Tissutal and Fluidic Aspects in Osteopathic Manual Therapy: A Narrative Review
Abstract
1. Introduction
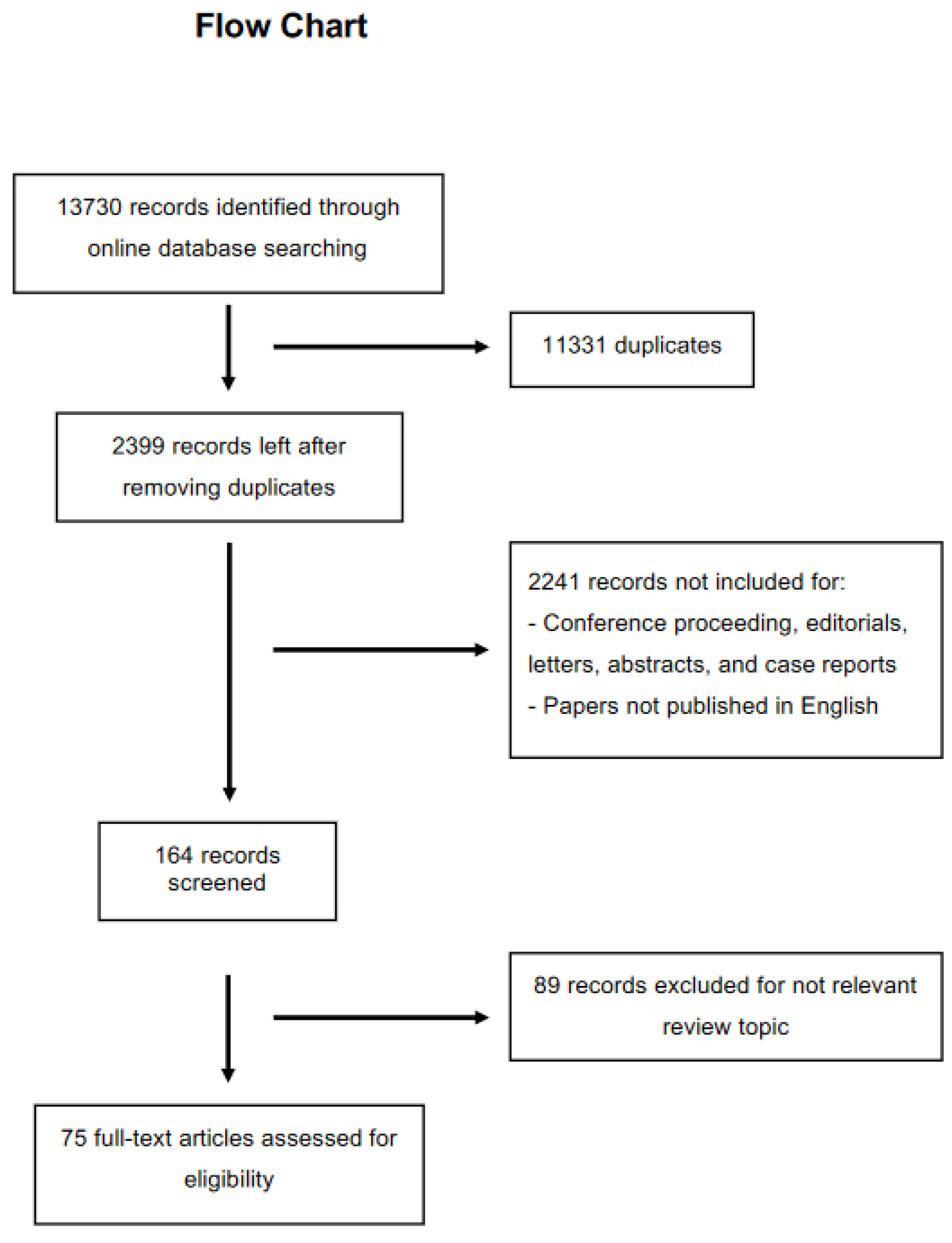
2. Methods
Inclusion Criteria of the Papers
3. Results
3.1. Redefine Inflammation
3.2. Some Water, Cells, and Body Fluids
3.3. Biophysics Aspects
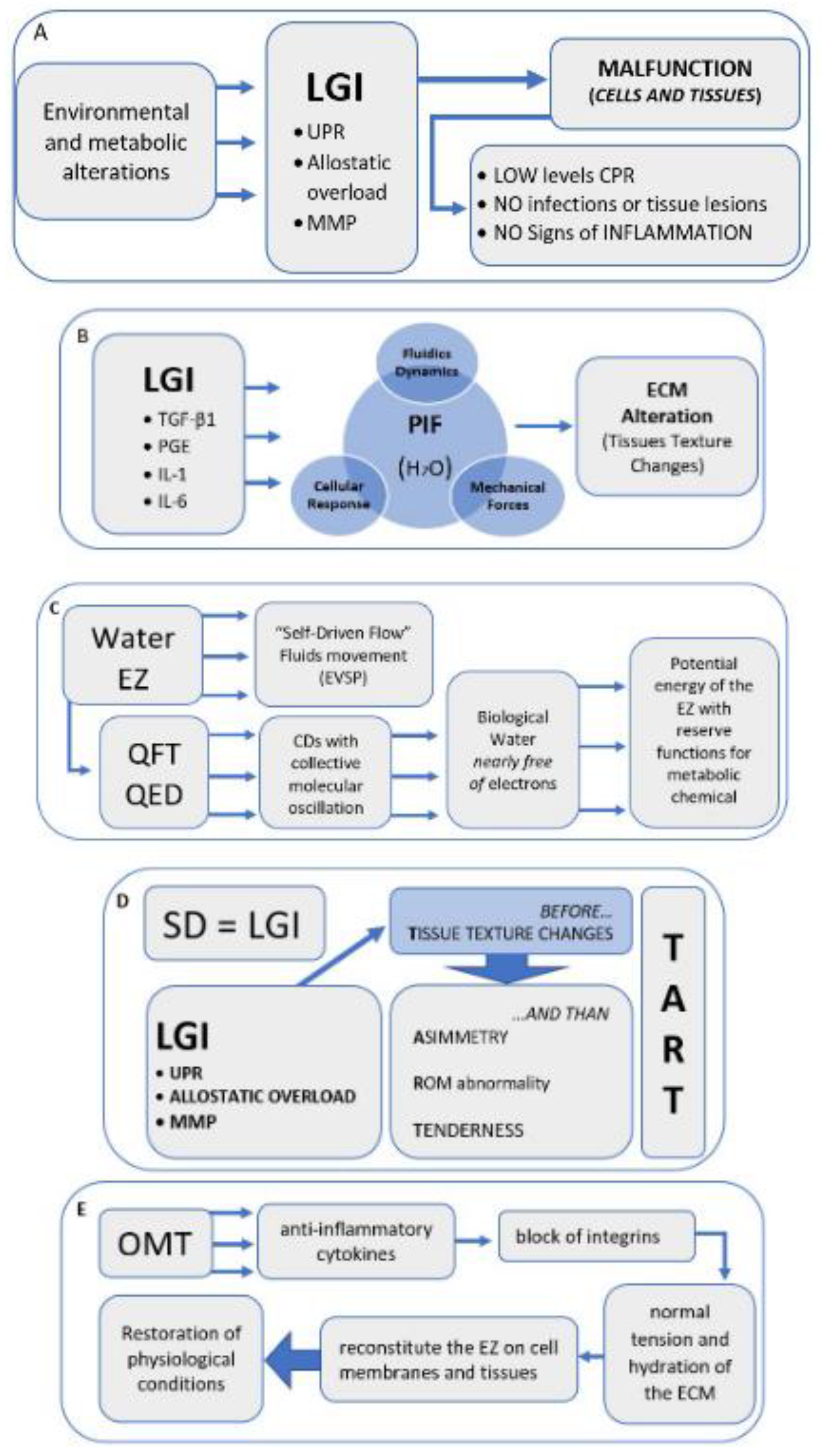
4. Discussion and Hypothesis
Hypothesis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
List of Abbreviations
| Somatic dysfunction | (SD) |
| Low-grade inflammation | (LGI) |
| Extracellular matrix | (ECM) |
| Exclusion zones | (EZ), |
| International classification of diseases | (ICD) |
| Osteopathic manipulative treatment | (OMT) |
| C-reactive protein | (CRP) |
| Unfolded protein response | (UPR) |
| Endoplasmic reticulum | (ER) |
| Matrix metalloproteinases | (MMP) |
| Fibroblasts | (FB) |
| Myofibroblasts | (MFB) |
| Interstitial fluid | (IF) |
| Fluid shear stress | (FSS) |
| Interstitial fluid pressure | (IFP) |
| Aquaporins | (AQP) |
| Electrokinetic vascular streaming potential | (EVSP) |
| Quantum field theory | (QFT) |
| Quantum electrodynamics | (QED) |
| Electromagnetic field | (EMF) |
| Coherence domain | (CD) |
| Tenderness, asymmetry, range of motion abnormality, and tissue texture changes | (TART) |
| Primary afferent nociceptors | (PAN) |
| Calcitonin gene-related peptide | (CGRP) |
| Unfolded protein response | (UPR) |
| Electromagnetic homeostasis | (EH) |
| Primary respiratory mechanism | (PRM) |
References
- Fryer, G. Somatic dysfunction: An osteopathic conundrum. Int. J. Osteopath. Med. 2016, 22, 52–63. [Google Scholar] [CrossRef]
- Chaitow, L. Somatic dysfunction and fascia’s gliding-potential. J. Bodyw. Mov. Ther. 2014, 18, 1–3. [Google Scholar] [CrossRef]
- Moran, R. Somatic dysfunction—Conceptually fascinating, but does it help us address health needs? Int. J. Osteopath. Med. 2016, 22, 1–2. [Google Scholar] [CrossRef]
- World Health Organization (WHO). International Statistical Classification of Diseases and Related Health Problems (ICD 11); WHO: Geneva, Switzerland, 2018. [Google Scholar]
- Ehrenfeuchter, W.C.; Kappler, R.E. Palpatory Examination. In Foundations of Osteopathic Medicine, 3rd ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2011; pp. 401–409. [Google Scholar]
- Kimberly, P.E.; Halma, K. Kirksville College of Osteopathic Medicine, Department of Osteopathic Theory and Methods. Outline of Osteopathic Manipulative Procedures: The Kimberly Manual 2006; Walsworth Pub. Co.: Marceline, MO, USA, 2008. [Google Scholar]
- Di Giovanna, E.L.; Schiowitz, S.; Dowling, D.J. An Osteopathic Approach to Diagnosis and Treatment; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2005; 746p. [Google Scholar]
- Giusti, R.; American Association of Colleges of Osteopathic Medicine; Educational Council on Osteopathic Principles. Glossary of Osteopathic Terminology; American Association of Colleges of Osteopathic Medicine: Chevy Chase, MD, USA, 2017. [Google Scholar]
- Licciardone, J.C.; Nelson, K.E.; Glonek, T.; Sleszynski, S.L.; Cruser, d.A. Osteopathic manipulative treatment of somatic dysfunction among patients in the family practice clinic setting: A retrospective analysis. J. Osteopath. Med. 2005, 105, 537–544. [Google Scholar]
- Tramontano, M.; Tamburella, F.; Dal Farra, F.; Bergna, A.; Lunghi, C.; Innocenti, M.; Cavera, F.; Savini, F.; Manzo, V.; D’Alessandro, G. International Overview of Somatic Dysfunction Assessment and Treatment in Osteopathic Research: A Scoping Review. Healthcare 2021, 10, 28. [Google Scholar] [CrossRef]
- Snider, K.T.; Johnson, J.C.; Snider, E.J.; Degenhardt, B.F. Increased incidence and severity of somatic dysfunction in subjects with chronic low back pain. J. Am. Osteopath. Assoc. 2008, 108, 372–378. [Google Scholar]
