The Prevalence of High-Risk Prescribing of Oral Non-Steroidal Anti-Inflammatory Drugs in Primary Healthcare: A Single-Centre Retrospective Chart Review Study
Abstract
:1. Introduction
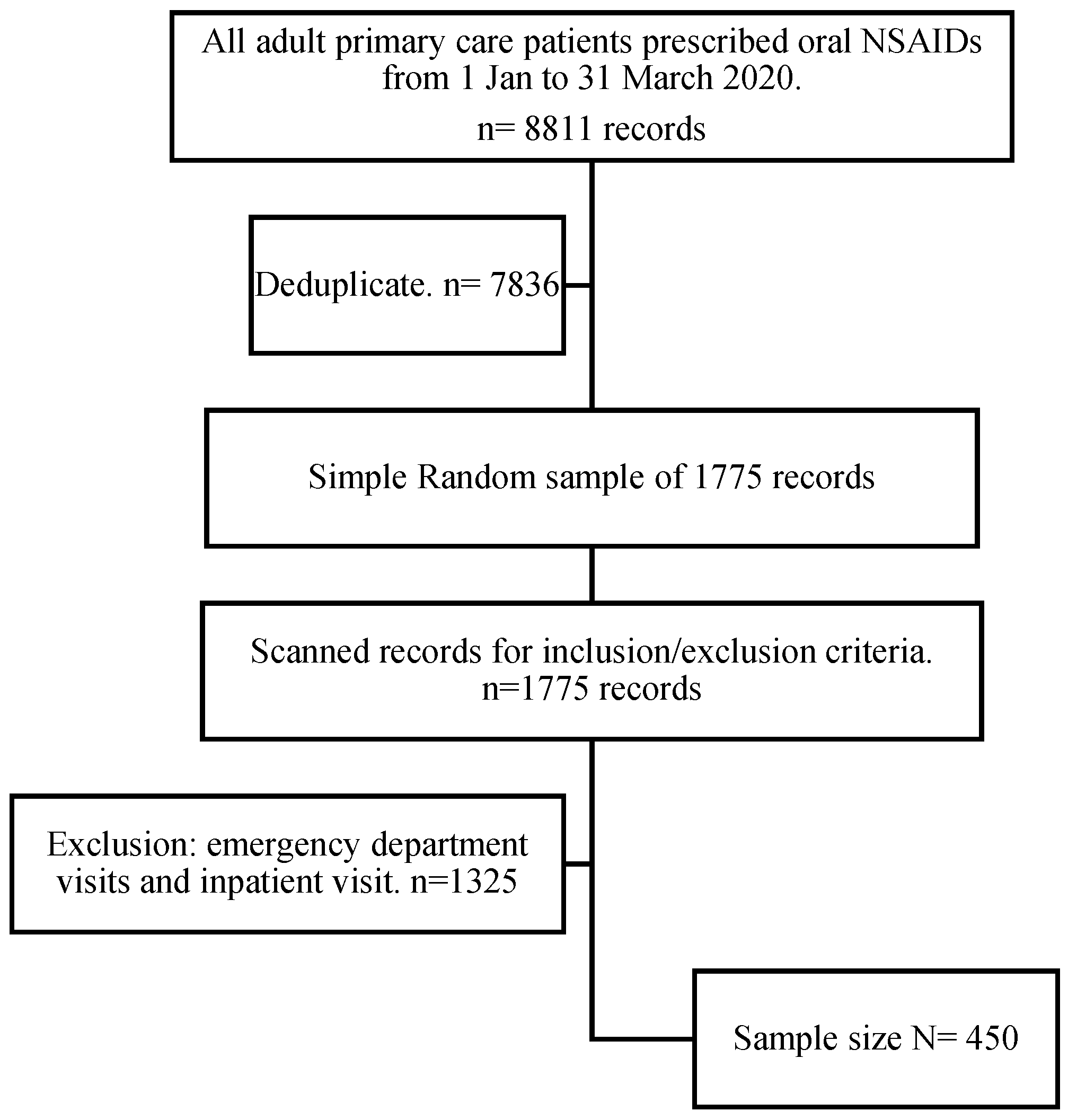
2. Materials and Methods
2.1. Study Design and Setting
Reporting
2.2. Ethical Considerations
2.3. Participants
2.3.1. Baseline Characteristics
2.3.2. Inclusion Criteria
2.3.3. Exclusion Criteria
2.4. Variables
- a.
