1. Introduction
With the increasing incidence and prevalence of chronic diseases, the demand for healthcare services is growing worldwide, exerting major funding pressures on constrained healthcare resources. Medicines are among the most common medical interventions for the treatment, prevention, and therapy of chronic diseases [
1]. The demand for medicines and therefore the pharmaceutical spending is increasing worldwide, typically at rates higher than the growth rates of other health spending categories, driving the growth in total healthcare expenditure [
2]. Croatia is no exception—between 2014 and 2018 the pharmaceutical spending increased on average 5% a year, while the growth of other health spending categories was slower [
3]. Countries apply various pricing and reimbursement policies to curb the growth in pharmaceutical spending and contain costs (as well as increase the overall cost-effectiveness of pharmaceutical spending). However, once the reimbursement process is finished, and the medicines are listed and available to patients (with or without copayments), the procedures which monitor different aspects of postlisting follow-up of medicines, including rational prescribing and rational use monitoring, are not always in place or are underdeveloped, as is the case in South-Eastern Europe [
4].
In Croatia, primary care physicians prescribe all outpatient medicines. The Croatian health insurance fund (CHIF), the main healthcare payer, strictly controls physicians’ prescribing behaviour, imposing fines if physicians do not comply with prescribing restrictions. To promote rational prescribing, CHIF’s restrictions determine which medicines can be prescribed for which diagnosis (Due to reference pricing, there is little pressure to prescribe generics). However, other aspects of rational prescribing (such as duplication of therapies, potential adverse drug events (ADEs), subtherapeutic dosage, and variations between prescribers) typically remain under the radar. Like rational prescribing, the rational use of prescribed medicines (such as monitoring polypharmacy in elderly patients and nonadherence) is also not promoted, in spite of considerable costs of inappropriate prescribing, ADEs, and nonadherence [
5,
6,
7,
8,
9,
10,
11,
12,
13,
14].
Comprehensive Medication Management (CMM) services provided by trained pharmacists can bridge this gap by increasing rational drug use, improving the prescribing of medicines, and reducing the unnecessary and often harmful use of medications and the resulting complications [
15,
16,
17,
18,
19]. Grounded in the practice of pharmaceutical care [
20,
21,
22] and promoted by major professional organizations [
19,
23,
24,
25], the standardised and internationally recognised CMM protocol proposed by Cipolle at all [
22] is an effective approach to resolving drug therapy problems (DTPs), improving clinical outcomes [
16,
17,
26,
27,
28,
29,
30,
31,
32,
33], reducing costs [
16,
34,
35], and improving patient and provider experience [
16,
36,
37], hence increasing the value of medicines used.
The CMM protocol of Cipolle et al. [
22] targets patients with diabetes type 2 (DMT2) and/or cardiovascular diseases (CVD) because these are among the most prevalent and costly chronic diseases worldwide, with CVD being the leading cause of global mortality [
38]. Croatia is no exception. Ischaemic heart disease and stroke are the two main causes of death in Croatia. The preventable mortality rates from ischaemic heart disease and stroke are twice the EU average [
39]. Unlike most EU countries, the mortality rate from ischaemic heart disease decreased only slightly between 2000 and 2016, while mortality rates from diabetes have increased sharply since 2000. The rise in mortality from treatable conditions such as diabetes should be a cause for concern and an argument for introducing CMM services. The same can be said for polypharmacy, a common occurrence in the elderly and chronically ill, which increases the risk of medication errors and DTPs, namely omissions, duplicate prescriptions, and harmful interactions. In the era of aging populations, polypharmacy, multiple chronic conditions, and complex and decreasingly manageable therapy regimens, CMM programmes are especially important for chronic elderly patients taking five or more medicines, who are at an increased risk of experiencing medication errors, ADEs, duplications of therapy, and detrimental interactions and who often fail to reach therapy goals (Although other pharmacist interventions, besides CMM, also have a positive impact on patient therapy goals, various studies have demonstrated the positive impact of CMM on the management of chronic diseases, by improving individual cardiovascular risk factors such as blood pressure [
30,
32,
33], glycosylated haemoglobin (HbA1c) [
17,
26,
30,
31,
32], and LDL cholesterol [
16,
17,
31,
32]). In turn, CMMs’ data could help payers to develop increasingly detailed prescribing guidelines and update their policies to monitor and enforce rational use, which would have a potential double benefit: fewer adverse events and lower overall prescribing costs.