- Snider, K.T.; Schneider, R.P.; Snider, E.J.; Danto, J.B.; Lehnardt, C.W.; Ngo, C.S.; Johnson, J.C.; Sheneman, T.A. Correlation of Somatic Dysfunction with Gastrointestinal Endoscopic Findings: An Observational Study. J. Am. Osteopath. Assoc. 2016, 116, 358–369. [Google Scholar] [CrossRef]
- Waddington, E.L.; Snider, K.T.; Lockwood, M.D.; Pazdernik, V.K. Incidence of Somatic Dysfunction in Healthy Newborns. J. Am. Osteopath. Assoc. 2015, 115, 654–665. [Google Scholar] [CrossRef]
- Ruffini, N.; D’Alessandro, G.; Cardinali, L.; Frondaroli, F.; Cerritelli, F. Osteopathic manipulative treatment in gynecology and obstetrics: A systematic review. Complement. Ther. Med. 2016, 26, 72–78. [Google Scholar] [CrossRef]
- Lanaro, D.; Ruffini, N.; Manzotti, A.; Lista, G. Osteopathic manipulative treatment showed reduction of length of stay and costs in preterm infants: A systematic review and meta-analysis. Medicine 2017, 96, e6408. [Google Scholar] [CrossRef]
- Cicchitti, L.; Martelli, M.; Cerritelli, F. Chronic inflammatory disease and osteopathy: A systematic review. PLoS ONE. 2015, 10, e0121327. [Google Scholar] [CrossRef]
- Cerritelli, F.; Ruffini, N.; Lacorte, E.; Vanacore, N. Osteopathic manipulative treatment in neurological diseases: Systematic review of the literature. J. Neurol. Sci. 2016, 369, 333–341. [Google Scholar] [CrossRef]
- Cicchitti, L.; Di Lelio, A.; Barlafante, G.; Cozzolino, V.; Di Valerio, S.; Fusilli, P.; Lucisano, G.; Renzetti, C.; Verzella, M.; Rossi, M.C. Osteopathic Manipulative Treatment in Neonatal Intensive Care Units. Med. Sci. 2020, 8, 24. [Google Scholar] [CrossRef]
- Bagagiolo, D.; Rosa, D.; Borrelli, F. Efficacy and safety of osteopathic manipulative treatment: An overview of systematic reviews. BMJ Open 2022, 12, e053468. [Google Scholar] [CrossRef]
- Greenman, P.E. Principles of Manual Medicine, 3rd ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2003. [Google Scholar]
- Di Giovanna, E.L.; Amen, C.G.; Burns, D.K. An Osteopathic Approach to Diagnosis and Treatment; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2020. [Google Scholar]
- Chila, A.G. Foundations of Osteopathic Medicine; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2010; 1152p. [Google Scholar]
- Nicholas Penney, J. The biopsychosocial model of pain and contemporary osteopathic practice. Int. J. Osteopath. Med. 2010, 13, 42–47. [Google Scholar] [CrossRef]
- Lunghi, C.; Baroni, F. Cynefin Framework for Evidence-Informed Clinical Reasoning and Decision-Making. J. Am. Osteopath. Assoc. 2019, 119, 312–321. [Google Scholar] [CrossRef]
- Lunghi, C.; Consorti, G.; Tramontano, M.; Esteves, J.E.; Cerritelli, F. Perspectives on tissue adaptation related to allostatic load: Scoping review and integrative hypothesis with a focus on osteopathic palpation. J. Bodyw. Mov. Ther. 2020, 24, 212–220. [Google Scholar] [CrossRef]
- Esteves, J.E.; Zegarra-Parodi, R.; Dun, P.; van Cerritelli, F.; Vaucher, P. Models and theoretical frameworks for osteopathic care—A critical view and call for updates and research. Int. J. Osteopath. Med. 2020, 35, 1–4. [Google Scholar] [CrossRef]
- Bergna, A.; Vismara, L.; Parravicini, G.; Dal Farra, F. A new perspective for Somatic Dysfunction in Osteopathy: The Variability Model. J. Bodyw. Mov. Ther. 2020, 24, 181–189. [Google Scholar] [CrossRef]
- Hodge, L.M.; Bearden, M.K.; Schander, A.; Huff, J.B.; Williams, A.; King, H.H.; Downey, H.F. Lymphatic pump treatment mobilizes leukocytes from the gut associated lymphoid tissue into lymph. Lymphat. Res. Biol. 2010, 8, 103–110. [Google Scholar] [CrossRef]
