Analysis of Factors Hindering the Dissemination of Medical Information Standards
Abstract
1. Introduction
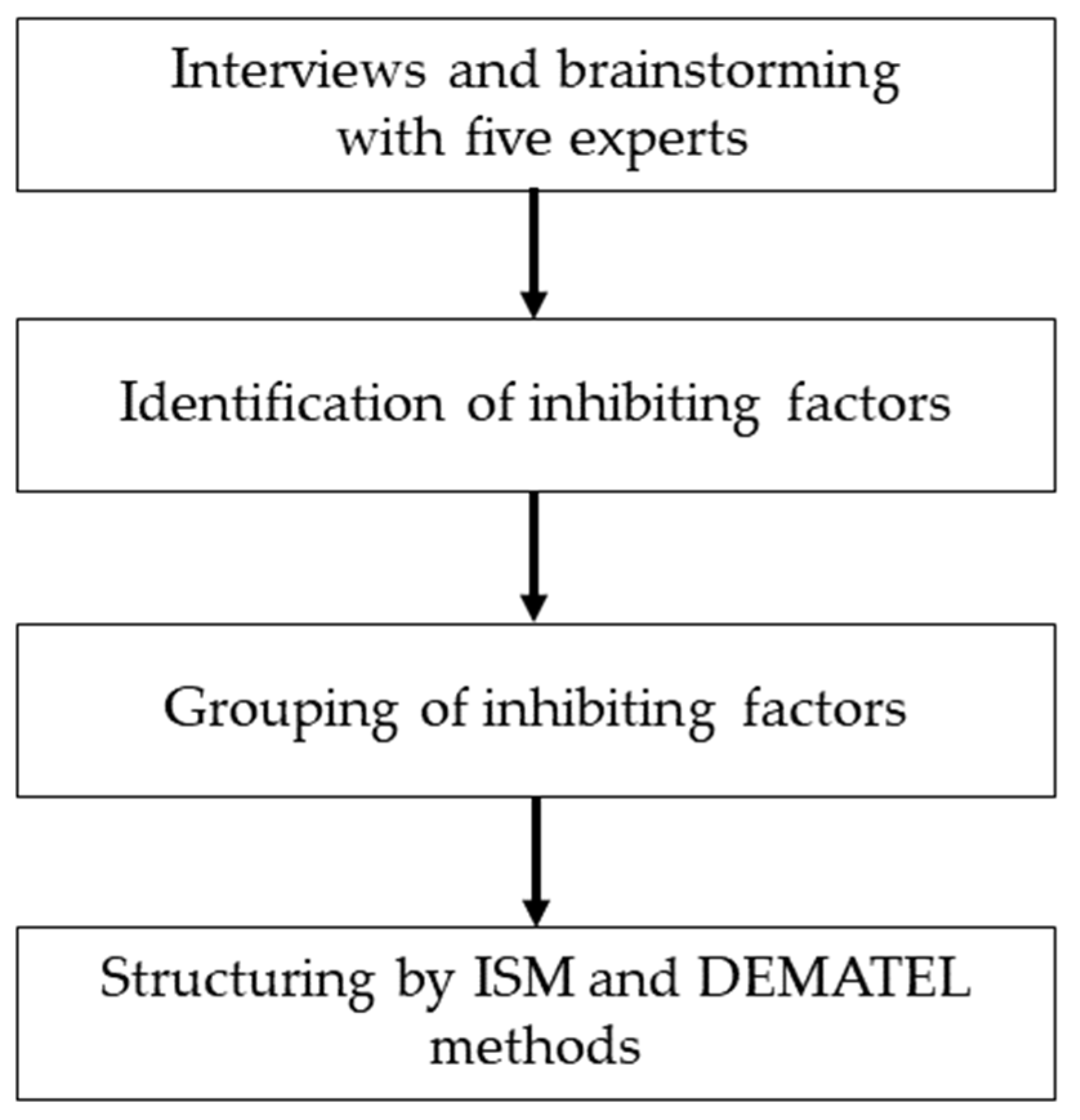
2. Materials and Methods
2.1. Extraction of Factors through Interviews
2.2. Extraction and Grouping of Dissemination Inhibiting Factors
