Breast Cancer—How Can Imaging Help?
Abstract
:1. Introduction
- BC expressing hormone receptor (estrogen or progesterone);
- BC expressing human epidermal receptor 2;
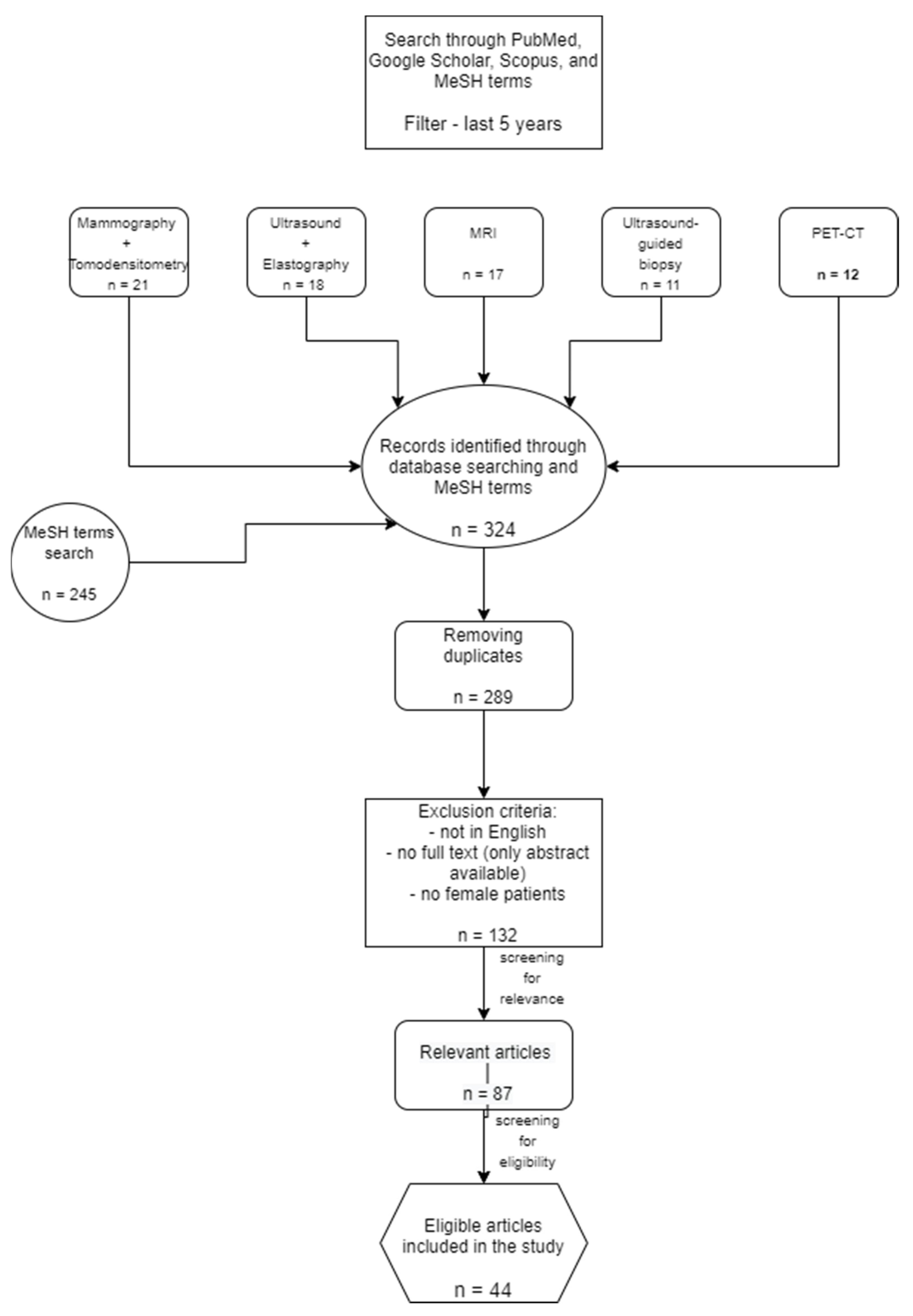
2. Materials and Methods
- Mammography and tomosynthesis in BC;
- Ultrasound and elastography in BC;
- MRI in BC;
- Ultrasound-guided biopsy in BC;
- PET-CT and PET-MRI in BC.