In January 2018, a standardised CMM service was introduced as a pilot project in the largest county health centre in Croatia—Health Centre Zagreb Centre [
40], making it the first health centre in Croatia and South-Eastern Europe to offer CMM. CMM was offered to eligible patients free of charge. The CMM patient care process followed Cipolle et al. [
22] methodology, as described in
Table 1. The same standardised CMM service protocol was previously applied in the US [
16,
20,
26,
28] and elsewhere [
27,
29,
30,
31], in the same patient groups (i.e., patients with DMT2 and/or CVD, as explained later on). These CMM services have demonstrated their ability to improve clinical outcomes [
16,
17,
26,
27,
28,
29,
30,
31,
32,
33] and reduce costs [
16,
34,
35]. However, it is unclear to what extent such standardised CMM interventions can be deemed affordable.
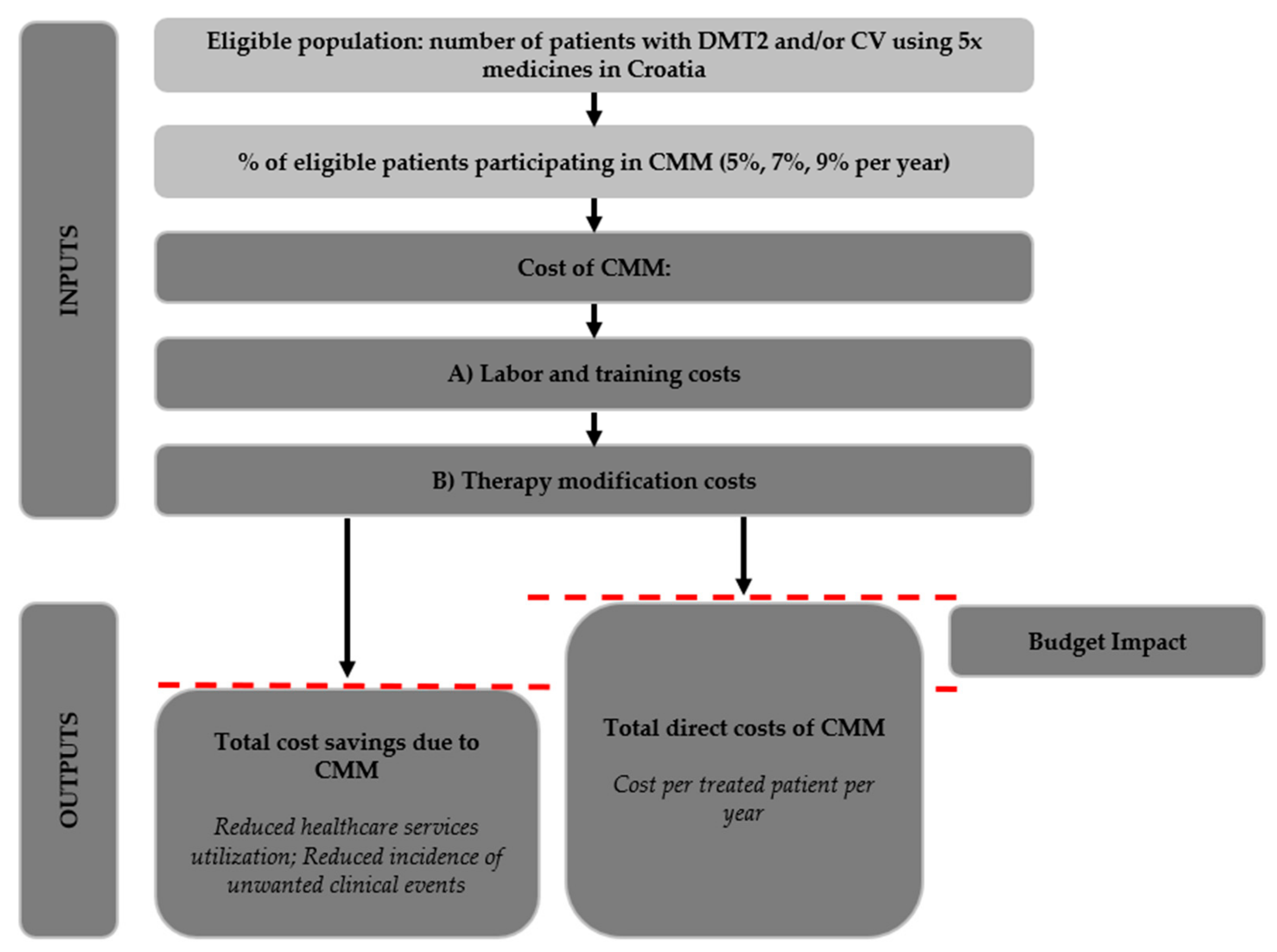
Budget impact analysis (BIA) assesses the affordability of interventions and helps policymakers decide whether the adoption of a new health intervention is within their means, given the resource and budget constraints of the context. So far, quantitative cost analyses and evaluations of pharmacist interventions have been in short supply [
9], and the question of CMM’s affordability remains unanswered. This paper reports the results of the BIA of CMM in the Croatian context to show whether, from the payer’s perspective (Croatia operates a single healthcare payer system, the Croatian health insurance fund (CHIF), which finances and contracts all public health services), introducing a nationwide CMM is affordable. Using data from various sources, the BIA identifies and models the costs, the savings, and the nonmonetary benefits [
41,
42,
43] associated with introducing and rolling-out a standardised nationwide CMM service [
22] in Croatia over a 3-year period (2022–2024) to predict CMM’s financial impact on the CHIF’s budget. Our study contributes to the literature by being the first budget impact analysis of CMM. As such, this study adds to the small body of literature by being among the few quantitative analyses and evaluations of pharmacist interventions more generally [
9].
