Saccharomyces cerevisiae after treatment with Saccharomyces boulardii. Am. J. Med. 1998, 105, 71–72. [Google Scholar] [CrossRef]
- Cherifi, S.; Robberecht, J.; Miendje, Y. Saccharomyces cerevisiae fungemia in an elderly patient with Clostridium difficile colitis. Acta Clin. Belg. 2004, 59, 223–224. [Google Scholar] [CrossRef]
- Ohishi, A.; Takahashi, S.; Ito, Y.; Ohishi, Y.; Tsukamoto, K.; Nanba, Y.; Ito, N.; Kakiuchi, S.; Saitoh, A.; Morotomi, M.; et al. Bifidobacterium septicemia associated with postoperative probiotic therapy in a neonate with omphalocele. J. Pediatrics 2010, 156, 679–681. [Google Scholar] [CrossRef] [PubMed]
- McFarland, L.V.; Surawicz, C.M.; Greenberg, R.N.; Elmer, G.W.; Moyer, K.A.; Melcher, S.A.; Bowen, K.E.; Cox, J.L. Prevention of beta-lactam-associated diarrhea by Saccharomyces boulardii compared with placebo. Am. J. Gastroenterol. 1995, 90, 439–448. [Google Scholar]
- Besselink, M.G.; van Santvoort, H.C.; Buskens, E.; Boermeester, M.A.; van Goor, H.; Timmerman, H.M.; Nieuwenhuijs, V.B.; Bollen, T.L.; van Ramshorst, B.; Witteman, B.J.; et al. Probiotic prophylaxis in predicted severe acute pancreatitis: A randomised, double-blind, placebo-controlled trial. Lancet 2008, 371, 651–659. [Google Scholar] [CrossRef]
- Vaarala, O. Immunological effects of probiotics with special reference to lactobacilli. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2003, 33, 1634–1640. [Google Scholar] [CrossRef] [PubMed]
- Veckman, V.; Miettinen, M.; Pirhonen, J.; Siren, J.; Matikainen, S.; Julkunen, I. Streptococcus pyogenes and Lactobacillus rhamnosus differentially induce maturation and production of Th1-type cytokines and chemokines in human monocyte-derived dendritic cells. J. Leukoc. Biol. 2004, 75, 764–771. [Google Scholar] [CrossRef]
- Dessart, S.R.; Steenson, S.L. High frequency intergeneric and intrageneric transfer conjugal transfer of drug resistance plasmids in Leuconostoc mesenteroides ssp. cremoris. J. Dairy Sci. 1991, 74, 2912–2919. [Google Scholar] [CrossRef]
- Guarner, F.; Sanders, M.E.; Eliakim, R.; Fedorak, R.; Gangl, A.; Garisch, J. WGO Practice Guideline: Probiotics and Prebiotics; World Gastroenterology Organisation: Milwaukee, WI, USA, 2017; pp. 1–35. [Google Scholar]
- Gray, C.; Dulong, C.; Argáez, C. Probiotics for Antibiotic-Associated Diarrhea in Pediatrics: A Review of Clinical Effectiveness and Guidelines; Canadian Agency for Drugs and Technologies in Health: Ottawa, ON, Canada, 2019; pp. 1–31. [Google Scholar]
- Szajewska, H.; Canani, R.B.; Guarino, A.; Hojsak, I.; Indrio, F.; Kolacek, S.; Orel, R.; Shamir, R.; Vandenplas, Y.; van Goudoever, J.B.; et al. Probiotics for the Prevention of Antibiotic-Associated Diarrhea in Children. J. Pediatr. Gastroenterol. Nutr. 2016, 62, 495–506. [Google Scholar] [CrossRef]
- Marchand, V. Using probiotics in the paediatric population. Paediatr. Child Health 2012, 17, 575–576. [Google Scholar] [CrossRef]
- Kelly, C.R.; Fischer, M.; Allegretti, J.R.; LaPlante, K.; Stewart, D.B.; Limketkai, B.N.; Stollman, N.H. ACG Clinical Guidelines: Prevention, Diagnosis, and Treatment of Clostridioides difficile Infections. Am. J. Gastroenterol. 2021, 116, 1124–1147. [Google Scholar] [CrossRef]
- Su, G.L.; Ko, C.W.; Bercik, P.; Falck-Ytter, Y.; Sultan, S.; Weizman, A.V.; Morgan, R.L. AGA Clinical Practice Guidelines on the Role of Probiotics in the Management of Gastrointestinal Disorders. Gastroenterology 2020, 159, 697–705. [Google Scholar] [CrossRef]
- McFarland, L.V.; Kullar, R.; Johnson, S.; Sniffen, J.C.; Woolard, K.; Goldstein, E.J.C. Why Do ACG and AGA Guidelines Differ for the Use of Probiotics and the Prevention of CDI? Am. J. Gastroenterol. 2022, 117, 501. [Google Scholar] [CrossRef] [PubMed]