- Outcome variables:
- b.
- Risk factors
- c.
- Exposure
2.5. Data Collection Data Source
2.6. Bias
2.7. Sample Size Estimation
- (1)
- Retrospective chart review study sample size:
- (2)
- Pilot sample size (10% of retrospective chart review total sample size) [30]: 10 × 380/100 = 38 records.
- (3)
- Final adjusted sample size:
2.8. Statistical Methods
2.9. Data Access and Cleaning Methods
2.10. Patient and Public Involvement
3. Results
3.1. Demographic Characteristics
3.2. Prescribing Safety Indicator Rate
3.3. Risk Factors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Howard, R.L.; Avery, A.J.; Slavenburg, S.; Royal, S.; Pipe, G.; Lucassen, P.; Pirmohamed, M. Which drugs cause preventable admissions to hospital? A systematic review. Br. J. Clin. Pharmacol. 2007, 63, 136–147. Available online: https://onlinelibrary.wiley.com/doi/10.1111/j.1365-2125.2006.02698.x (accessed on 1 April 2022). [CrossRef] [PubMed] [Green Version]
- Guthrie, B.; Yu, N.; Murphy, D.; Donnan, P.T.; Dreischulte, T. Measuring prevalence, reliability and variation in high-risk prescribing in general practice using multilevel modelling of observational data in a population database. Health Serv. Deliv. Res. 2015, 3, 1–140. Available online: https://www.journalslibrary.nihr.ac.uk/hsdr/hsdr03420/ (accessed on 15 November 2021). [CrossRef] [PubMed] [Green Version]
- Spencer, R.; Bell, B.; Avery, A.J.; Gookey, G.; Campbell, S.M. Royal College of General Practitioners. Identification of an updated set of prescribing—Safety indicators for GPs. Br. J. Gen. Pract. 2014, 64, 181–190. Available online: http://www.ncbi.nlm.nih.gov/pubmed/24686882 (accessed on 15 November 2021). [CrossRef] [PubMed] [Green Version]
- Curtis, H.J.; Dennis, J.M.; Shields, B.M.; Walker, A.J.; Bacon, S.; Hattersley, A.T.; Jones, A.G.; Goldacre, B. Time trends and geographical variation in prescribing of drugs for diabetes in England from 1998 to 2017. Diabetes Obes. Metab. 2018, 20, 2159–2168. Available online: https://onlinelibrary.wiley.com/doi/10.1111/dom.13346 (accessed on 15 November 2021).
- Curtis, H.J.; Walker, A.J.; Mahtani, K.R.; Goldacre, B. Time trends and geographical variation in prescribing of antibiotics in England 1998–2007. J. Antimicrob. Chemother. 2019, 74, 242–250. Available online: https://academic.oup.com/jac/advance-article/doi/10.1093/jac/dky377/5098536 (accessed on 15 November 2021).
- Bregnhøj, L.; Thirstrup, S.; Kristensen, M.B.; Sonne, J. Reliability of a modified medication appropriateness index in primary care. Eur. J. Clin. Pharmacol. 2005, 61, 769–773. Available online: http://link.springer.com/10.1007/s00228-005-0963-0 (accessed on 15 November 2021). [CrossRef]
- Hanlon, J.T.; Schmader, K.E. The Medication Appropriateness Index at 20: Where It Started, Where It Has Been, and Where It May Be Going. Drugs Aging 2013, 30, 893–900. Available online: http://link.springer.com/10.1007/s40266-013-0118-4 (accessed on 15 November 2021). [CrossRef] [Green Version]
- Guthrie, B.; McCowan, C.; Davey, P.; Simpson, C.R.; Dreischulte, T.; Barnett, K. High risk prescribing in primary care patients particularly vulnerable to adverse drug events: Cross Sectional Population Database analysis in Scottish General Practice. BMJ 2011, 342, d3514. Available online: https://www.bmj.com/lookup/doi/10.1136/bmj.d3514 (accessed on 15 November 2021). [CrossRef] [Green Version]
- Dreischulte, T.; Grant, A.M.; McCowan, C.; McAnaw, J.J.; Guthrie, B. Quality and safety of medication use in primary care: Consensus Validation of a New Set of Explicit Medication Assessment Criteria and Prioritisation of Topics for Improvement. BMC Clin. Pharmacol. 2012, 12, 5. Available online: http://link.springer.com/10.1186/1472-6904-12-5 (accessed on 15 November 2021). [CrossRef] [Green Version]
- Avery, A.J.; Dex, G.M.; Mulvaney, C.; Serumaga, B.; Spencer, R.; Lester, H.E.; Campbell, S.M. Development of prescribing-safety indicators for GPs using the RAND Appropriateness Method. Br. J. Gen. Pract. 2011, 61, 526–536. Available online: http://bjgp.org/lookup/doi/10.3399/bjgp11X588501 (accessed on 15 November 2021). [CrossRef] [Green Version]
- Babakor, S.D.; Al Ghamdi, M.M. Prevalence and Determinants of Over-the-Counter Analgesics Usage among Patients attending Primary Health Care Centers in Jeddah, Saudi Arabia. J. Young Pharm. 2018, 10, 91–97. Available online: https://www.jyoungpharm.org/article/1090 (accessed on 15 November 2021).
