Comparison of One-Stage and Two-Stage Intraoperative Uterine Artery Embolization during Cesarean Delivery for Placenta Accreta: Report of Two Clinical Cases at a Tertiary Referral Medical Center
Abstract
:1. Introduction
2. Case Report
2.1. Case 1
2.1.1. Patient Information
2.1.2. Clinical Findings and Diagnostic Assessment
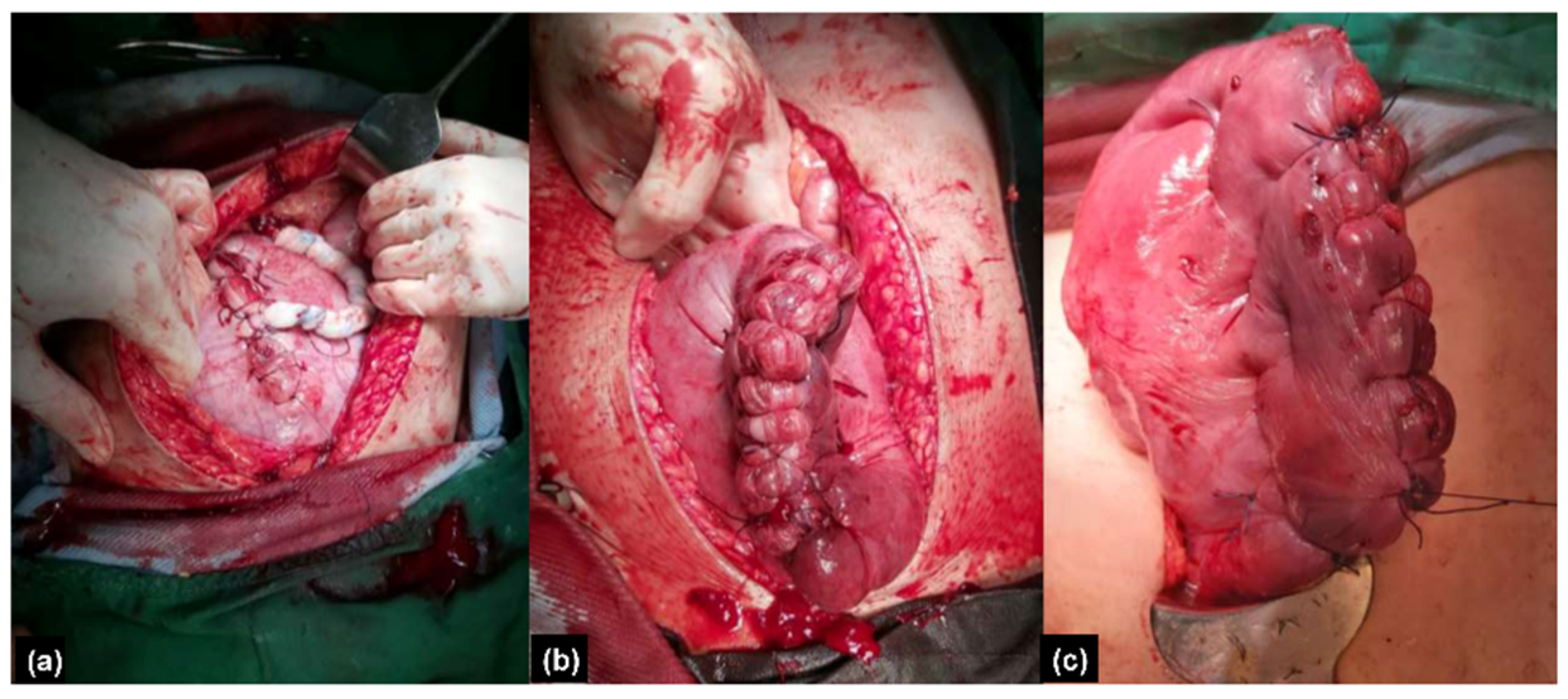
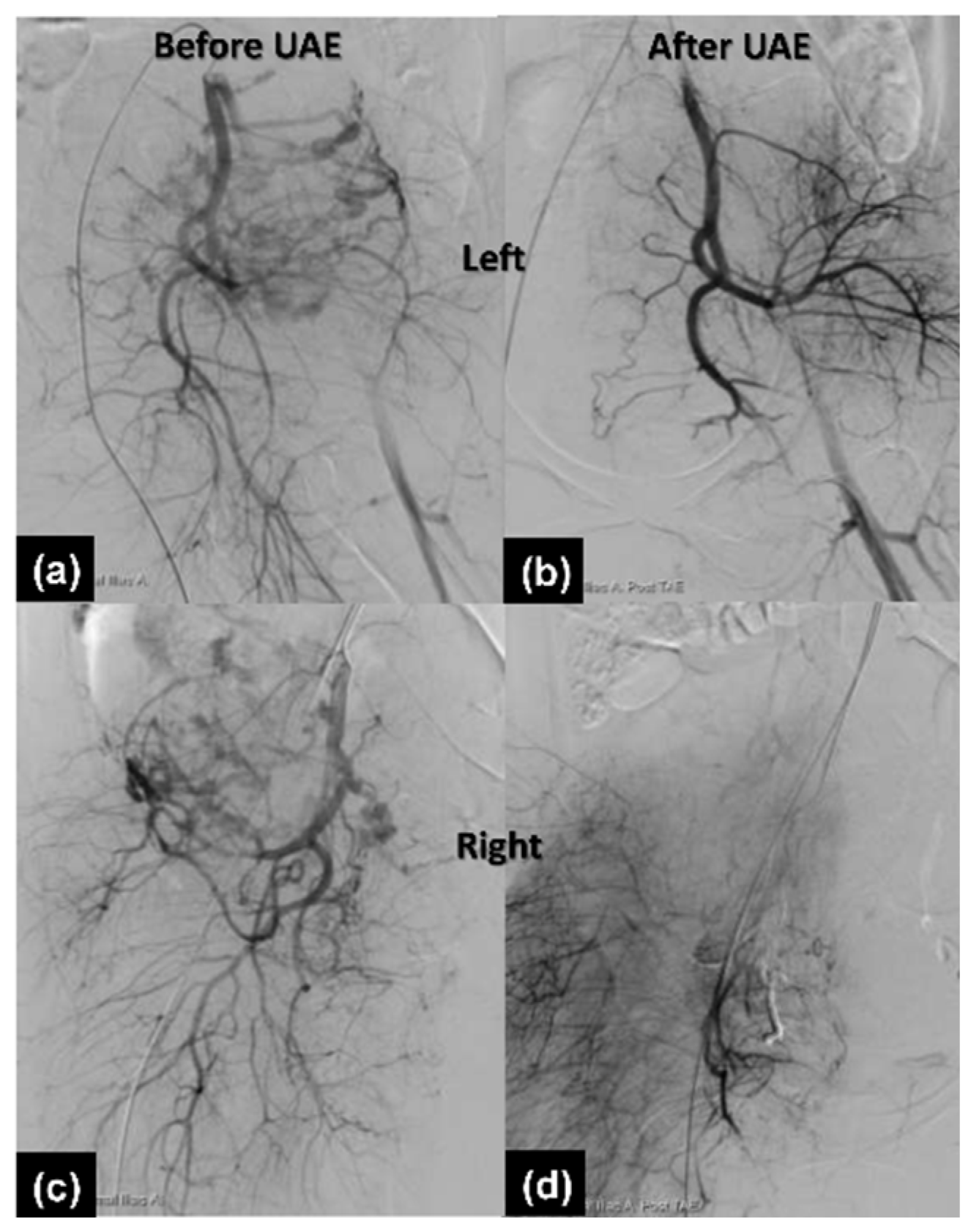
2.1.3. Therapeutic Intervention
2.1.4. Follow-Up and Outcomes
2.2. Case 2
2.2.1. Patient Information
2.2.2. Clinical Findings and Diagnostic Assessment
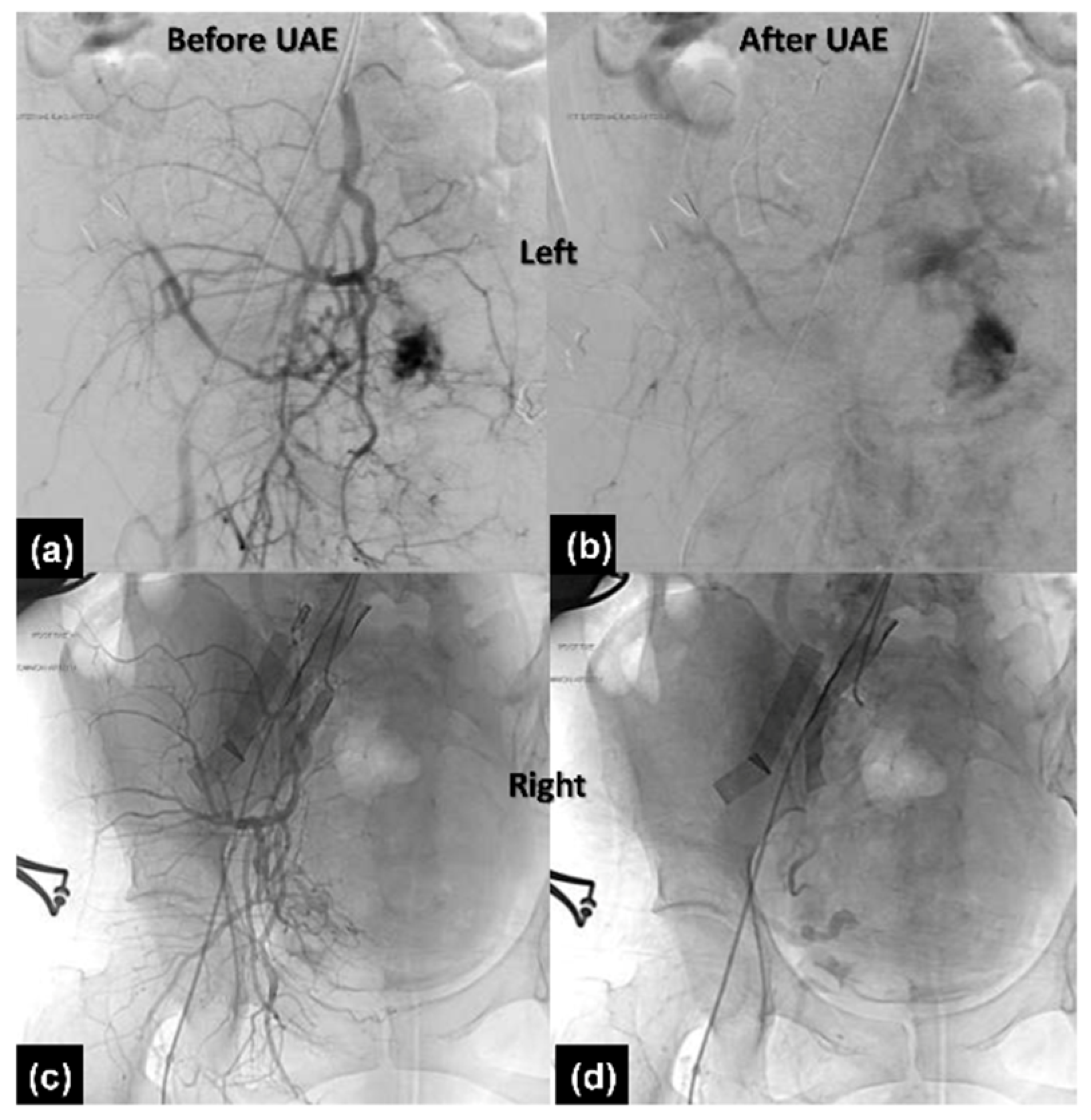
2.2.3. Therapeutic Intervention
2.2.4. Follow-Up and Outcomes
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Society of Gynecologic Oncology; American College of Obstetricians and Gynecologists and the Society for Maternal–Fetal Medicine; Cahill, A.G.; Beigi, R.; Heine, R.P.; Silver, R.M.; Wax, J.R. Placenta Accreta Spectrum. Am. J. Obstet. Gynecol. 2018, 219, B2–B6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cunningham, F.G.; Leveno, K.J.; Bloom, S.L.; Dashe, J.S.; Hoffman, B.L.; Casey, B.M.; Spong, C.Y. Obstetrical Hemorrhage. In Williams Obstetrics, 25th ed.; McGraw-Hill Education: New York, NY, USA, 2018. [Google Scholar]
- Bailit, J.L.; Grobman, W.A.; Rice, M.M.; Reddy, U.M.; Wapner, R.J.; Varner, M.W.; Leveno, K.J.; Iams, J.D.; Tita, A.T.N.; Saade, G.; et al. Morbidly adherent placenta treatments and outcomes. Obstet. Gynecol. 2015, 125, 683–689. [Google Scholar] [CrossRef] [PubMed]
