1. Introduction
Schizophrenia is a serious mental illness where patients have difficulty distinguishing between what is real and what is not [
1,
2]. Schizophrenia is a chronic brain disorder that affects a person’s thoughts, feelings, and behavior [
3]. People with schizophrenia often experience symptoms such as hallucinations, delusions, abnormal behavior, and disorganized speech [
4,
5]. Although the symptoms come and go, schizophrenia significantly affects the social lives, education, and professional performance of the affected individuals [
6].
Schizophrenia is a global disorder [
7] with a prevalence of approximately 1% worldwide, and it is reported that 20 million people are affected by schizophrenia [
8]. In addition, studies have shown that schizophrenia is more common in men than women [
9,
10]. The disorder usually starts between 18 and 25 years in men and between 25 and 35 years in women [
11]. The causes of schizophrenia are still unknown, but researchers have observed that genetics, brain chemistry, and environment may be associated with the development of the disorder [
12,
13]. Moreover, psychological factors are also known to trigger this disorder. Schizophrenia is treatable with medication and psychosocial support [
14]. Without treatment, people with schizophrenia can develop other mental health disorders and significant health problems. It is thus essential to establish a correct diagnosis and early treatment of the disease.
Schizophrenia (SZ) is diagnosed from the patient’s symptoms and a specialist’s opinion in mental health. In addition, techniques such as magnetic resonance imaging, computed tomography, and EEGs can also be used in the diagnostic phase [
15,
16]. In short, there is no single test method to diagnose SZ. These days, machine learning techniques are actively used to automatically interpret EEG signals [
17]. Due to the fact of these machine learning techniques, EEG data collected from patients are automatically classified, permitting early diagnosis of various diseases. One of these diseases is SZ, and there are various studies on automatic SZ classification in the literature as summarized in
Table 1.
Various data sources, such as EEG signals and MRI imaging, have been used to support schizophrenia diagnosis via machine learning. As shown in
Table 1, EEG signals are some of the most commonly used medical data for schizophrenia diagnosis.