3. Results
3.1. Mammography and Digital Tomosynthesis in Breast Cancer
3.2. Ultrasound and Elastography in Breast Cancer
3.3. MRI in Breast Cancer
3.4. Ultrasound-Guided Biopsy in Breast Cancer
3.5. PET-CT and PET-MRI in Breast Cancer
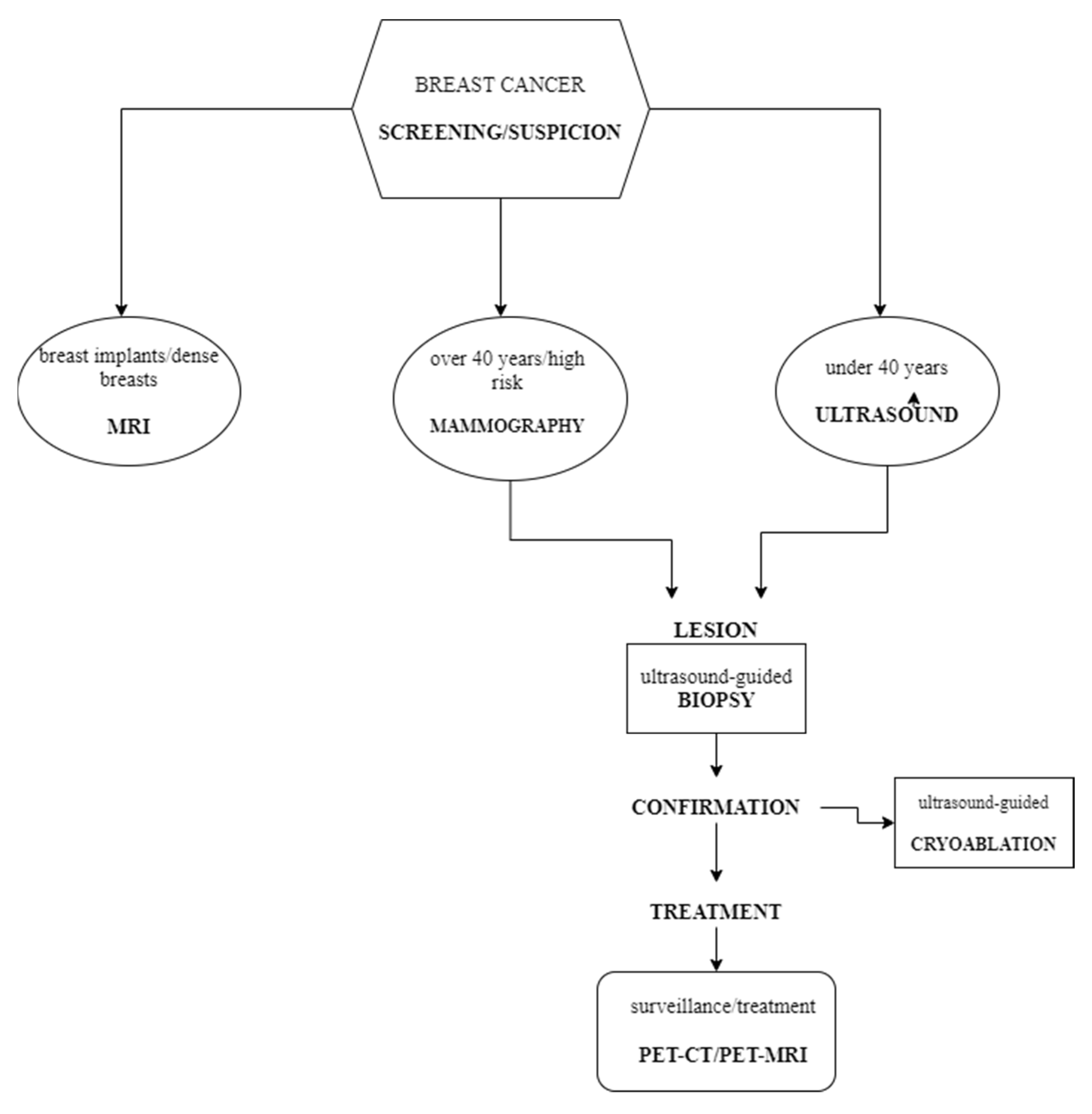
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Harbeck, N.; Gnant, M. Breast Cancer. Lancet 2017, 389, 1134–1150. [Google Scholar] [CrossRef]
- Iranmakani, S.; Mortezazadeh, T.; Sajadian, F.; Ghaziani, M.F.; Ghafari, A.; Khezerloo, D.; Musa, A.E. A Review of Various Modalities in Breast Imaging: Technical Aspects and Clinical Outcomes. Egypt J. Radiol. Nucl. Med. 2020, 51, 57. [Google Scholar] [CrossRef] [Green Version]
- Tagliafico, A.S.; Piana, M.; Schenone, D.; Lai, R.; Massone, A.M.; Houssami, N. Overview of Radiomics in Breast Cancer Diagnosis and Prognostication. Breast 2020, 49, 74–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coughlin, S.S. Epidemiology of Breast Cancer in Women. In Breast Cancer Metastasis and Drug Resistance; Advances in Experimental Medicine and Biology; Ahmad, A., Ed.; Springer International Publishing: Cham, Switzerland, 2019; Volume 1152, pp. 9–29. ISBN 978-3-030-20300-9. [Google Scholar]
- Lee, C.I.; Chen, L.E.; Elmore, J.G. Risk-Based Breast Cancer Screening. Med. Clin. N. Am. 2017, 101, 725–741. [Google Scholar] [CrossRef] [PubMed]
- Zavala, V.A.; Serrano-Gomez, S.J.; Dutil, J.; Fejerman, L. Genetic Epidemiology of Breast Cancer in Latin America. Genes 2019, 10, 153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barzaman, K.; Karami, J.; Zarei, Z.; Hosseinzadeh, A.; Kazemi, M.H.; Moradi-Kalbolandi, S.; Safari, E.; Farahmand, L. Breast Cancer: Biology, Biomarkers, and Treatments. Int. Immunopharmacol. 2020, 84, 106535. [Google Scholar] [CrossRef]
- Fragomeni, S.M.; Sciallis, A.; Jeruss, J.S. Molecular Subtypes and Local-Regional Control of Breast Cancer. Surg. Oncol. Clin. N. Am. 2018, 27, 95–120. [Google Scholar] [CrossRef]
- Nyayapathi, N.; Xia, J. Photoacoustic Imaging of Breast Cancer: A Mini Review of System Design and Image Features. J. Biomed. Opt. 2019, 24, 1. [Google Scholar] [CrossRef]
- Harbeck, N.; Penault-Llorca, F.; Cortes, J.; Gnant, M.; Houssami, N.; Poortmans, P.; Ruddy, K.; Tsang, J.; Cardoso, F. Breast Cancer. Nat. Rev. Dis. Primers 2019, 5, 66. [Google Scholar] [CrossRef]
- Funke, M. Bildgebende Diagnostik des Mammakarzinoms: Ein Update. Radiologe 2016, 56, 921–938. [Google Scholar] [CrossRef]
- Jafari, S.H.; Saadatpour, Z.; Salmaninejad, A.; Momeni, F.; Mokhtari, M.; Nahand, J.S.; Rahmati, M.; Mirzaei, H.; Kianmehr, M. Breast Cancer Diagnosis: Imaging Techniques and Biochemical Markers. J. Cell. Physiol. 2018, 233, 5200–5213. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Hashemi, H.; Gordon, P.; Warren, L.; Wang, Z.J.; Rohling, R.; Salcudean, T. Breast Cancer Detection Using Multimodal Time Series Features from Ultrasound Shear Wave Absolute Vibro-Elastography. IEEE J. Biomed. Health Inform. 2021, 26, 704–714. [Google Scholar] [CrossRef] [PubMed]