- El Gelany, S.; Mosbeh, M.H.; Ibrahim, E.M.; Mohammed, M.; Khalifa, E.M.; Abdelhakium, A.K.; Yousef, A.M.; Hassan, H.; Goma, K.; Alghany, A.A.; et al. Placenta Accreta Spectrum (PAS) disorders: Incidence, risk factors and outcomes of different management strategies in a tertiary referral hospital in Minia, Egypt: A prospective study. BMC Pregnancy Childbirth 2019, 19, 313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sentilhes, L.; Ambroselli, C.; Kayem, G.; Provansal, M.; Fernandez, H.; Perrotin, F.; Winer, N.; Pierre, F.; Benachi, A.; Dreyfus, M. Maternal outcome after conservative treatment of placenta accreta. Obstet. Gynecol. 2010, 115, 526–534. [Google Scholar] [CrossRef] [Green Version]
- Kayem, G.; Davy, C.; Goffinet, F.; Thomas, C.; Clément, D.; Cabrol, D. Conservative versus extirpative management in cases of placenta accreta. Obstet. Gynecol. 2004, 104, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Gatta, L.A.; Lee, P.S.; Gilner, J.B.; Weber, J.M.; Adkins, L.; Salinaro, J.R.; Habib, A.S.; Pabon-Ramos, W.; Strickland, K.C.; Ronald, J. Placental uterine artery embolization followed by delayed hysterectomy for placenta percreta: A case series. Gynecol. Oncol. Rep. 2021, 37, 100833. [Google Scholar] [CrossRef] [PubMed]
- Meller, C.H.; Garcia-Monaco, R.D.; Izbizky, G.; Lamm, M.; Jaunarena, J.; Peralta, O.; Otaño, L. Non-conservative management of placenta accreta spectrum in the hybrid operating room: A retrospective cohort study. Cardiovasc. Intervent. Radiol. 2019, 42, 365–370. [Google Scholar] [CrossRef]
- Shih, J.C.; Liu, K.L.; Kang, J.; Yang, J.H.; Lin, M.W.; Yu, C.U. ‘Nausicaa’ compression suture: A simple and effective alternative to hysterectomy in placenta accreta spectrum and other causes of severe postpartum haemorrhage. BJOG 2019, 126, 412–417. [Google Scholar] [CrossRef] [Green Version]