Table 1 also shows the various EEG signal-based schizophrenia detection models that were developed using hand-crafted, feature-based deep learning models. The deep learning models attained high classification performances but had high computational complexities. Moreover, most of the EEG signal-based schizophrenia detection models did not use LOSO cross-validation [
33]. The signals were segmented, which may cause overfitting with other validation techniques. On the other hand, real classification performance can be accurately calculated using LOSO cross-validation. Therefore, LOSO cross-validation is a critical validation technique, and in this work, we used LOSO cross-validation in our proposed model.
In this paper, two validation techniques (i.e., 10-fold cross-validation and LOSO validation) were used, and high classification accuracies were obtained with our developed model. Our proposed model can automatically classify EEG signals collected from schizophrenia patients and healthy individuals. The new feature extractor (i.e., CGP17Pat) was also tested on an open-access schizophrenia data set with much success.
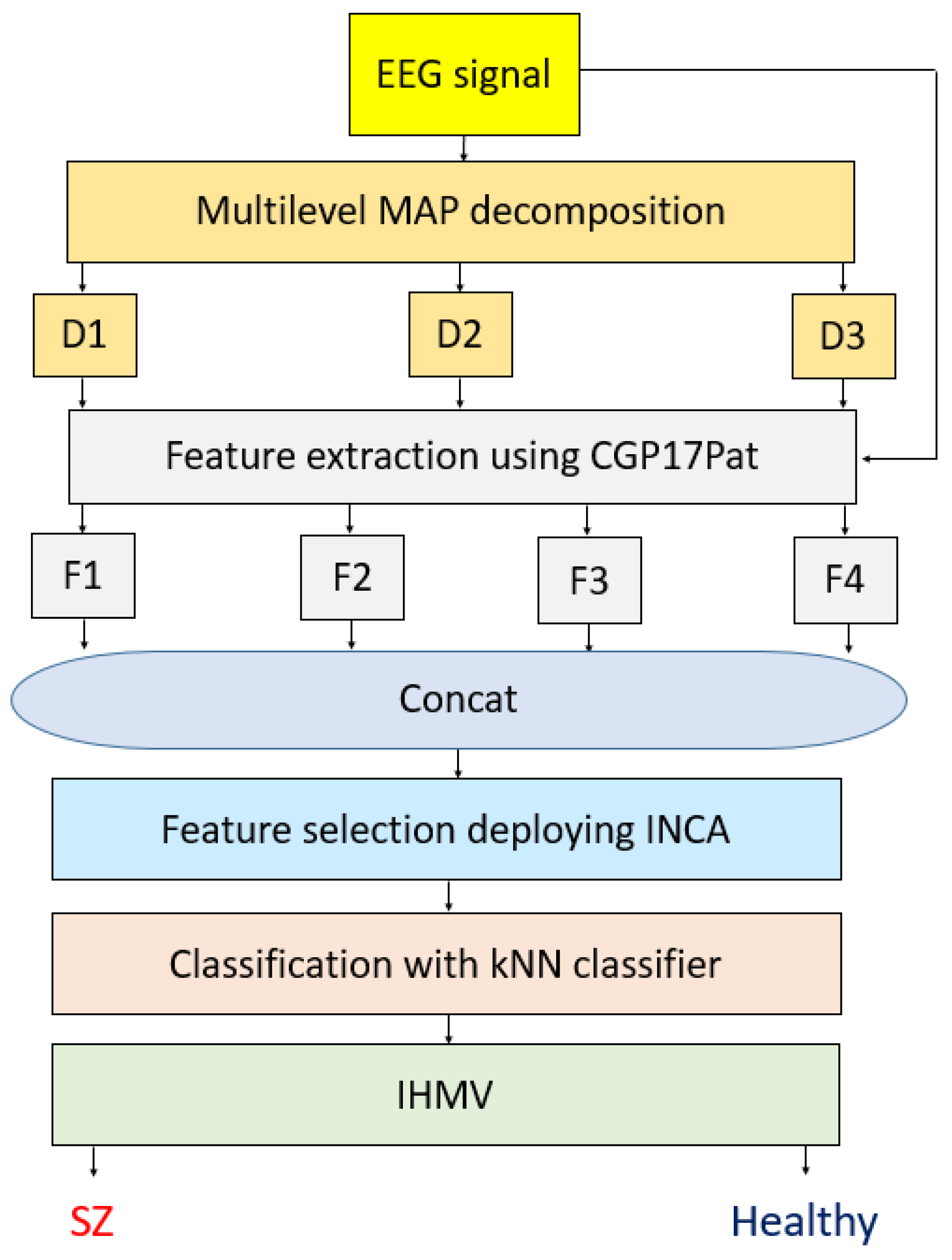