- Meltzer, K.R.; Standley, P.R. Modeled repetitive motion strain and indirect osteopathic manipulative techniques in regulation of human fibroblast proliferation and interleukin secretion. J. Am. Osteopath. Assoc. 2007, 107, 527–536. [Google Scholar]
- Dodd, J.G.; Good, M.M.; Nguyen, T.L.; Grigg, A.I.; Batia, L.M.; Standley, P.R. In vitro biophysical strain model for understanding mechanisms of osteopathic manipulative treatment. J. Am. Osteopath. Assoc. 2006, 106, 157–166. [Google Scholar]
- Zein-Hammoud, M.; Standley, P.R. Modeled Osteopathic Manipulative Treatments: A Review of Their in Vitro Effects on Fibroblast Tissue Preparations. J. Am. Osteopath. Assoc. 2015, 115, 490–502. [Google Scholar] [CrossRef]
- Cao, T.V.; Hicks, M.R.; Campbell, D.; Standley, P.R. Dosed myofascial release in three-dimensional bioengineered tendons: Effects on human fibroblast hyperplasia, hypertrophy, and cytokine secretion. J. Manip. Physiol. Ther. 2013, 36, 513–521. [Google Scholar] [CrossRef]
- Schander, A.; Downey, H.F.; Hodge, L.M. Lymphatic pump manipulation mobilizes inflammatory mediators into lymphatic circulation. Exp. Biol. Med. 2012, 237, 58–63. [Google Scholar] [CrossRef]
- Degenhardt, B.F.; Darmani, N.A.; Johnson, J.C.; Towns, L.C.; Rhodes, D.C.; Trinh, C.; McClanahan, B.; DiMarzo, V. Role of osteopathic manipulative treatment in altering pain biomarkers: A pilot study. J. Am. Osteopath. Assoc. 2007, 107, 387–400. [Google Scholar]
- Licciardone, J.C.; Kearns, C.M.; Hodge, L.M.; Bergamini, M.V. Associations of cytokine concentrations with key osteopathic lesions and clinical outcomes in patients with nonspecific chronic low back pain: Results from the OSTEOPATHIC Trial. J. Am. Osteopath. Assoc. 2012, 112, 596–605, Erratum in: J. Am. Osteopath. Assoc. 2017, 117, 350. [Google Scholar] [CrossRef]
- Robert, S.; Gicquel, T.; Victoni, T.; Valença, S.; Barreto, E.; Bailly-Maître, B.; Boichot, E.; Lagente, V. Involvement of matrix metalloproteinases (MMPs) and inflammasome pathway in molecular mechanisms of fibrosis. Biosci. Rep. 2016, 36, e00360. [Google Scholar] [CrossRef]
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef]
- McEwen, B.S.; Wingfield, J.C. What is in a name? Integrating homeostasis, allostasis and stress. Horm. Behav. 2010, 57, 105–111. [Google Scholar] [CrossRef]
- Sharma, A.; Adams, C.; Cashdollar, B.D.; Li, Z.; Nguyen, N.V.; Sai, H.; Shi, J.; Velchuru, G.; Zhu, K.Z.; Pollack, G.H. Effect of Health-Promoting Agents on Exclusion-Zone Size. Dose-Response Publ. Int. Hormesis Soc. 2018, 16, 1559325818796937. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Pollack, G.H. Healthy fats and exclusion-zone size. Food Chem. 2020, 316, 126305. [Google Scholar] [CrossRef] [PubMed]
- Tozzi, P. A unifying neuro-fasciagenic model of somatic dysfunction-underlying mechanisms and treatment-Part I. J Bodyw. Mov. Ther. 2015, 19, 310–326. [Google Scholar] [CrossRef] [PubMed]
- Del Giudice, E.; Tedeschi, A. Water and autocatalysis in living matter. Electromagn. Biol. Med. 2009, 28, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Pollack, G.H. The Fourth Phase of Water: A role in fascia? J. Bodyw. Mov. Ther. 2013, 17, 510–511. [Google Scholar] [CrossRef][Green Version]
- Gasparyan, A.Y.; Ayvazyan, L.; Blackmore, H.; Kitas, G.D. Writing a narrative biomedical review: Considerations for authors, peer reviewers, and editors. Rheumatol. Int. 2011, 31, 1409–1417. [Google Scholar] [CrossRef]
- Greenhalgh, T.; Peacock, R. Effectiveness and efficiency of search methods in systematic reviews of complex evidence: Audit of primary sources. BMJ 2005, 331, 1064–1065. [Google Scholar] [CrossRef]
- Karin, M.; Clevers, H. Reparative inflammation takes charge of tissue regeneration. Nature 2016, 529, 307–315. [Google Scholar] [CrossRef]
- Kumar, V.; Cotran, R.S. Robbins’ Basic Pathology; Saunders/Elsevier: Philadelphia, PA, USA, 2010. [Google Scholar]