- B-Lynch, C.; Coker, A.; Lawal, A.H.; Abu, J.; Cowen, M.J. The B-Lynch surgical technique for the control of massive postpartum haemorrhage: An alternative to hysterectomy? Five cases reported. Br. J. Obstet. Gynaecol. 1997, 104, 372–375. [Google Scholar] [CrossRef]
- Frederiksen, M.C.; Glassenberg, R.; Stika, C.S. Placenta previa: A 22-year analysis. Am. J. Obstet. Gynecol. 1999, 180, 1432–1437. [Google Scholar] [CrossRef]
- Roberts, C.L.; Algert, C.S.; Warrendorf, J.; Olive, E.C.; Morris, J.M.; Ford, J.B. Trends and recurrence of placenta praevia: A population-based study. Aust. N. Z. J. Obstet. Gynaecol. 2012, 52, 483–486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Räisänen, S.; Kancherla, V.; Kramer, M.R.; Gissler, M.; Heinonen, S. Placenta previa and the risk of delivering a small-for-gestational-age newborn. Obstet. Gynecol. 2014, 124, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Usta, I.M.; Hobeika, E.M.; Musa, A.A.; Gabriel, G.E.; Nassar, A.H. Placenta previa-accreta: Risk factors and complications. Am. J. Obstet. Gynecol. 2005, 193, 1045–1049. [Google Scholar] [CrossRef] [PubMed]
- Klar, M.; Michels, K.B. Cesarean section and placental disorders in subsequent pregnancies--a meta-analysis. J. Perinat. Med. 2014, 42, 571–583. [Google Scholar] [CrossRef] [PubMed]
- Downes, K.L.; Hinkle, S.N.; Sjaarda, L.A.; Albert, P.S.; Grantz, K.L. Previous prelabor or intrapartum cesarean delivery and risk of placenta previa. Am. J. Obstet. Gynecol. 2015, 212, e1–e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collins, S.L.; Ashcroft, A.; Braun, T.; Calda, P.; Langhoff-Roos, J.; Morel, O.; Stefanovic, V.; Tutschek, B.; Chantraine, F. Proposal for standardized ultrasound descriptors of abnormally invasive placenta (AIP). Ultrasound Obstet. Gynecol. 