3. Results
Total direct costs (
Table 9) of labour and training amount to EUR 2,667,098 for 3 years. CMM is expected to increase the cost of medication prescribed to patients by EUR 5,182,864 in 3 years, amounting to the total CMM costs of EUR 7,849,962 for 138,308 patients over 3 years. CMM’s cost per treated patient per year is therefore EUR 57. The annual cost increase is driven by the increase in the patient population covered by CMM (5% in year 1; 7% in year 2; and 9% in year 3) as well as the predicted 1% rise in the prevalence of CVD and DMT2 (
Table 2).
CMM is expected to reduce the utilisation rates and costs of healthcare service utilisation (
Table 10) and the incidence of unwanted clinical events (
Table 11), leading to a total 3-year reduction in healthcare costs of EUR 7,787,765.60. Given the total CMM costs of EUR 7,849,962, CMM’s 3-year budget impact equals EUR 92,869. Per treated patient incremental cost of CMM is therefore EUR 0.67.
Based on the incidence rates and the reduced individual risk rates due to CMM for a given event per disease group (
Table 5), CMM’s benefits can also be expressed in terms of the number of avoided unwanted clinical events. The number of avoided events per year in the group of patients participating in CMM are presented in
Table 12, totalling 2742 cases over 3 years (Some double counting may arise. The number of avoided events was calculated as an individual risk rate for any and all individuals in the sample but some individuals probably face a risk of developing two or more conditions simultaneously. The risk rates of combined conditions are not known). In preventing other severe conditions (stroke, nonfatal myocardial infarction, and others), CMM can contribute to saving and prolonging lives as well as increasing the quality of life and productivity of patients and their caregivers.
In the sensitivity analysis, we investigated CMM’s budget impact of the rates of avoided healthcare services as well as the risk reduction of unwanted events in case these were 5% under- or overestimated as well as 20% and 40% overestimated (
Table 13). Relative to the baseline budget impact estimates, the combined effect of 40% overestimation of both rates yields a budget impact of EUR 3.2 million and the incremental cost per patient of EUR 23. Alternatively, if the benefits of CMM are underestimated by mere 5%, the budget impact would be negative, making CMM the dominant intervention.
4. Discussion
CMM—as modelled in our budget impact analysis—is a large-scale intervention that would encompass over 138,000 patients over 3 years and employ 41 new pharmacists. CMM’s net budget impact—at just over EUR 92,000 for 3 years and EUR 0.67 incremental cost per patient—can be considered modest. That said, CMM appears to be a good investment also because of Croatia’s health and healthcare system profile. As already mentioned, ischaemic heart disease and stroke are the two main causes of death in Croatia, with preventable mortality rates from ischaemic heart disease and stroke twice the EU average [
39]. Mortality rates from diabetes have increased sharply since 2000. The rise in mortality from treatable conditions such as diabetes should be a cause for concern and an argument for introducing CMM services, which can help patients and physicians achieve desired health outcomes more efficiently.
There are various arguments for introducing CMM services into our healthcare systems. CMM is an intersectoral programme, requiring the coordination of GPs and pharmacists. As such, CMM could contribute to strengthening otherwise weak intersectoral policies and contribute to addressing key determinants of ill health, which in turn contribute to high rates of death from preventable and treatable causes. Moreover, CMM can help healthcare payers throughout Europe improve the postlisting value-for-money of prescription medicines. The fact that prescription medicine volumes are rising throughout Europe is not necessarily surprising given our aging populations, but this fact emphasises the need to further promote rational use. Greater efforts need to be made to ensure that medications are appropriately prescribed and coordinated to avoid DTPs, namely omissions, duplicate prescriptions, and harmful interactions, and CMM can be a great asset. As mentioned before, CMM can be used as a basis for developing increasingly detailed prescribing guidelines to monitor and enforce rational medicine use, which would have a double effect: fewer adverse events and lower overall prescribing costs.According to the results of our BIA model, the pilot CMM study could be transformed into a nationwide CMM service in Croatia, at a relatively modest price tag. CMM is not necessarily a dominant intervention in the sense that it reduces costs and generates incremental benefits, but these incremental benefits are generated at a modest cost. However, for CMM to become a reality in Croatia and elsewhere, both the policymakers and the payers need to support the development and implementation of CMM by reimbursing it and making it reproducible and sustainable over time. There need to be governments and health plans willing to support clinical pharmacists, namely professionals eager and capable to provide this service, as CMM will only live its full potential when we have well trained and experienced practitioners. The service is currently being piloted in Croatia although the pharmacists providing it are not being remunerated for their efforts. Considering the fact that CMM seems economically viable, both through this and previous analyses [
34], CMM is a highly recommended solution for addressing medication mismanagement and irrational drug use and as such should be a top priority for implementation in the healthcare system.