- Danesh, J. Smoldering arteries? Low-grade inflammation and coronary heart disease. JAMA 1999, 282, 2169–2171. [Google Scholar] [CrossRef]
- Festa, A.; D’Agostino, R.; Howard, G.; Mykkänen, L.; Tracy, R.P.; Haffner, S.M. Chronic subclinical inflammation as part of the insulin resistance syndrome: The Insulin Resistance Atherosclerosis Study (IRAS). Circulation 2000, 102, 42–47. [Google Scholar] [CrossRef]
- Guarner, V.; Rubio-Ruiz, M.E. Low-grade systemic inflammation connects aging, metabolic syndrome and cardiovascular disease. Interdiscip. Top. Gerontol. 2015, 40, 99–106. [Google Scholar] [PubMed]
- Żelechowska, P.; Agier, J.; Kozłowska, E.; Brzezińska-Błaszczyk, E. Mast cells participate in chronic low-grade inflammation within adipose tissue. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2018, 19, 686–697. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, M.; Kushner, I. It’s time to redefine inflammation. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2017, 31, 1787–1791. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S. Stress, adaptation, and disease. Allostasis and allostatic load. Ann. N. Y. Acad. Sci. 1998, 840, 33–44. [Google Scholar] [CrossRef]
- Sterling, P.; Eyer, J. Allostasis: A new paradigm to explain arousal pathology. In Handbook of Life Stress, Cognition and Health; John Wiley & Sons: Philadelphia, PA, USA, 1988; pp. 629–649. [Google Scholar]
- McEwen, B.S.; Wingfield, J.C. The concept of allostasis in biology and biomedicine. Horm. Behav. 2003, 43, 2–15. [Google Scholar] [CrossRef]
- Selye, H. The Stress of Life, 2nd ed.; McGraw-Hill Educations: New York, NY, USA, 1978. [Google Scholar]
- Amen, O.M.; Sarker, S.D.; Ghildyal, R.; Arya, A. Endoplasmic Reticulum Stress Activates Unfolded Protein Response Signaling and Mediates Inflammation, Obesity, and Cardiac Dysfunction: Therapeutic and Molecular Approach. Front. Pharmacol. 2019, 10, 977. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef]
- Gusev, E.Y.; Zotova, N.V. Cellular Stress and General Pathological Processes. Curr. Pharm. Des. 2019, 25, 251–297. [Google Scholar] [CrossRef]
- Todd, D.J.; Lee, A.-H.; Glimcher, L.H. The endoplasmic reticulum stress response in immunity and autoimmunity. Nat. Rev. Immunol. 2008, 8, 663–674. [Google Scholar] [CrossRef]
- Rohleder, N. Stress System Regulation of Chronic Low-grade Inflammation. Adv. Neuroimmune Biol. 2012, 3, 265–276. [Google Scholar] [CrossRef]
- Cohen, S.; Janicki-Deverts, D.; Miller, G.E. Psychological stress and disease. JAMA 2007, 298, 1685–1687. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A.; Vannella, K.M. Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity 2016, 44, 450–462. [Google Scholar] [CrossRef] [PubMed]
- Knipper, J.A.; Willenborg, S.; Brinckmann, J.; Bloch, W.; Maaß, T.; Wagener, R.; Krieg, T.; Sutherland, T.; Munitz, A.; Rothenberg, M.E.; et al. Interleukin-4 Receptor α Signaling in Myeloid Cells Controls Collagen Fibril Assembly in Skin Repair. Immunity 2015, 43, 803–816. [Google Scholar] [CrossRef] [PubMed]
- Malemud, C.J. Matrix metalloproteinases (MMPs) in health and disease: An overview. Front. Biosci. J. Virtual Libr. 2006, 11, 1696–1701. [Google Scholar] [CrossRef]
- Alameddine, H.S. Matrix metalloproteinases in skeletal muscles: Friends or foes? Neurobiol. Dis. 2012, 48, 508–518. [Google Scholar] [CrossRef]
- Bautista-Hernández, L.A.; Gómez-Olivares, J.L.; Buentello-Volante, B.; Bautista-de Lucio, V.M. Fibroblasts: The Unknown Sentinels Eliciting Immune Responses Against Microorganisms. Eur. J. Microbiol. Immunol. 2017, 7, 151–157. [Google Scholar] [CrossRef]