Feature engineering is one of the most important machine learning and classification issues. By deploying feature engineering methodologies, hand-crafted feature engineering models have been developed. In this work, we employed a cryptologic structure to propose a new nonlinear pattern. Cryptographic systems generally use finite groups that have been generated using various algorithms. The most popular finite group creation model is multiplication-based group creation. The prime numbers have been used to create cyclic finite group and is named the cyclic group of prime (CGP) order [
34,
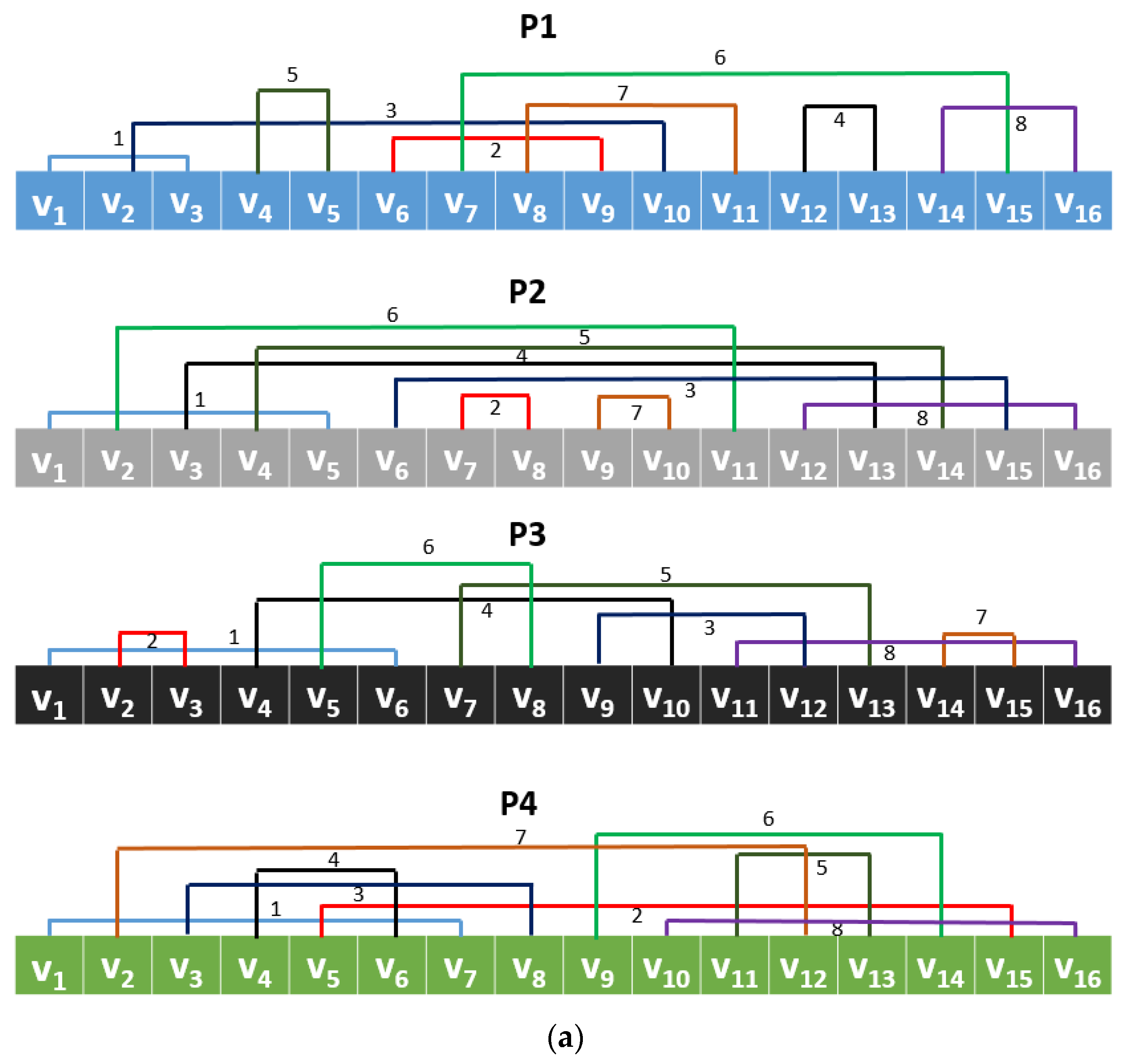
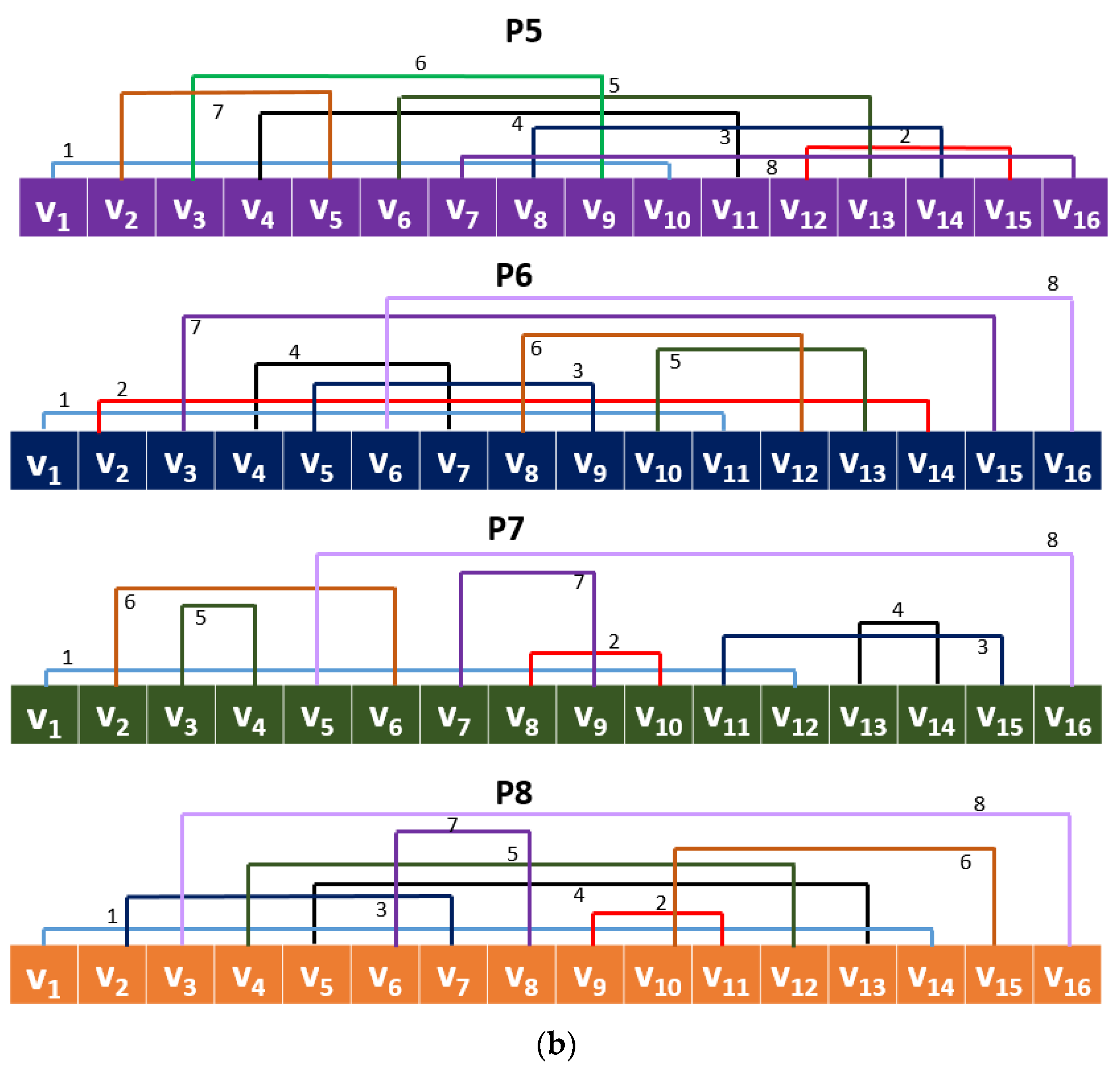
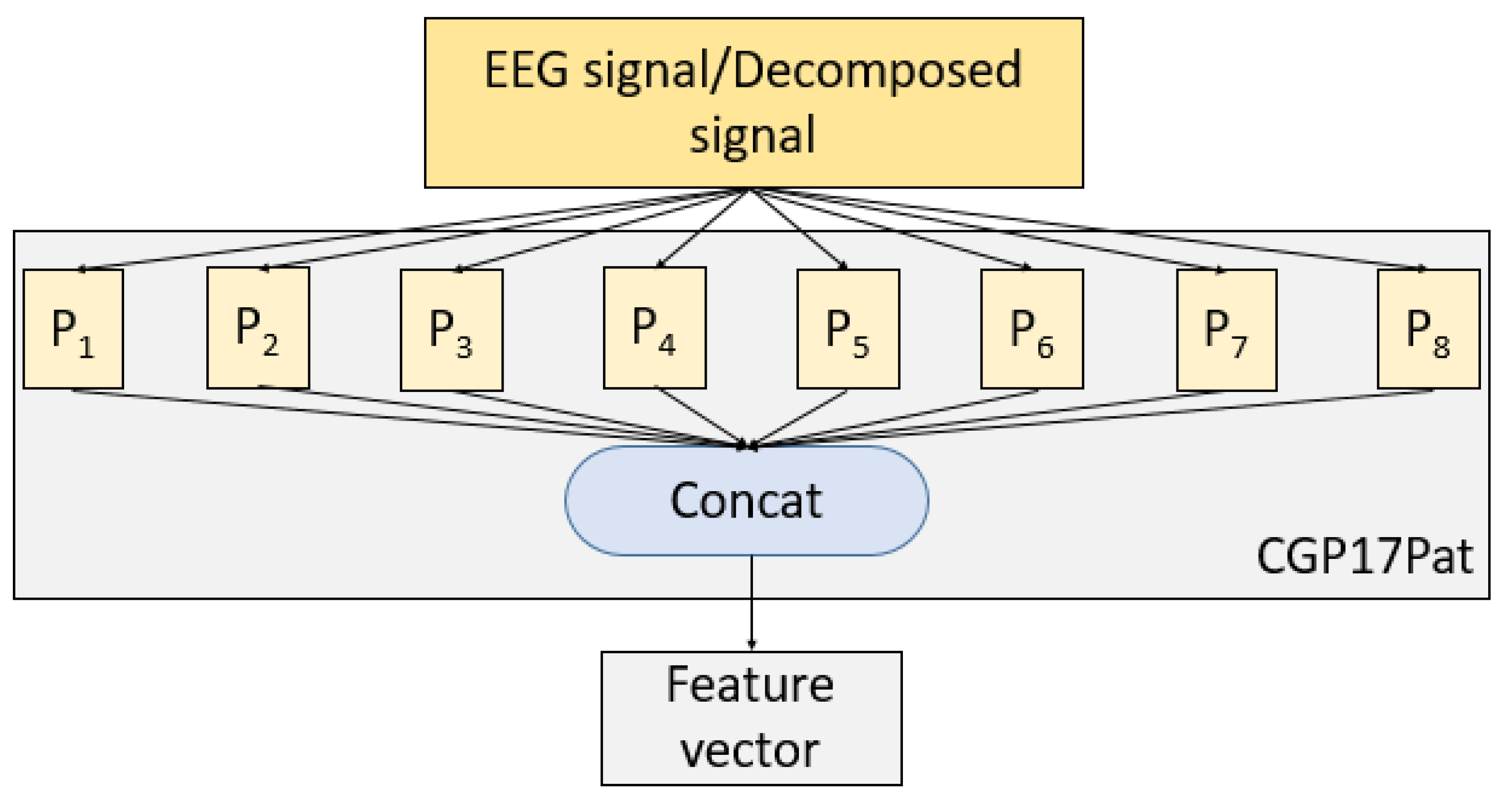
35]. Using the values of the group and a prime number, unique vectors are generated, and these vectors can be used to create permutation or substitution boxes. In this work, using these nonlinear values by generating CGP, a new local binary pattern, such as a signal descriptor, was generated. A local binary pattern and versions of it, generally, used a pattern to generate features. Using CGP with modulo 17, eight different patterns were created, and all of these patterns were applied to the signal to generate a feature vector. Thus, the proposed feature extraction function was named CGP17Pat.