- Eghtedari, M.; Chong, A.; Rakow-Penner, R.; Ojeda-Fournier, H. Current Status and Future of BI-RADS in Multimodality Imaging, from the AJR Special Series on Radiology Reporting and Data Systems. Am. J. Roentgenol. 2021, 216, 860–873. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Dong, Z.; Zhang, L.; Ning, C.; Li, Z.; Wang, D.; Liu, C.; Zhao, M.; Tian, J. Ultrasound Features of Breast Cancer for Predicting Axillary Lymph Node Metastasis: Ultrasound for Axillary Lymph Node Metastasis Prediction. J. Ultrasound Med. 2018, 37, 1354–1353. [Google Scholar] [CrossRef] [Green Version]
- Luo, W.; Huang, Q.; Huang, X.; Hu, H.; Zeng, F.; Wang, W. Predicting Breast Cancer in Breast Imaging Reporting and Data System (BI-RADS) Ultrasound Category 4 or 5 Lesions: A Nomogram Combining Radiomics and BI-RADS. Sci. Rep. 2019, 9, 11921. [Google Scholar] [CrossRef] [Green Version]
- Bevers, T.B.; Helvie, M.; Bonaccio, E.; Calhoun, K.E.; Daly, M.B.; Farrar, W.B.; Garber, J.E.; Gray, R.; Greenberg, C.C.; Greenup, R.; et al. Breast Cancer Screening and Diagnosis, Version 3.2018, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2018, 16, 1362–1389. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, T.A.; Lee, C.I.; Johnson, K.M. Breast Cancer Screening: Does Tomosynthesis Augment Mammography? CCJM 2017, 84, 522–527. [Google Scholar] [CrossRef] [Green Version]
- Milosevic, M.; Jankovic, D.; Milenkovic, A.; Stojanov, D. Early Diagnosis and Detection of Breast Cancer. THC 2018, 26, 729–759. [Google Scholar] [CrossRef]
- Phi, X.-A.; Tagliafico, A.; Houssami, N.; Greuter, M.J.W.; de Bock, G.H. Digital Breast Tomosynthesis for Breast Cancer Screening and Diagnosis in Women with Dense Breasts—A Systematic Review and Meta-Analysis. BMC Cancer 2018, 18, 380. [Google Scholar] [CrossRef] [Green Version]
- Yun, S.J.; Ryu, C.-W.; Rhee, S.J.; Ryu, J.K.; Oh, J.Y. Benefit of Adding Digital Breast Tomosynthesis to Digital Mammography for Breast Cancer Screening Focused on Cancer Characteristics: A Meta-Analysis. Breast Cancer Res. Treat. 2017, 164, 557–569. [Google Scholar] [CrossRef]
- Houssami, N.; Zackrisson, S.; Blazek, K.; Hunter, K.; Bernardi, D.; Lång, K.; Hofvind, S. Meta-Analysis of Prospective Studies Evaluating Breast Cancer Detection and Interval Cancer Rates for Digital Breast Tomosynthesis versus Mammography Population Screening. Eur. J. Cancer 2021, 148, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Sogani, J.; Mango, V.L.; Keating, D.; Sung, J.S.; Jochelson, M.S. Contrast-Enhanced Mammography: Past, Present, and Future. Clin. Imaging 2021, 69, 269–279. [Google Scholar] [CrossRef]