- Langevin, H.M.; Nedergaard, M.; Howe, A.K. Cellular control of connective tissue matrix tension. J. Cell. Biochem. 2013, 114, 1714–1719. [Google Scholar] [CrossRef]
- Janmey, P.A.; Winer, J.P.; Murray, M.E.; Wen, Q. The hard life of soft cells. Cell Migr. 2009, 66, 597–605. [Google Scholar] [CrossRef]
- Langevin, H.M.; Bouffard, N.A.; Fox, J.R.; Palmer, B.M.; Wu, J.; Iatridis, J.C.; Barnrs, W.D.; Badger, G.J.; Howe, A.K. Fibroblast cytoskeletal remodeling contributes to connective tissue tension. J. Cell. Physiol. 2011, 226, 1166–1175. [Google Scholar] [CrossRef]
- Schleip, R.; Gabbiani, G.; Wilke, J.; Naylor, I.; Hinz, B.; Zorn, A.; Jäger, H.; Breul, R.; Schreiner, S.; Klingler, W. Fascia Is Able to Actively Contract and May Thereby Influence Musculoskeletal Dynamics: A Histochemical and Mechanographic Investigation. Front. Physiol. 2019, 10, 336. [Google Scholar] [CrossRef]
- Schleip, R.; Klingler, W. Active contractile properties of fascia. Clin. Anat. N. Y. 2019, 32, 891–895. [Google Scholar] [CrossRef] [PubMed]
- Schleip, R.; Duerselen, L.; Vleeming, A.; Naylor, I.L.; Lehmann-Horn, F.; Zorn, A.; Jaeger, H.; Klingler, W. Strain hardening of fascia: Static stretching of dense fibrous connective tissues can induce a temporary stiffness increase accompanied by enhanced matrix hydration. J. Bodyw. Mov. Ther. 2012, 16, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Benias, P.C.; Wells, R.G.; Sackey-Aboagye, B.; Klavan, H.; Reidy, J.; Buonocore, D.; Miranda, M.; Kornacki, S.; Wayne, M.; Carr-Locke, D.L.; et al. Structure and Distribution of an Unrecognized Interstitium in Human Tissues. Sci. Rep. 2018, 8, 4947. [Google Scholar] [CrossRef] [PubMed]
- Varma, S.; Voldman, J. A cell-based sensor of fluid shear stress for microfluidics. Lab Chip 2015, 15, 1563–1573. [Google Scholar] [CrossRef] [PubMed]
- Adamo, L.; García-Cardeña, G. Directed stem cell differentiation by fluid mechanical forces. Antioxid. Redox Signal. 2011, 15, 1463–1473. [Google Scholar] [CrossRef] [PubMed]
- Hinz, B.; Phan, S.H.; Thannickal, V.J.; Prunotto, M.; Desmoulière, A.; Varga, J.; De Wever, O.; Mareel, M.; Gabbiani, G. Recent developments in myofibroblast biology: Paradigms for connective tissue remodeling. Am. J. Pathol. 2012, 180, 1340–1355. [Google Scholar] [CrossRef]
- Ingber, D.E. Tensegrity II. How structural networks influence cellular information processing networks. J. Cell Sci. 2003, 116 Pt 8, 1397–1408. [Google Scholar] [CrossRef]
- Ingber, D.E.; Tensegrity, I. Cell structure and hierarchical systems biology. J. Cell Sci. 2003, 116 Pt 7, 1157–1173. [Google Scholar] [CrossRef]
- Pedersen, J.A.; Lichter, S.; Swartz, M.A. Cells in 3D matrices under interstitial flow: Effects of extracellular matrix alignment on cell shear stress and drag forces. J. Biomech. 2010, 43, 900–905. [Google Scholar] [CrossRef]
- Ng, C.P.; Hinz, B.; Swartz, M.A. Interstitial fluid flow induces myofibroblast differentiation and collagen alignment in vitro. J Cell Sci. 2005, 118 Pt 20, 4731–4739. [Google Scholar] [CrossRef]
- Rodt, S.A.; Reed, R.K. Interstitial fluid pressure in rat skin becomes more negative in the initial phase of carrageenan-induced edema. Int. J. Microcirc. Clin. Exp. 1993, 12, 299–312. [Google Scholar] [PubMed]
- Berg, A.; Rubin, K.; Reed, R.K. Cytochalasin D induces edema formation and lowering of interstitial fluid pressure in rat dermis. Am. J. Physiol.-Heart Circ. Physiol. 2001, 281, H7–H13. [Google Scholar] [CrossRef] [PubMed]
- Reed, R.K.; Rubin, K. Transcapillary exchange: Role and importance of the interstitial fluid pressure and the extracellular matrix. Cardiovasc. Res. 2010, 87, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Meli, R.; Pirozzi, C.; Pelagalli, A. New Perspectives on the Potential Role of Aquaporins (AQPs) in the Physiology of Inflammation. Front. Physiol. 2018, 9, 101. [Google Scholar] [CrossRef] [PubMed]