- AlKhamees, O.A.; AlNemer, K.A.; Bin Maneea, M.W.; AlSugair, F.A.; AlEnizi, B.H.; Alharf, A.A. Top 10 most used drugs in the Kingdom of Saudi Arabia 2010–2015. Saudi Pharm. J. 2018, 26, 211–216. Available online: https://linkinghub.elsevier.com/retrieve/pii/S1319016417302177 (accessed on 15 November 2021). [CrossRef] [PubMed]
- Al-Harkan, A.; Al-Harkan, N.; Al-Najjar, A.; Al-Hunti, A.; Al-Rashidi, A.; Al-Themery, A. Investigation of Medication Errors in a Tertiary Care Hospitals in the Qassim Region, Saudi Arabia. Open Access Maced. J. Med. Sci. 2020, 8, 209–212. Available online: https://oamjms.eu/index.php/mjms/article/view/4330 (accessed on 15 November 2021). [CrossRef]
- Alharaibi, M.A.; Alhifany, A.A.; Asiri, Y.A.; Alwhaibi, M.M.; Ali, S.; Jaganathan, P.P.; Alhawassi, T.M. Prescribing errors among adult patients in a large tertiary care system in Saudi Arabia. Ann. Saudi Med. 2021, 41, 147–156. Available online: http://www.annsaudimed.net/doi/10.5144/0256-4947.2021.147 (accessed on 15 November 2021). [CrossRef] [PubMed]
- Babelghaith, S.D.; Alarifi, M.N.; Wajid, S.; Alhawassi, T.M.; Alqahtani, S.K.; Alghadeer, S.M. Knowledge of patients on safe medication use in relation to nonsteroidal anti-inflammatory drugs. Saudi J. Anaesth. 2019, 13, 106–111. Available online: http://www.ncbi.nlm.nih.gov/pubmed/31007655 (accessed on 15 November 2021).
- Qureshi, N.A.; Neyaz, Y.; Khoja, T.; Magzoub, M.A.; Haycox, A.; Walley, T. Physicians’ medication prescribing in primary care. in Riyadh City, Saudi Arabia. Literature review, part 3: Prescribing Errors. East. Mediterr. Health J. 2011, 17, 140–148. Available online: http://www.ncbi.nlm.nih.gov/pubmed/21735949 (accessed on 15 November 2021). [CrossRef]
- Zedan, H.S.; Avery, A.J. Prescribing safety in primary care: Comparing the United Kingdom and Saudi Arabia. Saudi J. Anaesth. 2008, 29, 1703–1710. Available online: http://www.ncbi.nlm.nih.gov/pubmed/19082217 (accessed on 15 November 2021).