Schizophrenia is a serious mental disorder, and diagnosing schizophrenia is not easy. Constant follow ups with suspected cases are required before a diagnosis can be made. Moreover, early diagnosis and treatment are vital for a better prognosis. Hence, to simplify the diagnosis process of schizophrenia, an automated EEG-based diagnosis model is proposed. Our machine learning method uses CGP17Pat as the feature extractor. A successful machine learning method needs an effective feature extractor, a feature selection function to choose the most discriminative ones, and an appropriate classifier. Our proposed model extracted 2048 features using eight patterns of the presented CGP17Pat. The MAP decomposer was used to create high-level features, while INCA [
36] was employed to choose the top features. kNN [
37] was employed to generate channel-wise results using 10-fold cross-validation and LOSO. Finally, iterative hard majority voting created the general results. The objectives of this study were (i) to show the feature generation ability of the proposed CGP17Pat, (ii) develop an automated schizophrenia detection model using EEG signals with a low time burden, and (iii) to analyze the schizophrenia detection ability using each EEG channel.
A new generation hand-modeled EEG signal classification method is proposed in this research, and the novelty of this research is the CGP17Pat. CGP has generally been used for cryptographic engineering models, since it is a finite group creator. CGP and 17 (a prime number) were used in this work to present a new feature extractor. The contributions of this work are given as below.
(i) A new one-dimensional feature extraction function using a cryptographic model is proposed. The main aim of this feature extractor was to suggest a nonlinear local feature extractor, and this feature extractor is a local binary pattern (LBP) feature extractor. Therefore, the proposed CGP17Pat generates textural features. The informative feature generation ability of CGP17Pat is demonstrated using EEG signals;
(ii) The CGP17Pat is the main feature extraction function of this model. An effective feature selector (i.e., INCA) was employed to decrease the number of features, and a shallow/conventional classifier (i.e., kNN) was deployed to obtain the classification results. Furthermore, two validations methods (i.e., 10-fold cross-validation and LOSO) were used to validate the robustness of the CGP17Pat-based EEG signal classification method. A schizophrenia data set with 19 channels was analyzed in this work.
3. Results
The performance of the presented CGP17Pat-based schizophrenia detection model is evaluated in this section.
3.1. Experimental Setup
The proposed parametric CGP17Pat-based EEG classification model used in this study contains four phases. To implement the CGP17Pat-based EEG classification model, a MATLAB (2021b) environment was used. The parameters used in this EEG classification model are listed in
Table 5.
3.2. Performance Metrics
By deploying the parameters above (
Table 5), the proposed CGP17Pat-based schizophrenia detection model was implemented using a MATLAB (2021b) environment. The model’s accuracy (
), sensitivity (
), specificity (
), and geometric mean (
) were calculated. Mathematical notations of the performance metrics are given in Equations (11)–(14) [
41,
42]:
Herein, , and are the number of true positives, false negatives, true negatives, and false positives.
3.3. Performance Evaluation
The results were calculated using 10-fold cross-validation and LOSO. Moreover, this data set contained 19 channels, and the channel-wise (channel by channel) results are listed in
Table 6.