- James, J.J.; Tennant, S.L. Contrast-Enhanced Spectral Mammography (CESM). Clin. Radiol. 2018, 73, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Ghaderi, K.F.; Phillips, J.; Perry, H.; Lotfi, P.; Mehta, T.S. Contrast-Enhanced Mammography: Current Applications and Future Directions. RadioGraphics 2019, 39, 1907–1920. [Google Scholar] [CrossRef] [PubMed]
- Knabben, L.; Mueller, M.D. Breast Cancer and Pregnancy. Horm. Mol. Biol. Clin. Investig. 2017, 32, 2017-0026. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, S.; Ding, L.; Liang, X.; Wang, Y.; Greuter, M.J.W.; de Bock, G.H.; Lu, W. Is Ultrasound an Accurate Alternative for Mammography in Breast Cancer Screening in an Asian Population? A Meta-Analysis. Diagnostics 2020, 10, 985. [Google Scholar] [CrossRef]
- Huang, Z.; Chen, L.; Wang, Y.; Fu, L.; Lv, R. Molecular Markers, Pathology, and Ultrasound Features of Invasive Breast Cancer. Clin. Imaging 2021, 79, 85–93. [Google Scholar] [CrossRef]
- Geisel, J.; Raghu, M.; Hooley, R. The Role of Ultrasound in Breast Cancer Screening: The Case for and against Ultrasound. Semin. Ultrasound CT MRI 2018, 39, 25–34. [Google Scholar] [CrossRef]
- Perhavec, A.; Miklavčič, M.; Perić, B.; Pilko, G.; Žgajnar, J. Is Preoperative Ultrasound of the Axilla Necessary in Screen-Detected Breast Cancer? Eur. J. Surg. Oncol. 2020, 46, 85–88. [Google Scholar] [CrossRef]
- Colakovic, N.; Zdravkovic, D.; Skuric, Z.; Mrda, D.; Gacic, J.; Ivanovic, N. Intraoperative Ultrasound in Breast Cancer Surgery—From Localization of Non-Palpable Tumors to Objectively Measurable Excision. World J. Surg. Oncol. 2018, 16, 184. [Google Scholar] [CrossRef]
- Leblond, M.; Duchesne, N.; Provencher, L.; Hogue, J.; Pinault, S. Is Contralateral Breast Ultrasound Worthwhile in Preoperative Staging of Breast Cancer? J. Clin. Ultrasound 2019, 47, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Forte, A.J.; Huayllani, M.T.; Boczar, D.; Cinotto, G.; Ciudad, P.; Manrique, O.J.; Lu, X.; McLaughlin, S.A. The Basics of Ultrasound Elastography for Diagnosis, Assessment, and Staging Breast Cancer-Related Lymphedema: A Systematic Review of the Literature. Gland. Surg. 2020, 9, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Barr, R.G. Future of Breast Elastography. Ultrasonography 2019, 38, 93–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mann, R.M.; Hooley, R.; Barr, R.G.; Moy, L. Novel Approaches to Screening for Breast Cancer. Radiology 2020, 297, 266–285. [Google Scholar] [CrossRef] [PubMed]
- Barr, R.G. Breast Elastography: How to Perform and Integrate into a “Best-Practice” Patient Treatment Algorithm. J. Ultrasound Med. 2020, 39, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Carlsen, J.F.; Hansen, K.L.; Ewertsen, C.; Nielsen, M.B. Elastography in Breast Imaging. Ultraschall Med. 2019, 40, 688–691. [Google Scholar] [CrossRef] [Green Version]