- Aljadhey, H.; Alenizi, R.; Mahmoud, M.A.; Hazzali, M.A. An Assessment of the Current Medication Safety Practices in the Primary Care Settings in Riyadh, Saudi Arabia. Pharmacoepidemiol. Drug Saf. 2014, 23, 478. [Google Scholar]
- Khawagi, W.Y.; Steinke, D.T.; Nguyen, J.; Pontefract, S.; Keers, R.N. Development of prescribing safety indicators related to mental health disorders and medications: Modified e-Delphi study. Br. J. Clin. Pharmacol. 2021, 87, 189–209. Available online: https://onlinelibrary.wiley.com/doi/10.1111/bcp.14391 (accessed on 1 April 2022). [CrossRef]
- Benchimol, E.I.; Smeeth, L.; Guttmann, A.; Harron, K.; Moher, D.; Petersen, I.; Sørensen, H.T.; Elm, E.V.; Langan, S.M. The Reporting of studies Conducted using Observational Routinely-collected health Data (RECORD) statement. PLoS Med. 2015, 12, e1001885. Available online: http://www.ncbi.nlm.nih.gov/pubmed/26440803 (accessed on 15 November 2021). [CrossRef]
- Vandenbroucke, J.P.; Elm, E.V.; Altman, D.G.; Gøtzsche, P.C.; Mulrow, C.D.; Pocock, S.J.; Poole, C.; Schlesselman, J.J.; Egger, M. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and Elaboration. Int. J. Surg. 2014, 12, 1500–1524. Available online: http://www.ncbi.nlm.nih.gov/pubmed/25046751 (accessed on 15 November 2021). [CrossRef] [Green Version]
- Masnoon, N.; Shakib, S.; Kalisch-Ellett, L.; Caughey, G.E. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017, 17, 230. Available online: http://bmcgeriatr.biomedcentral.com/articles/10.1186/s12877-017-0621-2 (accessed on 1 April 2022). [CrossRef] [PubMed] [Green Version]
- Stocks, S.J.; Kontopantelis, E.; Akbarov, A.; Rodgers, S.; Avery, A.J.; Ashcroft, D.M. Examining variations in prescribing safety in UK general practice: Cross Sectional Study Using the Clinical Practice Research Datalink. BMJ 2015, 351, 5501. Available online: https://www.bmj.com/lookup/doi/10.1136/bmj.h5501 (accessed on 15 November 2021). [CrossRef] [PubMed] [Green Version]
- Assiri, G.A.; Alkhenizan, A.H.; Al-Khani, S.A.; Grant, L.M.; Sheikh, A. Investigating the epidemiology of medication errors in adults in community care settings. A retrospective cohort study in central Saudi Arabia. Saudi Med. J. 2019, 40, 158–167. Available online: http://www.ncbi.nlm.nih.gov/pubmed/30723861 (accessed on 15 November 2021). [CrossRef] [PubMed]
- Alhawassi, T.M.; Alatawi, W.; Alwhaibi, M. Prevalence of potentially inappropriate medications use among older adults and risk factors using the 2015 American Geriatrics Society Beers criteria. BMC Geriatr. 2019, 19, 154. Available online: https://bmcgeriatr.biomedcentral.com/articles/10.1186/s12877-019-1168-1 (accessed on 15 November 2021). [CrossRef] [PubMed]
- Hernández-Díaz, S.; Rodríguez, L.A.G. Association Between Nonsteroidal Anti-inflammatory Drugs and Upper Gastrointestinal Tract Bleeding/Perforation. Arch. Intern. Med. 2000, 160, 2093–2099. Available online: http://archinte.jamanetwork.com/article.aspx?doi=10.1001/archinte.160.14.2093 (accessed on 15 November 2021). [CrossRef] [PubMed]
- Verheij, R.A.; Curcin, V.; Delaney, B.C.; McGilchrist, M.M. Possible Sources of Bias in Primary Care Electronic Health Record Data Use and Reuse. J. Med. Internet Res. 2018, 20, 185. Available online: http://www.ncbi.nlm.nih.gov/pubmed/29844010 (accessed on 15 November 2021). [CrossRef] [PubMed]
- Pannucci, C.J.; Wilkins, E.G. Identifying and Avoiding Bias in Research. Plast. Reconstr. Surg. 2010, 126, 619–625. Available online: http://journals.lww.com/00006534-201008000-00034 (accessed on 15 November 2021). [CrossRef]
- Hajian-Tilaki, K. Sample size estimation in epidemiologic studies. Casp. J. Intern. Med. 2011, 2, 289–298. Available online: http://www.ncbi.nlm.nih.gov/pubmed/24551434 (accessed on 15 November 2021).
- Lackey, N.R.; Wingate, A.L. The pilot study: One Key to Research Success. Kans. State Nurses Assoc. 1986, 61, 6–7. Available online: http://www.ncbi.nlm.nih.gov/pubmed/3642076 (accessed on 15 November 2021).