- Zannetti, A.; Benga, G.; Brunetti, A.; Napolitano, F.; Avallone, L.; Pelagalli, A. Role of Aquaporins in the Physiological Functions of Mesenchymal Stem Cells. Cells 2020, 9, 2678. [Google Scholar] [CrossRef]
- Igarashi, H.; Tsujita, M.; Kwee, I.L.; Nakada, T. Water influx into cerebrospinal fluid is primarily controlled by aquaporin-4, not by aquaporin-1: 17O JJVCPE MRI study in knockout mice. NeuroReport 2014, 25, 39–43. [Google Scholar] [CrossRef]
- Verkman, A.S. Aquaporins. Curr. Biol. Gennaio 2013, 23, R52–R55. [Google Scholar] [CrossRef]
- Mariajoseph-Antony, L.F.; Kannan, A.; Panneerselvam, A.; Loganathan, C.; Shankar, E.M.; Anbarasu, K.; Prahalathan, C. Role of Aquaporins in Inflammation-a Scientific Curation. Inflammation 2020, 43, 1599–1610. [Google Scholar] [CrossRef]
- Hoffmann, E.K.; Lambert, I.H.; Pedersen, S.F. Physiology of Cell Volume Regulation in Vertebrates. Physiol. Rev. 2009, 89, 193–277. [Google Scholar] [CrossRef]
- Ling, G.N. A New Theoretical Foundation for the Polarized-Oriented Multilayer Theory of Cell Water and for Inanimate Systems Demonstrating Long-range Dynamic Structuring of Water Molecules. Physiol. Chem. Phys. Med. NMR 2003, 35, 91–130. [Google Scholar]
- Zheng, J.; Pollack, G.H. Long-range forces extending from polymer-gel surfaces. Phys. Rev. E 2003, 68, 031408. [Google Scholar] [CrossRef] [PubMed]
- De Ninno, A.; Pregnolato, M. Electromagnetic homeostasis and the role of low-amplitude electromagnetic fields on life organization. Electromagn. Biol. Med. 2017, 36, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Pollack, G.H. Cells, Gels and the Engines of Life. (A New, Unifying Approach to Cell Function), 1st ed.; Ebner and Sons Publisher: Seattle, DC, USA, 2001; 305p. [Google Scholar]
- Pizzitutti, F.; Marchi, M.; Sterpone, F.; Rossky, P.J. How protein surfaces induce anomalous dynamics of hydration water. J. Phys. Chem. B 2007, 111, 7584–7590. [Google Scholar] [CrossRef]
- Seneff, S.; Nigh, G. Sulfate’s Critical Role for Maintaining Exclusion Zone Water: Dietary Factors Leading to Deficiencies. Water 2019, 11, 22–42. [Google Scholar]
- Kerch, G. Distribution of tightly and loosely bound water in biological macromolecules and age-related diseases. Int. J. Biol. Macromol. 2018, 118 Pt A, 1310–1318. [Google Scholar] [CrossRef]
- Li, Z.; Pollack, G.H. Surface-induced flow: A natural microscopic engine using infrared energy as fuel. Sci. Adv. 2020, 6, eaba0941. [Google Scholar] [CrossRef]
- Seneff, S.; Davidson, R.M.; Lauritzen, A.; Samsel, A.; Wainwright, G. A novel hypothesis for atherosclerosis as a cholesterol sulfate deficiency syndrome. Theor. Biol. Med. Model. 2015, 12, 9. [Google Scholar] [CrossRef]
- Trivedi, D.P.; Hallock, K.J.; Bergethon, P.R. Electric fields caused by blood flow modulate vascular endothelial electrophysiology and nitric oxide production. Bioelectromagnetics 2013, 34, 22–30. [Google Scholar] [CrossRef]
- Preparata, G. Qed Coherence in Matter; World Scientific: Singapore, 1995; 251p. [Google Scholar]
- Germano, R. Water’s quantum structures and life. Electromagn. Biol. Med. 2015, 34, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Brizhik, L.S.; Del Giudice, E.; Popp, F.-A.; Maric-Oehler, W.; Schlebusch, K.-P. On the dynamics of self-organization in living organisms. Electromagn. Biol. Med. 2009, 28, 28–40. [Google Scholar] [CrossRef]
- Del Giudice, E.; Preparata, G. Coherent dynamics in water as a possible explanation of biological membranes formation. J. Biol. Phys. 1994, 20, 105–116. [Google Scholar] [CrossRef]
- Arani, R.; Bono, I.; Del Giudice, E.; Preparata, G. QED Coherence and the Thermodynamics of Water; World Scientific Publishing Company: Singapore, 1995; Volume 9. [Google Scholar]