2.3. ISM and DEMATEL Methods
3. Results
3.1. Extraction of Factors from Interviews and a Summary of Elements
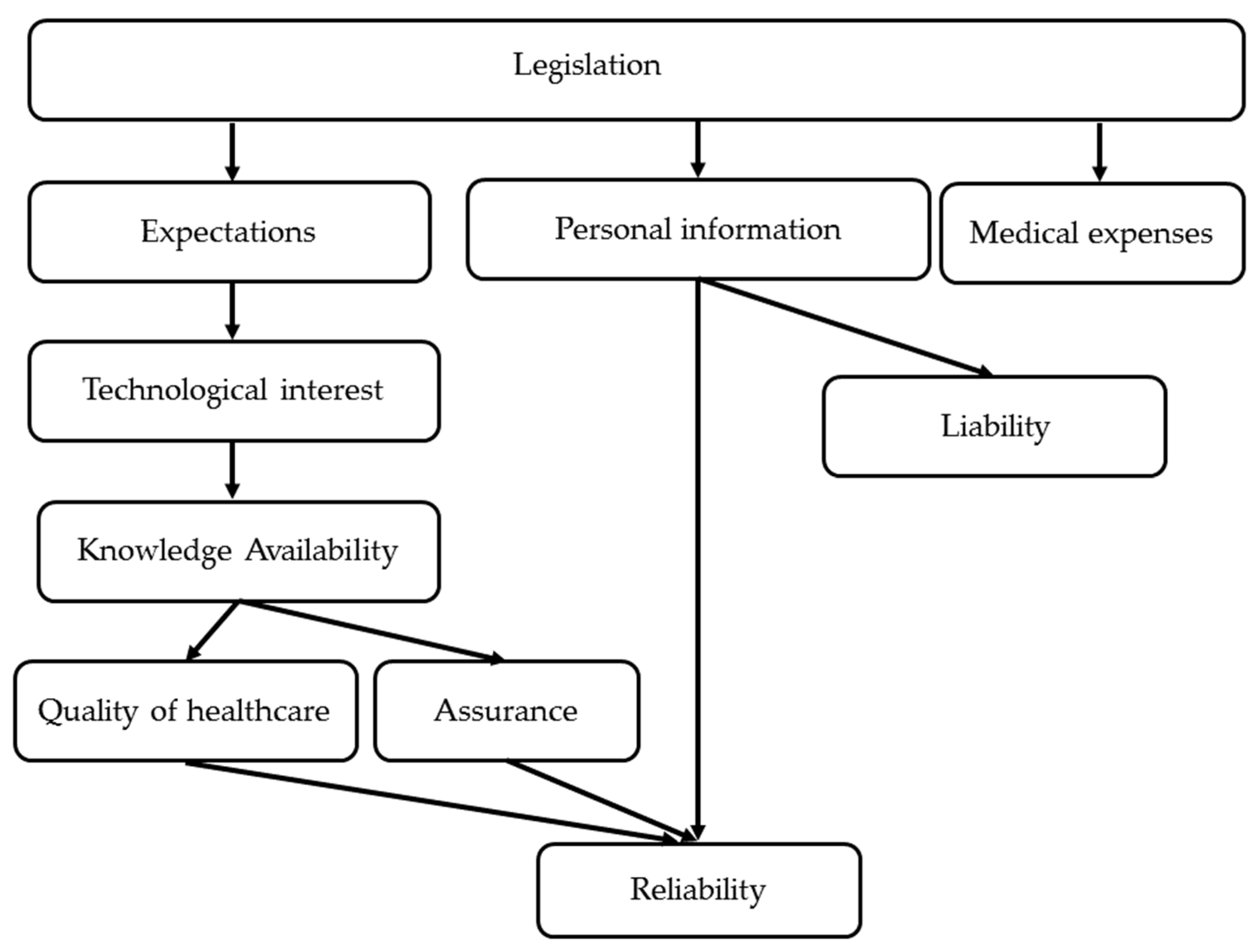
3.2. ISM
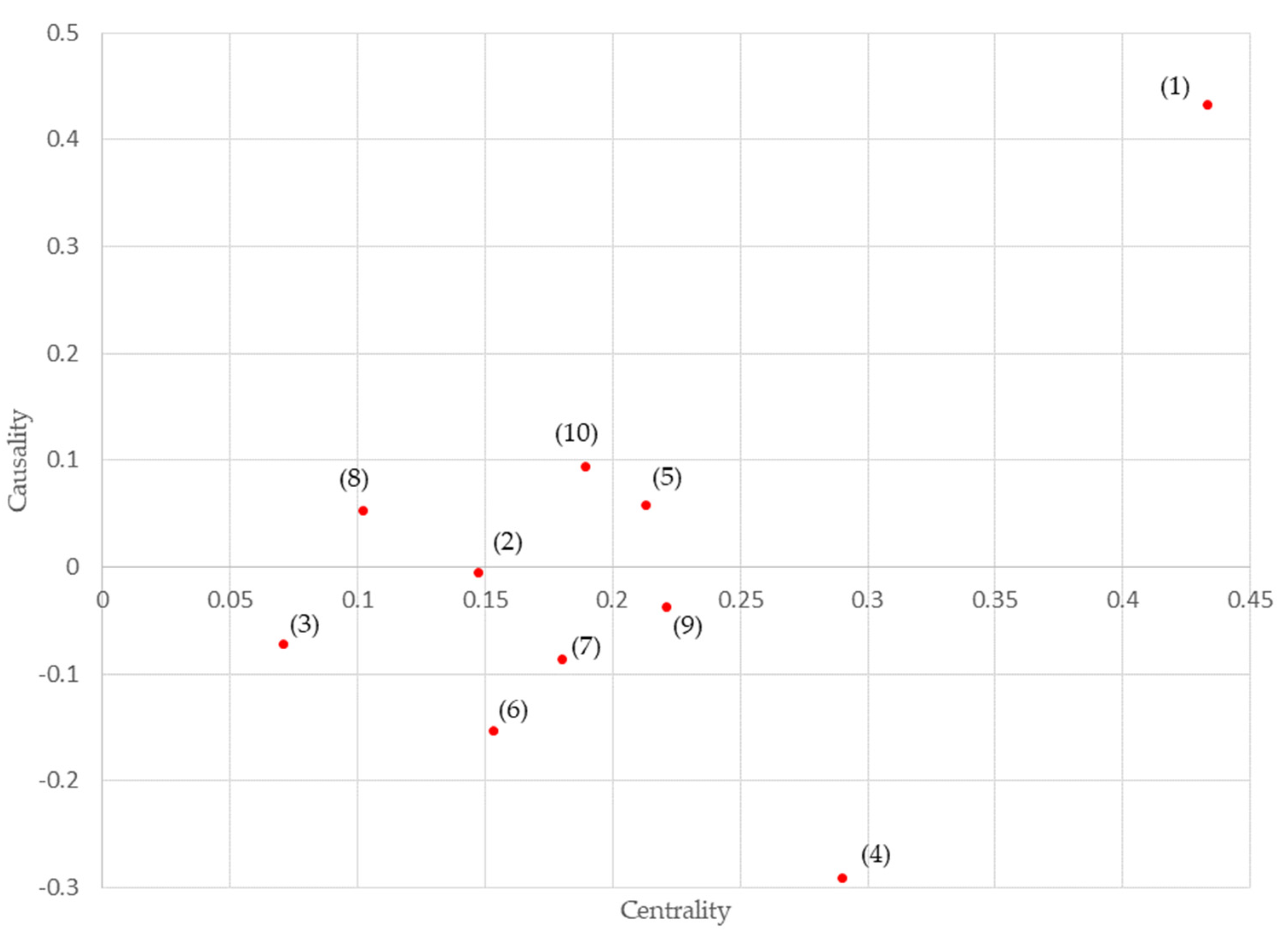
3.3. DEMATEL
4. Discussion
5. Conclusions
- (1)
- We structured the factors inhibiting the dissemination of medical information standards and determined the significance of the factors using ISM (interpretive structural modeling) and the DEMATEL (decision-making trial and evaluation laboratory) method, respectively.