2016, 47, 271–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salmanian, B.; Fox, K.A.; Arian, S.E.; Erfani, H.; Clark, S.L.; Aagaard, K.M.; Detlefs, S.E.; Aalipour, S.; Espinoza, J.; Nassr, A.A.; et al. In vitro fertilization as an independent risk factor for placenta accreta spectrum. Am. J. Obstet. Gynecol. 2020, 223, e1–e568. [Google Scholar] [CrossRef]
- Sentilhes, L.; Seco, A.; Azria, E.; Beucher, G.; Bonnet, M.-P.; Branger, B.; Carbillon, L.; Chiesa, C.; Crenn-Hebert, C.; Dreyfus, M. Conservative management or cesarean hysterectomy for placenta accreta spectrum: The PACCRETA prospective study. Am. J. Obstet. Gynecol. 2021. [Google Scholar] [CrossRef]
- Yang, C.-C.; Chou, Y.-C.; Kuo, T.-N.; Liou, J.-Y.; Cheng, H.-M.; Kuo, Y.-T. Prophylactic Intraoperative Uterine Artery Embolization During Cesarean Section or Cesarean Hysterectomy in Patients with Abnormal Placentation: A Systematic Review and Meta-Analysis. CardioVasc. Interv. Radiol. 2022, 45, 488–501. [Google Scholar] [CrossRef]
- Lo, T.K.; Yung, W.; Lau, W.; Law, B.; Lau, S.; Leung, W. Planned conservative management of placenta accreta–experience of a regional general hospital. J. Matern. Fetal Neonatal Med. 2014, 27, 291–296. [Google Scholar] [CrossRef]
- Soncini, E.; Pelicelli, A.; Larini, P.; Marcato, C.; Monaco, D.; Grignaffini, A. Uterine artery embolization in the treatment and prevention of postpartum hemorrhage. Int. J. Gynecol. Obstet. 2007, 96, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Wong, T. Arterial embolisation in intractable primary post-partum haemorrhage: Case series. Hong Kong Med. J. 2004, 10, 301–306. [Google Scholar]