- Kelly, C.R.; Fischer, M.; Allegretti, J.R.; LaPlante, K.; Stewart, D.B.; Limketkai, B.N.; Stollman, N. Response to McFarland et al. Am. J. Gastroenterol. 2022, 117, 501–502. [Google Scholar] [CrossRef] [PubMed]
- Debast, S.B.; Bauer, M.P.; Kuijper, E.J. European Society of Clinical Microbiology and Infectious Diseases: Update of the treatment guidance document for Clostridium difficile infection. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2014, 20 (Suppl. 2), 1–26. [Google Scholar] [CrossRef] [PubMed]
- Stavropoulou, E.; Bezirtzoglou, E. Probiotics in Medicine: A Long Debate. Front. Immunol. 2020, 11, 2192. [Google Scholar] [CrossRef]
- Cremonini, F.; Di Caro, S.; Nista, E.C.; Bartolozzi, F.; Capelli, G.; Gasbarrini, G.; Gasbarrini, A. Meta-analysis: The effect of probiotic administration on antibiotic-associated diarrhoea. Aliment. Pharmacol. Ther. 2002, 16, 1461–1467. [Google Scholar] [CrossRef]
- McFarland, L.V. Meta-analysis of probiotics for the prevention of antibiotic associated diarrhea and the treatment of Clostridium difficile disease. Am. J. Gastroenterol. 2006, 101, 812–822. [Google Scholar] [CrossRef]
- D’Souza, A.L.; Rajkumar, C.; Cooke, J.; Bulpitt, C.J. Probiotics in prevention of antibiotic associated diarrhoea: Meta-analysis. BMJ 2002, 324, 1361. [Google Scholar] [CrossRef]
| Probiotic Genus and Strain | Outcome of RCTs |
|---|---|
| Lactobacillus acidophilus | It significantly shortened the duration of diarrhea [46] and improved gastrointestinal complaints [47].The probiotic preparation did not consistently prevent amoxicillin-induced diarrhea in a pediatric population [48] or reduce the side effects of H. pylori triple therapy [49]. |
| Lactobacillus rhamnosus GG | Lactobacillus GG overall significantly reduced stool frequency and increased stool consistency during antibiotic therapy by the tenth day compared with the placebo group [50]. The absolute risk reduction of diarrhea was 11% within two weeks of antibiotic therapy [51]. Patients using this probiotic showed improvements with respect to the number of bowel movements (p < 0.10) and feces consistency ratings by the investigators (p < 0.05) at the study end [52]. |
| Lactobacillus reuteri | L. reuteri significantly reduced the diarrhea rate by 24 h during antibiotic use. It also increased the eradication rate in H. pylori patients and decreased the occurrence of the most common side effects that are observed with antibiotic treatment [53]. |
| Bacillus subtilis and Streptococcus faecium | Supplementation with probiotic strains, composed of Bacillus subtilis and Streptococcus faecium, was shown to improve drug compliance, reduce side effects, and enhance the intention-to-treat eradication rate of H. pylori [54]. |
| Saccharomyces boulardii | S. boulardii decreased the diarrhea rate from 32.3% to 11.4% in a pediatric group receiving sulbactam-ampicillin [55], prevented AAD and decreased side effects when given with H. pylori treatments [56,57,58,59], and significantly reduced the rate of AAD when given prophylactically with a beta-lactam antibiotic [59]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kopacz, K.; Phadtare, S. Probiotics for the Prevention of Antibiotic-Associated Diarrhea. Healthcare 2022, 10, 1450. https://doi.org/10.3390/healthcare10081450
Kopacz K, Phadtare S. Probiotics for the Prevention of Antibiotic-Associated Diarrhea. Healthcare. 2022; 10(8):1450. https://doi.org/10.3390/healthcare10081450
Chicago/Turabian StyleKopacz, Kira, and Sangita Phadtare. 2022. "Probiotics for the Prevention of Antibiotic-Associated Diarrhea" Healthcare 10, no. 8: 1450. https://doi.org/10.3390/healthcare10081450
APA StyleKopacz, K., & Phadtare, S. (2022). Probiotics for the Prevention of Antibiotic-Associated Diarrhea. Healthcare, 10(8), 1450. https://doi.org/10.3390/healthcare10081450