- Naing, L.; Winn, T.; Rusli, B. Practical Issues in Calculating the Sample Size for Prevalence Studies. Arch. Orofac. Sci. 2006, 1, 9–14. [Google Scholar]
- Dreischulte, T.; Guthrie, B. High-risk prescribing and monitoring in primary care: How Common Is It, and How Can It Be Improved? Adv. Drug Saf. 2012, 3, 175–184. Available online: http://journals.sagepub.com/doi/10.1177/2042098612444867 (accessed on 15 November 2021). [CrossRef] [PubMed] [Green Version]
- Williams, R.; Keers, R.; Gude, W.T.; Jeffries, M.; Davies, C.; Brown, B.; Kontopantelis, E.; Avery, A.J.; Ashcroft, D.M.; Peek, N. SMASH! The Salford medication safety dashboard. BMJ Health Care Inform. 2018, 25, 183–193. Available online: https://informatics.bmj.com/lookup/doi/10.14236/jhi.v25i3.1015 (accessed on 15 November 2021). [CrossRef] [PubMed] [Green Version]
- Vincent, C.A.; Coulter, A. Patient safety: What About the Patient? Qual. Saf. Health Care 2002, 11, 76–80. Available online: https://qualitysafety.bmj.com/lookup/doi/10.1136/qhc.11.1.76 (accessed on 15 November 2021). [CrossRef] [PubMed] [Green Version]
- Neves, A.L.; Freise, L.; Laranjo, L.; Carter, A.W.; Darzi, A.; Mayer, E. Impact of providing patients access to electronic health records on quality and safety of care: A Systematic Review and Meta-Analysis. BMJ Qual. Saf. 2020, 29, 1019–1032. Available online: https://qualitysafety.bmj.com/lookup/doi/10.1136/bmjqs-2019-010581 (accessed on 15 November 2021). [CrossRef]
| Outcome | Categories | Number (%) |
|---|---|---|
| Age | 18–64 years | 288 (64) |
| ≥65 years | 162 (36) | |
| Mean age: 62; standard deviation 11.2 | - | |
| Gender | Male | 132 (29.3) |
| Female | 318 (70.7) | |
| Nationality | Saudi | 450 (100) |
| Non-Saudi | 0 (0) | |
| Polypharmacy | Yes, ≥5 concurrent medications | 407 (90.4) |
| No, 1–4 concurrent medications | 43 (9.6) | |
| NSAIDs | Celecoxib 200 mg | 238 (52.9) |
| Diclofenac 50 mg | 36 (8) | |
| Ibuprofen 400 mg | 18 (4) | |
| Meloxicam 7.5 mg | 40 (8.9) | |
| Meloxicam 15 mg | 118 (26.2) | |
| Duration of NSAID use | 1–30 days | 114 (25.3) |
| 31–90 days | 277(61.6) | |
| 91–180 days | 54 (12) | |
| 181–270 days | 3 (0.7) | |
| ≥270 days | 2 (0.4) | |
| Pre-existing conditions | ||
| Arthritic disorder | Osteoarthritis | 191 (42.4) |
| Cardiac and vascular disorders | Dyslipidaemia | 276 (61.3) |
| Essential hypertension | 338 (75.1) | |
| Heart failure | 7 (1.6) | |
| Endocrine disorder | Diabetes mellitus | 316 (70.2) |
| Gastrointestinal disorder | Ulcer | 3 (0.7) |
| Renal disorder | Chronic kidney disease | 50 (11.1) |
| Co-prescribed drugs | ||
| Oral anticoagulant | 14 (3.1) | |
| Antiplatelet | 200 (44.4) | |
| Aspirin | 185 (92.5) | |
| Clopidogrel | 15 (7.5) | |
| ACE/ARB | 230 (51.1) | |
| Diuretics | 101 (22.4) | |
| Prescribing Safety Indicator (PSI) Name | Numerator Definition | Number | Denominator Definition | Number | Proportion of PSI (%); 95%CI | |
|---|---|---|---|---|---|---|
| 1 | Non-steroidal anti-inflammatory drug (NSAID) prescribed to person with history of peptic ulcer, without co-prescription of gastroprotection | Prescribed oral NSAID during quarter and not prescribed gastroprotective drug in 12 weeks before NSAID prescription | 0 | Diagnosed with peptic ulcer before the quarter | 3 | 0 |
| 2 | NSAID prescribed to person aged 75 years or over, without co-prescription of gastroprotection | Prescribed oral NSAID during quarter and not prescribed a gastroprotective drug in 12 weeks before NSAID prescription | 20 | Age 75 years before the quarter | 62 | 32.3; 20.29–44.23 |