- Cottier, J.; Fignon, A.; Tranquart, F.; Herbreteau, D. Uterine necrosis after arterial embolization for postpartum hemorrhage. Obstet. Gynecol. 2002, 100, 1074–1077. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Zhou, X.; Yang, Z.; Cui, S.; De, W.; Sun, L. Retrospective cohort study of prophylactic intraoperative uterine artery embolization for abnormally invasive placenta. Int. J. Gynecol. Obstet. 2017, 137, 45–50. [Google Scholar] [CrossRef]
- Mohan, B.; Wander, G.; Bansal, R.; Mutti, J.; Tandon, P.; Juneja, S.; Puri, S. Intra-operative uterine artery embolization with caesarean delivery in an adjoining operating theatre and catheter lab (OT/CL) complex vs. conventional management in patients with abnormally invasive placenta: A retrospective case control study. J. Obstet. Gynaecol. 2020, 40, 324–329. [Google Scholar] [CrossRef]
- Konishi, Y.; Yamamoto, S.; Sugiki, K.; Sakamoto, H.; Sawamura, S. A Novel and Multidisciplinary Strategy for Cesarean Delivery With Placenta Percreta: Intraoperative Embolization in a Hybrid Suite. A A Case Rep. 2016, 7, 135–138. [Google Scholar] [CrossRef]
- Chen, W.S.; Chiang, M.H.; Hung, K.C.; Lin, K.L.; Wang, C.H.; Poon, Y.Y.; Luo, S.D.; Wu, S.C. Adverse respiratory events with sevoflurane compared with desflurane in ambulatory surgery: A systematic review and meta-analysis. Eur. J. Anaesthesiol. 2020, 37, 1093–1104. [Google Scholar] [CrossRef]

| Characteristic | Classic Two-Step Procedure | One-Step Procedure |
|---|---|---|
| Age | 32 | 41 |
| Gravid Para | G1P0 | G2P1 |
| Prior uterine surgery | No | myomectomy, polypectomy |
| PAS sign: sonography | abnormal placental lacunae and uterovesical hypervascularity | abnormal placental lacunae, placenta bulging, and uterovesical hypervascularity |