- (2)
- The results showed that “legislation” and “reliability” were important inhibiting factors for the dissemination of medical information standards.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eisenhauer, E.A. Real-world evidence in the treatment of ovarian cancer. Ann. Oncol. 2017, 28 (Suppl. 8), viii61–viii65. [Google Scholar] [CrossRef] [PubMed]
- Pasello, G.; Pavan, A.; Attili, I.; Bortolami, A.; Bonanno, L.; Menis, J.; Conte, P.; Guarneri, V. Real world data in the era of Immune Checkpoint Inhibitors (ICIs): Increasing evidence and future applications in lung cancer. Cancer Treat. Rev. 2020, 87, 102031. [Google Scholar] [CrossRef] [PubMed]
- Feinberg, B.A.; Gajra, A.; Zettler, M.E.; Phillips, T.D.; Phillips, E.G., Jr.; Kish, J. KUse of real-world evidence to support FDA approval of oncology drugs. Value Health 2020, 23, 1358–1365. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.A.; Noh, J.M.; Sun, J.M.; Lee, S.H.; Ahn, J.S.; Ahn, M.J.; Pyob, H.; Ahn, Y.C.; Park, K. Real world data of durvalumab consolidation after chemoradiotherapy in stage III non-small-cell lung cancer. Lung Cancer 2020, 146, 23–29. [Google Scholar] [CrossRef]
- Kraus, A.L.; Yu-Kite, M.; Mardekian, J.; Cotter, M.J.; Kim, S.; Decembrino, J.; Snow, T.; Carson, K.R.; Rockland, J.M.; Tursi, J.M.; et al. Real-world data of palbociclib in combination with endocrine therapy for the treatment of metastatic breast cancer in men. Clin. Pharmacol. Ther. 2022, 111, 302–309. [Google Scholar] [CrossRef]
- Brufsky, A.; Liu, X.; Li, B.; McRoy, L.; Layman, R.M. Real-world tumor response of Palbociclib plus Letrozole versus Letrozole for metastatic breast cancer in US Clinical Practice. Target. Oncol. 2021, 16, 601–611. [Google Scholar] [CrossRef]
- Japanese Ministry of Health. Labour and Welfare. An Outline of the Japanese Medical System. Available online: https://www.mhlw.go.jp/bunya/iryouhoken/iryouhoken01/dl/01_eng.pdf (accessed on 15 March 2020).
- Yamamoto, R. Current Status and Prospects of Standardization of Medical Information in Japan and Abroad. Information Management. Jpn. J. Inf. Process Manag. 2017, 60, 619–628. [Google Scholar]
- Sakai, A. Efforts to Support Standard Codes for Data Utilization. In Proceedings of the 40th Joint Conference on Medical Informatics, Hamamatsu, Japan, 18–22 November 2020. [Google Scholar]
- Translational Research Network Program. Available online: https://www.amed.go.jp/program/list/05/01/001.html (accessed on 14 May 2022).
- Kishiba, M.; Yamada, R.; Ota, Y.; Yinyama, T.; Yamaguchi, M.; Uyama, Y. The Process and Importance of Data Standardization in MID-NET® to Promote Drug Safety Measures. Jpn. J. Clin. Pathol. 2019, 67, 698–707. [Google Scholar]
- Yamaguchi, M.; Inomata, S.; Harada, S.; Matsuzaki, Y.; Kawaguchi, M.; Kishiba, M.; Fujimura, Y.; Kimura, M.; Murata, K.; Nakashima, N.; et al. Establishment of the MID-NET® medical information database network as a reliable and valuable database for drug safety assessments in Japan. Pharmacoepidemiol. Drug Saf. 2019, 28, 1395–1404. [Google Scholar] [CrossRef]
- Kishiba, M.; Ota, Y.; Kageyama, T.; Yamaguchi, M.; Uyama, Y. Data Standardization Efforts in MID-NET®. JAPIC News 2019, 426, 4–7. [Google Scholar]
- Yamada, R. Clinical Laboratory Technician at the Pharmaceuticals and Medical Devices Agency (PMDA) Standardization of specimen test data in MID-NET® as an example. Mod. Med. Lab. 2021, 49, 474–478. [Google Scholar]
- Health Information and Communication Standards. Available online: http://helics.umin.ac.jp/MhlwTsuuchi.html (accessed on 4 June 2022).