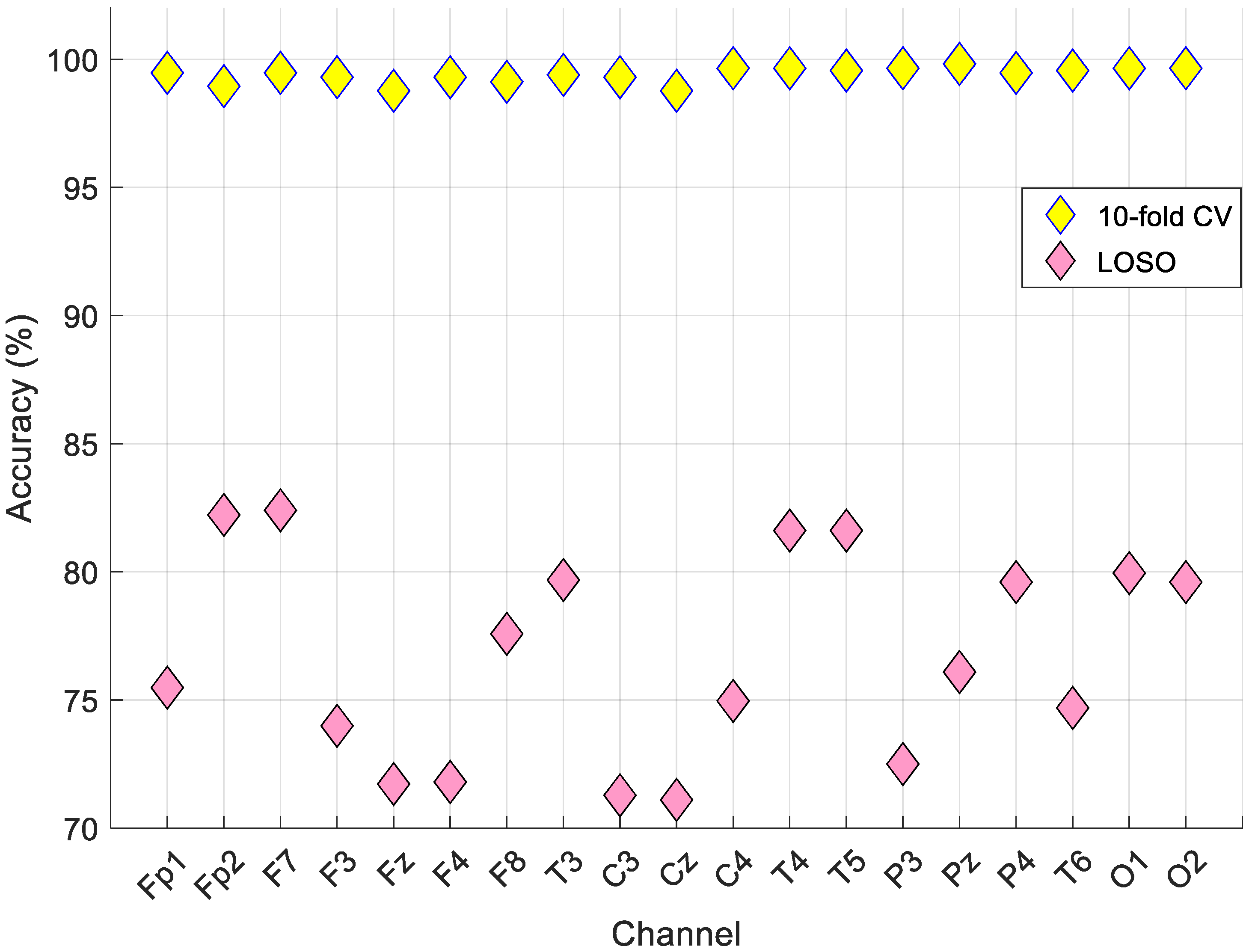
The best results are noted in bold font type. According to the results in
Table 6, the best accurate channel was Pz based on the 10-fold cross-validation, and our proposed model reached a 99.82% classification accuracy and geometric mean. Furthermore, our model yielded an 82.40% classification accuracy using LOSO validation on the F7 channel.
The EEG data set had 19 channels, and the channel-wise results were also calculated using the presented CGP17Pat-based EEG signal classification model. To calculate the general (channel-wise) results, iterative hard majority voting was applied to the prediction vectors. The calculated voted results are tabulated in
Table 7.
Table 7 shows that iterative hard majority voting algorithm increased classification accuracy from 99.82% to 99.91% for the 10-fold cross-validation and from 82.40% to 84.33% for the LOSO validation.