Limitations
The first point we wish to address is the realistic representation of healthcare costs. As explained in the methods section, the cost savings of reduced incidence of treating unwanted events are DRG-based. In Croatia, the price of DRGs is considerably lower than in neighbouring EU member states, and their price fluctuates often, depending on the financial situation in the healthcare system [
66,
67]. Moreover, the DRGs contain only inpatient costs, while the treatment of conditions such as stroke requires additional medications, rehabilitation, and many other (direct and indirect) follow-up costs, which are not considered in the hospital-based DRG. There is no national costing catalogue. To correct (at least partly) for the underestimation of the total DRG-based inpatient cost of the treatment of unwanted clinical events, we added the cost of one-time rehabilitation lasting 21 days to all events although at a fraction of its price. Hence, it is reasonable to expect that the inclusion of all costs of treatments would lead to higher cost savings related to CMM and consequently lower its budget impact. However, even our conservative estimate of the cost-saving impact of CMM shows that CMM can be affordable, even at unrealistically low costs for treating expensive conditions.
The second point we wish to address is the issue of using data from published sources. One may argue that the risk rates and the utilisation rates employed in our analysis—although taken from published sources—may not necessarily be the best representation of the Croatian epidemiological, clinical, and utilisation data. Ideally, we would calculate the actual and detailed costs of treating unwanted clinical events and the costs of particular healthcare services in the Croatian population and multiply those by the actual rates of healthcare services used and the incidence rates of unwanted clinical events per patient group receiving CMM and a group not receiving CMM (and subtract the difference). However, the rate of healthcare service utilisation and incidence rates of unwanted clinical events relevant for our study are not available in Croatia (let alone, for the target patient group). To reduce the risk associated with using published results in our BIA model, several measures were taken. With regard to the utilisation and clinical event rates, we used the study, which was methodologically and results-wise comparable to our CMM study, using the same CMM protocol as the one used in Croatia and a comparable patient population [
16]. With respect to the incidence rates of unwanted clinical events [
45,
46,
47,
48,
49,
50,
51,
52,
53,
54,
55,
56,
57,
58,
59,
60,
61,
62], these were discussed with key opinion leaders to confirm their applicability in the Croatian context. Finally, the sensitivity analysis was developed precisely to test the effect of overestimating the individual risk and rates of utilisation, to obtain a sense of the effect of over- or underestimation of these parameters as possible consequences of using data from different sources. Under the unfavourable assumption of 40% overestimation of the risk and utilisation rate reductions due to CMM, the budget impact reaches around EUR 3.2 mil for 3 years. Nevertheless, even with this relatively high budget impact, when we take into account the large number of patients included in CMM, the incremental cost per patient remains relatively low.
Third, the usefulness of data from the US and its transferability to the Croatian context may be hampered by the differences in healthcare payments and health insurance coverage (and the related accessibility of CMM). The United States has Medicare, a government-provided insurance for older individuals compared to more private insurance options for younger individuals. The coverage differences result in varying use of health care services. Unlike the US, Croatia operates a generous universal health insurance covering all citizens, funded from income-based contributions. Healthcare is free at the point of entry, except for certain medicines which require copayments. Hence, there is little variability in age-related access and use of healthcare, which would be determined by insurance coverage since healthcare is accessible and free at the point of entry for all (including CMM). In that sense, the US data may offer a conservative outlook on CMM’s benefits relative to its potential in Croatia.
The fourth point we wish to address is the scope of the BIA model. CMM is an intervention that could be intended for all patient groups irrespective of the condition they suffer from. The BIA conducted in this study included CVD and DMT2 patients only, rendering the budget impact relevant exclusively for this patient group. Future research should be focused on evaluating the impact of CMM on a broader range of health conditions.
Finally, the implementation of CMM initially leads to medication cost increase, as the main drug therapy problems typically identified and addressed by CMM service are the need for additional drug therapy and subtherapeutic dosage requiring increasing the doses and introducing new therapies. However, in the course of time, most often within the first year of CMM introduction, the related cost savings resulting from the reduction in the use of healthcare services and the incidence of unwanted clinical events balance and exceed this initial cost increase.