- Thakur, V.; Anbanandam, R. Healthcare waste management: An interpretive structural modeling approach. Int. J. Health Care Qual. Assur. 2016, 29, 559–581. [Google Scholar] [CrossRef] [PubMed]
- Sarikhani, Y.; Shojaei, P.; Rafiee, M.; Delavari, S. Analyzing the interaction of main components of hidden curriculum in medical education using interpretive structural modeling method. BMC Med. Educ. 2020, 20, 176. [Google Scholar] [CrossRef] [PubMed]
- Attri, R. Interpretive Structural Modeling (ISM) approach: An overview. Res. J. Manag. Sci. 2013, 2, 3–8. [Google Scholar]
- Janes, F.R. Interpretive Structural Modeling (ISM): A methodology for structuring complex issue. Trans. Inst. Meas. Control 1988, 10, 145–154. [Google Scholar] [CrossRef]
- Lee, A.H.I.; Kang, H.Y. A multi-criteria decision-making model for evaluating senior daycare center locations. Int. J. Environ. Res. Public Health 2019, 16, 5031. [Google Scholar] [CrossRef]
- Masaoka, A.; Kawamata, M.; Ishigaki, R.; Ota, S.; Goko, D.; Tsujimoto, T.; Matsumura, M.; Sakamoto, H.; Banno, T.; Yokohama, N.; et al. Analysis of workflow and structuring of the problem element for workflow database construction of radiological technologists in radiation therapy. Nihon Hoshasen Gijutsu Gakkai Zasshi 2019, 75, 1286–1296. [Google Scholar] [CrossRef]
- Karamat, J.; Shurong, T.; Ahmad, N.; Afridi, S.; Khan, S.; Mahmood, K. Promoting healthcare sustainability in developing countries: Analysis of knowledge management drivers in public and private hospitals of Pakistan. Int. J. Environ. Res. Public Health 2019, 16, 508. [Google Scholar] [CrossRef]
- Karamat, J.; Shurong, T.; Ahmad, N.; Waheed, A.; Mahmood, K. Enablers supporting the implementation of knowledge management in the healthcare of Pakistan. Int. J. Environ. Res. Public Health 2018, 15, 2816. [Google Scholar] [CrossRef]
- Bahadori, M.; Teymourzadeh, E.; Tajik, H.; Ravangard, R.; Raadabadi, M.; Hosseini, S.M. Factors affecting strategic plan implementation using interpretive structural modeling (ISM). Int. J. Health Care Qual. Assur. 2018, 31, 406–414. [Google Scholar] [CrossRef]
- Dwyer, C.P.; McKenna-Plumley, P.E.; Durand, H.; Gormley, E.M.; Slattery, B.W.; Harney, O.M.; MacNeela, P.; McGuire, B.E. Factors influencing the application of a biopsychosocial perspective in clinical judgement of chronic pain: Interactive management with medical students. Pain Physician 2017, 20, E951–E960. [Google Scholar] [CrossRef] [PubMed]
- Kudo, N.; Kurowarabi, K.; Terashita, T.; Nishimoto, N.; Ogasawara, K. Hierarchy structuring for mammography technique by interpretive structural modeling method. Nihon Hoshasen Gijutsu Gakkai Zasshi 2009, 65, 1385–1390. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ogasawara, K.; Yamashina, H.; Kamiya, T.; Jiang, G.; Sakurai, T. Interpretive Structural Modeling for introducing of image information system at the middle-scale hospitals. AMIA Annu. Symp. Proc. 2003, 2003, 957. [Google Scholar]
- Mahdinia, M.; Mohammadfam, I.; Soltanzadeh, A.; Aliabadi, M.M.; Aghaei, H. A fuzzy Bayesian network DEMATEL model for predicting safety behavior. Int. J. Occup. Saf. Ergon. 2021, 29, 1–8. [Google Scholar] [CrossRef]
- Maqbool, A.; Khan, N.Z. Analyzing barriers for implementation of public health and social measures to prevent the transmission of COVID-19 disease using DEMATEL method. Diabetes Metab. Syndr. 2020, 14, 887–892. [Google Scholar] [CrossRef]
- Xie, K.; Liu, Z. Factors influencing escalator-related incidents in China: A systematic analysis using ISM-DEMATEL method. Int. J. Environ. Res. Public Health. 2019, 16, 2478. [Google Scholar] [CrossRef]
- Booth, C.M.; Karim, S.; Mackillop, W.J. Real-world data: Towards achieving the achievable in cancer care. Nat. Rev. Clin. Oncol. 2019, 16, 312–325. [Google Scholar] [CrossRef]
- Flatiron OncoEMR. Available online: https://flatiron.com/oncology/oncology-ehr/ (accessed on 14 May 2022).