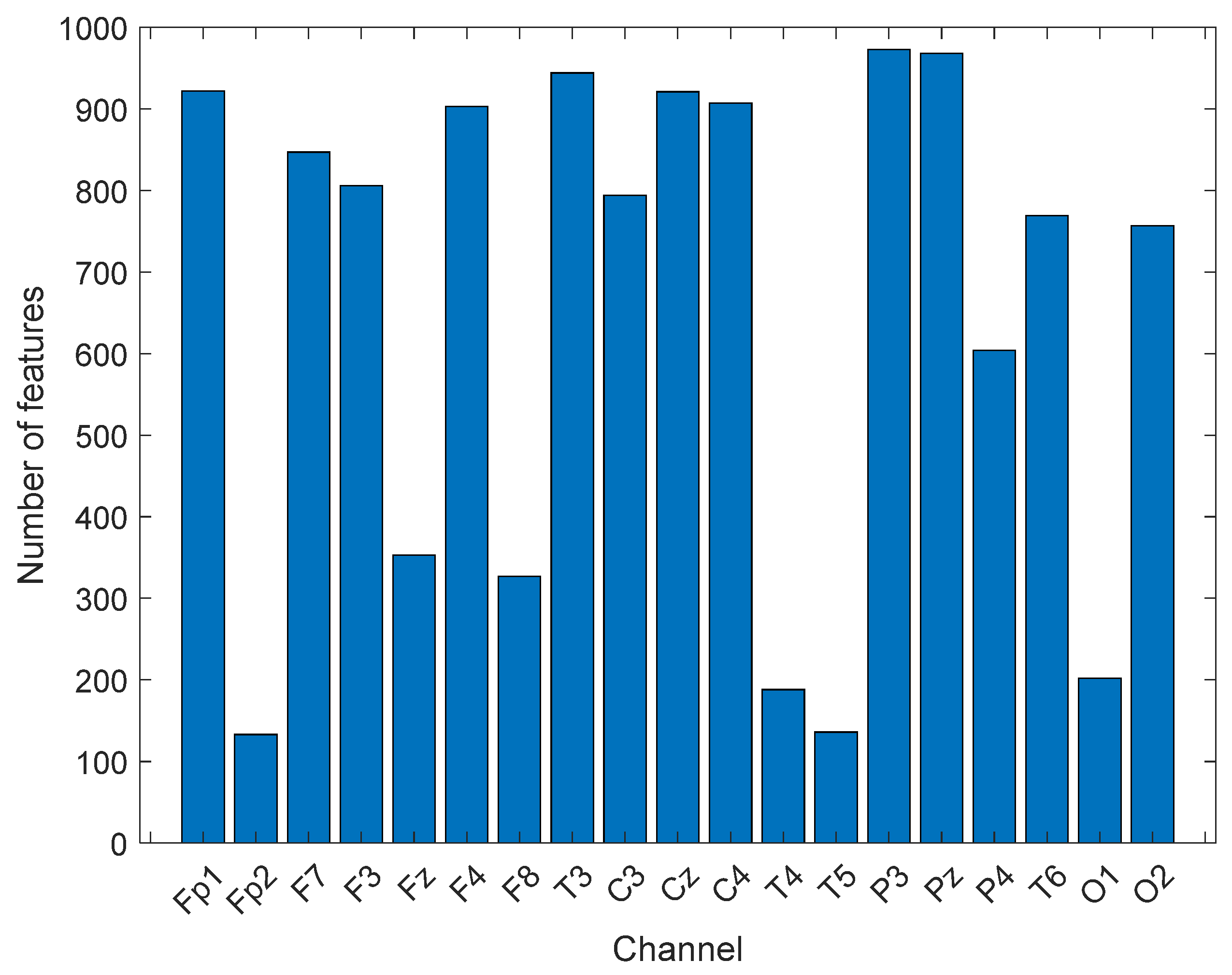
CGP is the most widely used mathematical model to create cyclic groups for cryptographic applications. Here, CGP was utilized to propose a new generation nonlinear pattern. By using 17 as modulo, eight cyclic groups were created. Each group’s creation was considered to create a center symmetric local feature extractor. The presented CGP17Pat created 2048 features. The most valuable feature vector was chosen using the INCA selector. The range of the length of the selected optimal feature vectors was [133–973]. INCA chose 133 features for the Fp2 channel, and 973 features for the P3 channel. The number of the selected features from each channel chosen by INCA are depicted in
Figure 4.
These feature vectors were then classified using kNN. By using the kNN classifier, the results of all channels were calculated. Iterative hard majority voting was used to calculate the general classification results, and 99.91% and 84.33% classification accuracies were obtained using 10-fold cross-validation and LOSO cross-validation, respectively.
The second evaluation parameter was time/computational complexity. Big O notation was used to calculate the time complexity of our proposed CGP17Pat-based model, and the phase-by-phase results are given below.
Feature extraction: In this phase, a decomposition model and CGP17Pat feature generation function were used. The time burden of the CGP17Pat was equal to . Furthermore, this feature extractor (CGP17Pat) generates features from decomposed signals. Therefore, is calculated as the time complexity of the CGP17Pat-based multilevel feature extraction method. Here, represents the length of the signal, and defines the number of instances.
Feature selection: In the feature chosen phase, the INCA function was used, and it uses two parameters: loop range and loss function. Moreover, NCA was applied to calculate the indexes qualified of the features. Considering these parameters, the complexity of the INCA was calculated as . Herein, is the complexity coefficient of the NCA, and defines the number of feature vectors.
Classification: kNN was employed to obtain the classification results. The time complexity of the kNN is .
The computational/time complexity of this model is equal to . This result demonstrates that our proposed CGP17Pat-based schizophrenia classification model has linear complexity. Therefore, this model is a lightweight classification model.