- Del Giudice, E.; Spinetti, P.R.; Tedeschi, A. Water Dynamics at the Root of Metamorphosis in Living Organisms. Water 2010, 2, 566–586. [Google Scholar] [CrossRef]
- Montagnier, L.; Aissa, J.; Del Giudice, E.; Lavallee, C.; Tedeschi, A.; Vitiello, G. DNA waves and water. J. Phys. Conf. Ser. 2011, 306, 012007. [Google Scholar] [CrossRef]
- Santana-Blank, L.; Rodríguez-Santana, E.; Santana-Rodríguez, K.E. Photobiomodulation of aqueous interfaces as selective rechargeable bio-batteries in complex diseases: Personal view. Photomed. Laser Surg. 2012, 30, 242–249. [Google Scholar] [CrossRef]
- Del Giudice, E.; Pulselli, R.M.; Tiezzi, E. Thermodynamics of irreversible processes and quantum field theory: An interplay for the understanding of ecosystem dynamics. Ecol. Model. 2009, 220, 1874–1879. [Google Scholar] [CrossRef]
- Marchettini, N.; Del Giudice, E.; Voeikov, V.; Tiezzi, E. Water: A medium where dissipative structures are produced by a coherent dynamics. J. Theor. Biol. 2010, 265, 511–516. [Google Scholar] [CrossRef]
- Vladimir, V.; Del Giudice, E. Water respiration—The basis of the living state. Water 2009, 1, 52–75. [Google Scholar]
- Barbieri, M. The Organic Codes. An Introduction to Semantic Biology; Cambridge University Press: Cambridge, UK, 2003. [Google Scholar]
- Oesper, P. Bioenergetics (Szent-Gyorgyi, Albert). J. Chem. Educ. 1957, 34, 627. [Google Scholar] [CrossRef][Green Version]
- Degenhardt, B.F.; Snider, K.T.; Snider, E.J.; Johnson, J.C. Interobserver reliability of osteopathic palpatory diagnostic tests of the lumbar spine: Improvements from consensus training. J. Am. Osteopath. Assoc. 2005, 105, 465–473. [Google Scholar]
- Fryer, G.; Gibbons, P.; Morris, T. The relation between thoracic paraspinal tissues and pressure sensitivity measured by a digital algometer. J. Osteopath. Med. 2004, 7, 64–69. [Google Scholar] [CrossRef]
- Brink, R.C.; Schlösser, T.P.C.; Colo, D.; Vincken, K.L.; van Stralen, M.; Hui, S.C.N.; Chu, W.C.W.; Cheng, J.C.Y.; Castelein, R.M. Asymmetry of the Vertebral Body and Pedicles in the True Transverse Plane in Adolescent Idiopathic Scoliosis: A CT-Based Study. Spine Deform. 2017, 5, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Kanchan, T.; Mohan Kumar, T.S.; Pradeep Kumar, G.; Yoganarasimha, K. Skeletal asymmetry. J. Forensic Leg. Med. 2008, 15, 177–179. [Google Scholar] [CrossRef]
- Thevenot, J.; Pulkkinen, P.; Kuhn, V.; Eckstein, F.; Jämsä, T. Structural asymmetry between the hips and its relation to experimental fracture type. Calcif. Tissue Int. 2010, 87, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Howell, J.N.; Willard, F. Nociception: New Understandings and Their Possible Relation to Somatic Dysfunction and Its Treatment. Ohio. Res. Clin. Rev. 2005, 15, 12–15. [Google Scholar]
- D’Alessandro, G.; Cerritelli, F.; Cortelli, P. Sensitization and Interoception as Key Neurological Concepts in Osteopathy and Other Manual Medicines. Front. Neurosci. 2016, 10, 100. [Google Scholar] [CrossRef]
- Sorkin, L.S.; Eddinger, K.A.; Woller, S.A.; Yaksh, T.L. Origins of antidromic activity in sensory afferent fibers and neurogenic inflammation. Semin. Immunopathol. 2018, 40, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Xanthos, D.N.; Sandkühler, J. Neurogenic neuroinflammation: Inflammatory CNS reactions in response to neuronal activity. Nat. Rev. Neurosci. 2014, 15, 43–53. [Google Scholar] [CrossRef]
- Brain, S.D. Sensory neuropeptides: Their role in inflammation and wound healing. Immunopharmacology 1997, 37, 133–152. [Google Scholar] [CrossRef]
- Craig, A.D. How do you feel? Interoception: The sense of the physiological condition of the body. Nat. Rev. Neurosci. 2002, 3, 655–666. [Google Scholar] [CrossRef]