| 3 | NSAID prescribed to person taking an antiplatelet drug, without co-prescription of gastroprotection | Prescribed oral NSAID during quarter and not prescribed gastroprotective drug in 12 weeks before NSAID prescription | 92 | Prescribed antiplatelet drug during quarter | 200 | 46; 39.03–52.97 |
| 4 | NSAID prescribed to person taking an oral anticoagulant (OAC), without co-prescription of gastroprotection | Prescribed oral NSAID during quarter and not prescribed gastroprotective drug in 12 weeks before NSAID prescription | 4 | Prescribed OAC during quarter | 14 | 28.6; 1.50–55.64 |
| 5 | NSAID prescribed to person aged 65 years or over prescribed angiotensin-converting enzyme (ACE) inhibitor/angiotensin II receptor blocker (ARB) and diuretic (“‘triple whammy”) | Prescribed oral NSAID in same quarter | 34 | Age 65 years or over before start of quarter and prescribed ACE inhibitor/ARB and diuretic during quarter | 100 | 34; 24.55–43.45 |
| 6 | NSAID prescribed to patient aged over 65 years with estimated glomerular filtration rate (GFR) <60 | Prescribed NSAID during quarter | 45 | No. of patients aged ≥65 years with stage 3, 4, or 5 renal impairment (estimated GFR <60) | 45 | 100; 100–100 |
| 7 | NSAID prescribed to patient with heart failure | Prescribed NSAID during quarter | 7 | Diagnosed with heart failure at time of last prescription | 7 | 100; 100–100 |
| Overall proportion of prescribing safety indicator | Total number of positive numerators | 202 | Total number of positive denominators | 431 | 46.9; 42.12–51.61 | |
| Overall period prevalence | Number of patients with at least one positive PSI | 153 | Total number of patients (N) | 450 | 34; 29.60–38.39 |
| Risk Factor | OR; 95% CI | p-Value | |
|---|---|---|---|
| Age (≥65 or 18–64 years) | 5.22; 3.32–8.21 | <0.001 * | |
| Gender (female or male) | 0.49; 0.31–0.79 | 0.003 * | |
| Polypharmacy (yes or no) | 2.97; 1.17–7.55 | 0.022 * | |
| Duration of NSAID use | 31–90 days | 0.44; 0.26–0.74 | 0.002 * |
| 91–180 days | 0.36; 0.16–0.82 | 0.015 * | |
| 181–270 days | 1 | ||
| ≥270 days | 0.32; 0.02–5.56 | 0.431 | |
| Overall | 0.29; 0.11–0.79 | 0.017 * | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Assiri, G.A.; Alanazi, B.M.; AlRuthia, Y. The Prevalence of High-Risk Prescribing of Oral Non-Steroidal Anti-Inflammatory Drugs in Primary Healthcare: A Single-Centre Retrospective Chart Review Study. Healthcare 2022, 10, 867. https://doi.org/10.3390/healthcare10050867
Assiri GA, Alanazi BM, AlRuthia Y. The Prevalence of High-Risk Prescribing of Oral Non-Steroidal Anti-Inflammatory Drugs in Primary Healthcare: A Single-Centre Retrospective Chart Review Study. Healthcare. 2022; 10(5):867. https://doi.org/10.3390/healthcare10050867
Chicago/Turabian StyleAssiri, Ghadah Asaad, Bashayr Mohammed Alanazi, and Yazed AlRuthia. 2022. "The Prevalence of High-Risk Prescribing of Oral Non-Steroidal Anti-Inflammatory Drugs in Primary Healthcare: A Single-Centre Retrospective Chart Review Study" Healthcare 10, no. 5: 867. https://doi.org/10.3390/healthcare10050867
APA StyleAssiri, G. A., Alanazi, B. M., & AlRuthia, Y. (2022). The Prevalence of High-Risk Prescribing of Oral Non-Steroidal Anti-Inflammatory Drugs in Primary Healthcare: A Single-Centre Retrospective Chart Review Study. Healthcare, 10(5), 867. https://doi.org/10.3390/healthcare10050867