| PAS sign: MRI | prominent vascularity around the uterus | low signal intensity representing hemorrhage in the placenta |
| Anesthesia | spinal then general by Sevoflurane | general by Desflurane |
| Time | 338 min | 280 min |
| Complications | blood transfusion; pulmonary edema | no |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, Z.-W.; Lee, W.-Y.; Huang, Y.-C.; Wu, W.-J.; Chen, M. Comparison of One-Stage and Two-Stage Intraoperative Uterine Artery Embolization during Cesarean Delivery for Placenta Accreta: Report of Two Clinical Cases at a Tertiary Referral Medical Center. Healthcare 2022, 10, 774. https://doi.org/10.3390/healthcare10050774
Lim Z-W, Lee W-Y, Huang Y-C, Wu W-J, Chen M. Comparison of One-Stage and Two-Stage Intraoperative Uterine Artery Embolization during Cesarean Delivery for Placenta Accreta: Report of Two Clinical Cases at a Tertiary Referral Medical Center. Healthcare. 2022; 10(5):774. https://doi.org/10.3390/healthcare10050774
Chicago/Turabian StyleLim, Zhu-Wei, Wei-Yang Lee, Yuan-Chun Huang, Wan-Ju Wu, and Ming Chen. 2022. "Comparison of One-Stage and Two-Stage Intraoperative Uterine Artery Embolization during Cesarean Delivery for Placenta Accreta: Report of Two Clinical Cases at a Tertiary Referral Medical Center" Healthcare 10, no. 5: 774. https://doi.org/10.3390/healthcare10050774
APA StyleLim, Z.-W., Lee, W.-Y., Huang, Y.-C., Wu, W.-J., & Chen, M. (2022). Comparison of One-Stage and Two-Stage Intraoperative Uterine Artery Embolization during Cesarean Delivery for Placenta Accreta: Report of Two Clinical Cases at a Tertiary Referral Medical Center. Healthcare, 10(5), 774. https://doi.org/10.3390/healthcare10050774