- Wang, J.; Ren, Y.; Zou, X.; Fang, L.; Willis, W.D.; Lin, Q. Sympathetic influence on capsaicin-evoked enhancement of dorsal root reflexes in rats. J. Neurophysiol. 2004, 92, 2017–2026. [Google Scholar] [CrossRef][Green Version]
- Denslow, J.S. Pathophysiologic evidence for the osteopathic lesion: The known, unknown, and controversial. J. Am. Osteopath. Assoc. 1975, 75, 415–421. [Google Scholar]
- Korr, I.M. The neural basis of the osteopathic lesion. J. Am. Osteopath. Assoc. 1947, 47, 191–198. [Google Scholar]
- Walkowski, S.; Singh, M.; Puertas, J.; Pate, M.; Goodrum, K.; Benencia, F. Osteopathic manipulative therapy induces early plasma cytokine release and mobilization of a population of blood dendritic cells. PLoS ONE 2014, 9, e90132. [Google Scholar] [CrossRef]
- Ruffini, N.; D’Alessandro, G.; Mariani, N.; Pollastrelli, A.; Cardinali, L.; Cerritelli, F. Variations of high frequency parameter of heart rate variability following osteopathic manipulative treatment in healthy subjects compared to control group and sham therapy: Randomized controlled trial. Front. Neurosci. 2015, 9, 272. [Google Scholar] [CrossRef] [PubMed]
- Giles, P.D.; Hensel, K.L.; Pacchia, C.F.; Smith, M.L. Suboccipital decompression enhances heart rate variability indices of cardiac control in healthy subjects. J. Altern. Complement. Med. N. Y. 2013, 19, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Cenaj, O.; Allison, D.H.R.; Imam, R.; Zeck, B.; Drohan, L.M.; Chiriboga, L.; Llewellyn, J.; Liu, C.Z.; Park, Y.N.; Wells, R.G.; et al. Evidence for continuity of interstitial spaces across tissue and organ boundaries in humans. Commun. Biol. 2021, 4, 436. [Google Scholar] [CrossRef] [PubMed]
- Pollack, G.H. The Fourth Phase of Water: Beyond Solid, Liquid, and Vapor; Ebner and Sons Publisher: Seattle, DC, USA, 2013. [Google Scholar]
- Parravicini, G.; Bergna, A. Biological effects of direct and indirect manipulation of the fascial system. Narrative review. J. Bodyw. Mov. Ther. 2017, 21, 435–445. [Google Scholar] [CrossRef]
- Castillo, R.; Schander, A.; Hodge, L.M. Lymphatic Pump Treatment Mobilizes Bioactive Lymph That Suppresses Macrophage Activity In Vitro. J. Am. Osteopath. Assoc. 2018, 118, 455–461. [Google Scholar] [CrossRef]
- Henderson, A.T.; Fisher, J.F.; Blair, J.; Shea, C.; Li, T.S.; Bridges, K.G. Effects of rib raising on the autonomic nervous system: A pilot study using noninvasive biomarkers. J. Am. Osteopath. Assoc. 2010, 110, 324–330. [Google Scholar]
- Sutherland, W.G. The cranial bowl. 1944. J. Am. Osteopath. Assoc. 2000, 100, 568–573. [Google Scholar]
- Rollin, E.B. Life in Motion; Stillness Press LLC.: Portland, OR, USA, 1997. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verzella, M.; Affede, E.; Di Pietrantonio, L.; Cozzolino, V.; Cicchitti, L. Tissutal and Fluidic Aspects in Osteopathic Manual Therapy: A Narrative Review. Healthcare 2022, 10, 1014. https://doi.org/10.3390/healthcare10061014
Verzella M, Affede E, Di Pietrantonio L, Cozzolino V, Cicchitti L. Tissutal and Fluidic Aspects in Osteopathic Manual Therapy: A Narrative Review. Healthcare. 2022; 10(6):1014. https://doi.org/10.3390/healthcare10061014
Chicago/Turabian StyleVerzella, Marco, Erika Affede, Luca Di Pietrantonio, Vincenzo Cozzolino, and Luca Cicchitti. 2022. "Tissutal and Fluidic Aspects in Osteopathic Manual Therapy: A Narrative Review" Healthcare 10, no. 6: 1014. https://doi.org/10.3390/healthcare10061014
APA StyleVerzella, M., Affede, E., Di Pietrantonio, L., Cozzolino, V., & Cicchitti, L. (2022). Tissutal and Fluidic Aspects in Osteopathic Manual Therapy: A Narrative Review. Healthcare, 10(6), 1014. https://doi.org/10.3390/healthcare10061014