- Chang, W.; Jia, W.; Shi, J.; Yuan, C.; Zhang, Y.; Chen, M. Role of Elastography in Axillary Examination of Patients with Breast Cancer: Elastography in Axillary Examinations of Patients with Breast Cancer. J. Ultrasound Med. 2018, 37, 699–707. [Google Scholar] [CrossRef] [Green Version]
- Chang, J.M.; Won, J.-K.; Lee, K.-B.; Park, I.A.; Yi, A.; Moon, W.K. Comparison of Shear-Wave and Strain Ultrasound Elastography in the Differentiation of Benign and Malignant Breast Lesions. Am. J. Roentgenol. 2013, 201, W347–W356. [Google Scholar] [CrossRef]
- Scaranelo, A.M. What’s Hot in Breast MRI. Can. Assoc. Radiol. J. 2022, 73, 125–140. [Google Scholar] [CrossRef]
- Adrada, B.E.; Candelaria, R.; Rauch, G.M. MRI for the Staging and Evaluation of Response to Therapy in Breast Cancer. Top. Magn. Reson. Imaging 2017, 26, 211–218. [Google Scholar] [CrossRef]
- van Bodegraven, E.A.; van Raaij, J.C.; Van Goethem, M.; Tjalma, W.A.A. Guidelines and Recommendations for MRI in Breast Cancer Follow-up: A Review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 218, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Saadatmand, S.; Geuzinge, H.A.; Rutgers, E.J.T.; Mann, R.M.; de Roy van Zuidewijn, D.B.W.; Zonderland, H.M.; Tollenaar, R.A.E.M.; Lobbes, M.B.I.; Ausems, M.G.E.M.; van ’t Riet, M.; et al. MRI versus Mammography for Breast Cancer Screening in Women with Familial Risk (FaMRIsc): A Multicentre, Randomised, Controlled Trial. Lancet Oncol. 2019, 20, 1136–1147. [Google Scholar] [CrossRef] [Green Version]
- Hayashi, M.; Yamamoto, Y.; Iwase, H. Clinical Imaging for the Prediction of Neoadjuvant Chemotherapy Response in Breast Cancer. Chin. Clin. Oncol. 2020, 9, 31. [Google Scholar] [CrossRef] [PubMed]
- Mann, R.M.; Kuhl, C.K.; Moy, L. Contrast-enhanced MRI for Breast Cancer Screening. J. Magn. Reson. Imaging 2019, 50, 377–390. [Google Scholar] [CrossRef]
- Schoub, P.K. Understanding Indications and Defining Guidelines for Breast Magnetic Resonance Imaging. S. Afr. J. Radiol. 2018, 22, 2. [Google Scholar] [CrossRef] [PubMed]
- Patel, B.K.; Samreen, N.; Zhou, Y.; Chen, J.; Brandt, K.; Ehman, R.; Pepin, K. MR Elastography of the Breast: Evolution of Technique, Case Examples, and Future Directions. Clin. Breast Cancer 2021, 21, e102–e111. [Google Scholar] [CrossRef] [PubMed]
- Leithner, D.; Wengert, G.J.; Helbich, T.H.; Thakur, S.; Ochoa-Albiztegui, R.E.; Morris, E.A.; Pinker, K. Clinical Role of Breast MRI Now and Going Forward. Clin. Radiol. 2018, 73, 700–714. [Google Scholar] [CrossRef] [PubMed]
- Reig, B.; Heacock, L.; Lewin, A.; Cho, N.; Moy, L. Role of MRI to Assess Response to Neoadjuvant Therapy for Breast Cancer. J. Magn. Reason. Imaging 2020, 52, jmri.27145. [Google Scholar] [CrossRef]
- Bhatt, A.A.; Whaley, D.H.; Lee, C.U. ULTRASOUND-GUIDED Breast Biopsies: Basic and New Techniques. J. Ultrasound Med. 2021, 40, 1427–1443. [Google Scholar] [CrossRef]