| Experts | Factors |
|---|---|
| Administrative institutions and researchers | No incentive for standardization No regulations or penalties for non-standardized systems No direct benefit to small hospitals Low compatibility with existing systems Data structure differs from facility to facility due to operational differences The significance of the secondary use of medical information is not understood by clinical staff Some products are not standardized at the vendor level Standardized product costs are high (in many cases, they are optional) The optimal data granularity differs between standardization standards and medical institutions Limited situations of medical collaboration using personal information data The benefits of standardization from the viewpoint of medical institutions are not well recognized Low awareness of the initiative among the Ministry of Health, Labour and Welfare, academic societies, and council initiatives Cases where there are no personnel within the medical institution or vendor who can promote standardization Lack of activities to promote the standardization of medical information |
| National research institute and researchers | Vendors’ responses are mixed Costly (expensive) to deal with and not linked to income, such as medical fees Servers (SS-MIX server) may be necessary Complexity of the master maintenance Difficult to deal with when there is no one dedicated to supporting the system There are many standard codes depending on the field, making it difficult to understand Unclear to what extent one should respond User indifference No incentive for standardization |
| Elements | Definition (Contents) |
|---|---|
| Legislation | The need for legislation and guideline maintenance as an environment when using standardization technology in medicine |
| Quality of healthcare | Expectations for the improvement of medical care quality and treatment through standardized technology or assurance of medical care quality |
| Medical expenses | The impact of standardized technology use on healthcare costs, or bearing the costs for medical care and treatment using technology |
| Reliability | Ensuring confidence in medical and medical practice based on standardized technology and its methods |
| Technological interest | Interest in and understanding of standardization technologies and the means to increase this interest |
| Liability | Organizing the breakdown of responsibilities for medical care and treatment that use standardized technology |
| Assurance | Assurance that the system reflects standardized technology, technical response to the fact that the standardized technology itself is being updated, and organization of the maintenance scope |
| Expectations | Improvement of the medical care quality by the use of standardization technology in medical care, and motivation to use standardization technology in medical care |
| Knowledge availability | Understanding the standardization technology, and experience of examples of implementation in other fields and its necessity |
| Personal information | Organizing the handling of personal information as data when developing standardization technologies, and the risks to personal and private information when using standardization technologies |
| Legislation | Quality of Healthcare | Medical Expenses | Reliability | Technological Interest | Liability | Assurance | Expectations | Knowledge Availability | Personal Information | |
|---|---|---|---|---|---|---|---|---|---|---|
| Legislation | 0 | 0 | 3 | 0 | 1 | 4 | 4 | 1 | 0 | 2 |
| Quality of healthcare | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 |
| Medical expenses | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Reliability | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Technological interest | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 0 |
| Liability | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Assurance | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 |
| Expectations | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 |
| Knowledge availability | 0 | 2 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Personal information | 0 | 0 | 0 | 4 | 0 | 2 | 0 | 0 | 0 | 0 |
| Inhibition Factor | Affected Degree (a) | Influence Degree (b) | Centrality (a + b) | Causality (b − a) | |
|---|---|---|---|---|---|
| (1) | legislation | 0 | 0.433 | 0.433 | 0.433 |
| (2) | quality of healthcare | 0.076 | 0.071 | 0.147 | −0.005 |
| (3) | medical expenses | 0.071 | 0 | 0.071 | −0.071 |
| (4) | reliability | 0.29 | 0 | 0.29 | −0.29 |
| (5) | technological interest | 0.077 | 0.136 | 0.213 | 0.059 |
| (6) | liability | 0.153 | 0 | 0.153 | −0.153 |
| (7) | assurance | 0.133 | 0.047 | 0.18 | −0.086 |
| (8) | expectations | 0.024 | 0.078 | 0.102 | 0.054 |
| (9) | knowledge availability | 0.129 | 0.092 | 0.221 | −0.037 |
| (10) | personal information | 0.047 | 0.142 | 0.189 | 0.095 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mukai, M.; Ogasawara, K. Analysis of Factors Hindering the Dissemination of Medical Information Standards. Healthcare 2022, 10, 1248. https://doi.org/10.3390/healthcare10071248
Mukai M, Ogasawara K. Analysis of Factors Hindering the Dissemination of Medical Information Standards. Healthcare. 2022; 10(7):1248. https://doi.org/10.3390/healthcare10071248
Chicago/Turabian StyleMukai, Masami, and Katsuhiko Ogasawara. 2022. "Analysis of Factors Hindering the Dissemination of Medical Information Standards" Healthcare 10, no. 7: 1248. https://doi.org/10.3390/healthcare10071248
APA StyleMukai, M., & Ogasawara, K. (2022). Analysis of Factors Hindering the Dissemination of Medical Information Standards. Healthcare, 10(7), 1248. https://doi.org/10.3390/healthcare10071248