- Ji, X.; Li, D.; Gao, D.; Lv, X.; Feng, Y.; Zhang, D.; Ye, W. Value of Ultrasound-Guided Biopsy in Evaluating Internal Mammary Lymph Node Metastases in Breast Cancer. Clin. Breast Cancer 2021, 21, 532–538. [Google Scholar] [CrossRef]
- Versaggi, S.L.; De Leucio, A. Breast Biopsy; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Newell, M.S.; Mahoney, M.C. Ultrasound-Guided Percutaneous Breast Biopsy. Tech. Vasc. Interv. Radiol. 2014, 17, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Ward, R.C.; Lourenco, A.P.; Mainiero, M.B. Ultrasound-Guided Breast Cancer Cryoablation. Am. J. Roentgenol. 2019, 213, 716–722. [Google Scholar] [CrossRef] [PubMed]
- Ariaratnam, N.S.; Little, S.T.; Whitley, M.A.; Ferguson, K. Digital Breast Tomosynthesis Vacuum Assisted Biopsy for Tomosynthesis-Detected Sonographically Occult Lesions. Clin. Imaging 2018, 47, 4–8. [Google Scholar] [CrossRef] [PubMed]
- Ulaner, G.A. PET/CT for Patients with Breast Cancer: Where Is the Clinical Impact? Am. J. Roentgenol. 2019, 213, 254–265. [Google Scholar] [CrossRef]
- Paydary, K.; Seraj, S.M.; Zadeh, M.Z.; Emamzadehfard, S.; Shamchi, S.P.; Gholami, S.; Werner, T.J.; Alavi, A. The Evolving Role of FDG-PET/CT in the Diagnosis, Staging, and Treatment of Breast Cancer. Mol. Imaging Biol. 2019, 21, 1–10. [Google Scholar] [CrossRef]
- Caresia Aroztegui, A.P.; García Vicente, A.M.; Alvarez Ruiz, S.; Delgado Bolton, R.C.; Orcajo Rincon, J.; Garcia Garzon, J.R.; de Arcocha Torres, M.; Garcia-Velloso, M.J. 18F-FDG PET/CT in Breast Cancer: Evidence-Based Recommendations in Initial Staging. Tumour Biol. 2017, 39, 101042831772828. [Google Scholar] [CrossRef] [Green Version]
- Sarikaya, I. Breast Cancer and PET Imaging. Nucl. Med. Rev. Cent. East. Eur. 2021, 24, 16–26. [Google Scholar] [CrossRef]
- Hulikal, N.; Gajjala, S.R.; Kalawat, T.; Kadiyala, S.; Kottu, R. Predicting Response to Neoadjuvant Chemotherapy Using 18F FDG PET-CT in Patients with Locally Advanced Breast Cancer. Asian Pac. J. Cancer Prev. 2020, 21, 93–98. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Yao, L.; Jin, P.; Hu, L.; Li, X.; Guo, T.; Yang, K. MRI and PET/CT for Evaluation of the Pathological Response to Neoadjuvant Chemotherapy in Breast Cancer: A Systematic Review and Meta-Analysis. Breast 2018, 40, 106–115. [Google Scholar] [CrossRef]
- Beaton, L.; Nica, L.; Tyldesley, S.; Sek, K.; Ayre, G.; Aparicio, M.; Gondara, L.; Speers, C.; Nichol, A. PET/CT of Breast Cancer Regional Nodal Recurrences: An Evaluation of Contouring Atlases. Radiat. Oncol. 2020, 15, 136. [Google Scholar] [CrossRef]
- Fowler, A.M.; Strigel, R.M. Clinical Advances in PET–MRI for Breast Cancer. Lancet Oncol. 2022, 23, e32–e43. [Google Scholar] [CrossRef]
- Ming, Y.; Wu, N.; Qian, T.; Li, X.; Wan, D.Q.; Li, C.; Li, Y.; Wu, Z.; Wang, X.; Liu, J.; et al. Progress and Future Trends in PET/CT and PET/MRI Molecular Imaging Approaches for Breast Cancer. Front. Oncol. 2020, 10, 1301. [Google Scholar] [CrossRef] [PubMed]
| Imaging Technique | Advantages | Disadvantages |
|---|---|---|
| Mammography | Screening—reduces mortality by up to 33% [18]; high sensitivity [17]. | Ionizing radiation [18]; low specificity in dense breasts [23]. |
| Tomosynthesis | Detection of early invasive breast cancer [18,20]; can detect small lesions and distortions [18,20]. | Doubles the radiation exposure compared to mammography [18,21]. |
| Contrast-enhanced mammography | Higher sensitivity and diagnosis performance compared to mammography [24,25]; can identify cancer in dense breasts [25]; tumor staging (for patients with claustrophobia or MRI-incompatible implants) [25]; preoperative estimation of disease extent [23]. | Allergy to contrast agents [23]; higher radiation dose than mammography [23]. |
| Breast ultrasound | Both in screening and diagnosis [27]; helpful in dense breast tissue; non-irradiating [27]; repetitive [27]; preoperative examination of the axilla [30]. | Does not detect small breast calcifications [27,29]. |
| Breast MRI | Higher accuracy in detecting lesions in women with dense breasts [40]; early detection of cancer recurrence [42]; evaluation of residual disease after neoadjuvant chemotherapy [41]. | Low specificity for both benign and malign lesions [47]; women with claustrophobia [25]; implants or other materials not compatible with MRI [25]. |
| Ultrasound-guided breast biopsy | Confirmation of neoplasia and its cellularity type [50,51,52]; lower risks and side effects compared to surgical biopsies [51]; high accuracy in detecting metastatic lymph nodes [51]; reduces unnecessary surgery [53]. | Associated risks: bruising and swelling, infection, bleeding [51,52]. |
| PET-CT | Systemic staging of the disease [56]; detection of distant metastases [57]; after neoadjuvant chemotherapy, can avoid radical mastectomy by detecting the presence of residual neoplasia better than MRI [61]. | Low sensitivity for primary breast tumors [56,57]; not able to detect cancers under 1 cm [57]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iacob, R.; Manolescu, D.L.; Stoicescu, E.R.; Fabian, A.; Malita, D.; Oancea, C. Breast Cancer—How Can Imaging Help? Healthcare 2022, 10, 1159. https://doi.org/10.3390/healthcare10071159
Iacob R, Manolescu DL, Stoicescu ER, Fabian A, Malita D, Oancea C. Breast Cancer—How Can Imaging Help? Healthcare. 2022; 10(7):1159. https://doi.org/10.3390/healthcare10071159
Chicago/Turabian StyleIacob, Roxana, Diana Luminita Manolescu, Emil Robert Stoicescu, Antonio Fabian, Daniel Malita, and Cristian Oancea. 2022. "Breast Cancer—How Can Imaging Help?" Healthcare 10, no. 7: 1159. https://doi.org/10.3390/healthcare10071159
APA StyleIacob, R., Manolescu, D. L., Stoicescu, E. R., Fabian, A., Malita, D., & Oancea, C. (2022). Breast Cancer—How Can Imaging Help? Healthcare, 10(7), 1159. https://doi.org/10.3390/healthcare10071159