4. Discussion
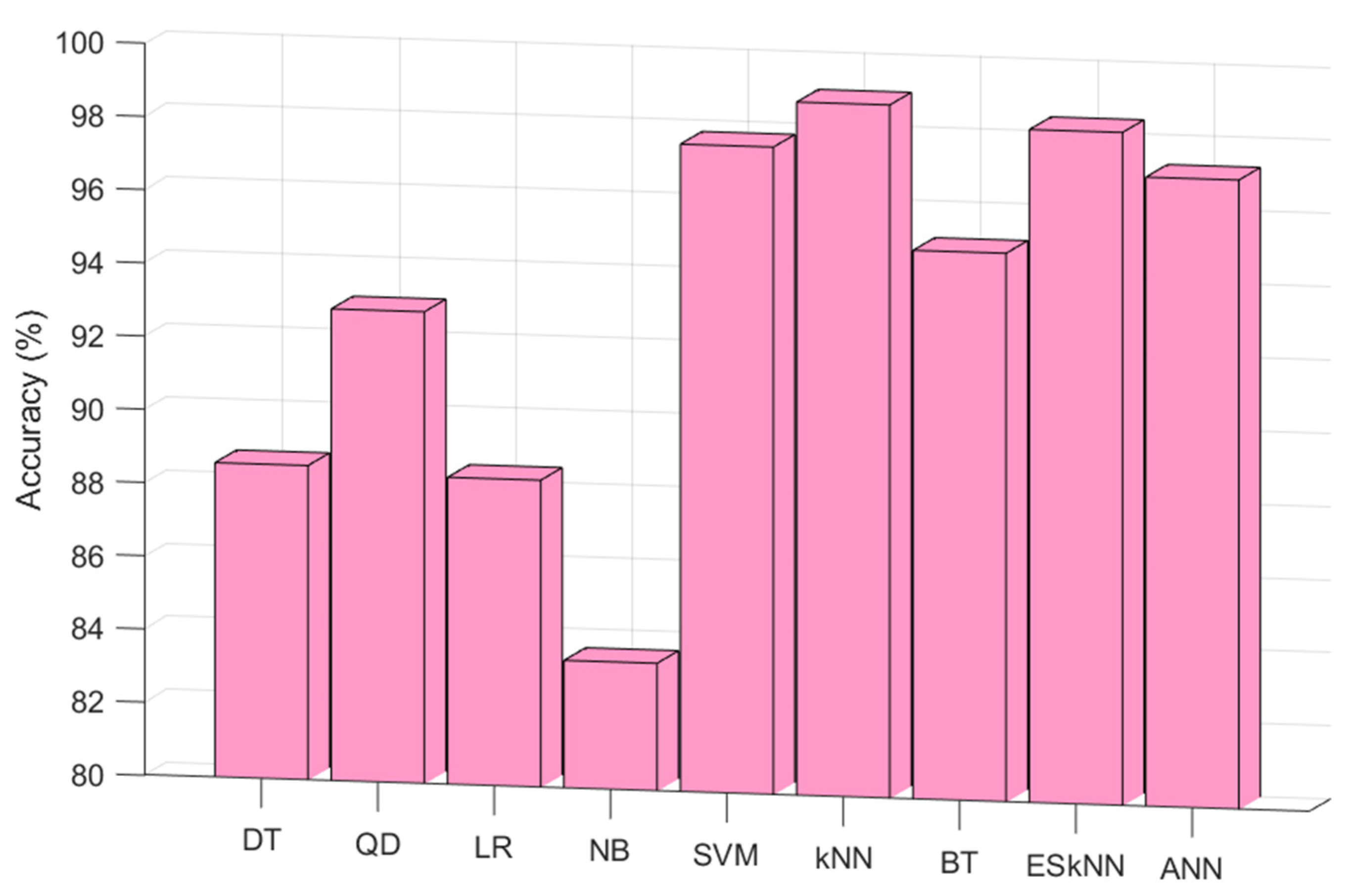
In this work, a hand-crafted feature extraction function (CGP17Pat)-based EEG signal classification model was presented to detect schizophrenia automatically. The proposed hand-modeled learning method uses kNN as both the INCA classifier and loss value generator. This classifier was selected according to the experiments. According to the results of the shallow classifiers (testing results), the best classifier was Fine kNN. The test results of the tested classifier on the Fp2 by employing a 10-fold cross-validation are depicted in
Figure 5.
From
Figure 4, the best classifier was weighted kNN for this data set. Therefore, kNN was used as the classifier in this research. Moreover, two validation techniques were used, and they were 10-fold cross-validation and LOSO cross-validation. The classification results according to the validation techniques are shown in
Figure 6.
From
Figure 6, the best accurate validation technique was the 10-fold cross-validation. This model attained the best results on the Pz channel for 10-fold cross-validation and the F7 channel for LOSO validation. Iterative hard majority voting was applied to these results, and the general results calculated are denoted in
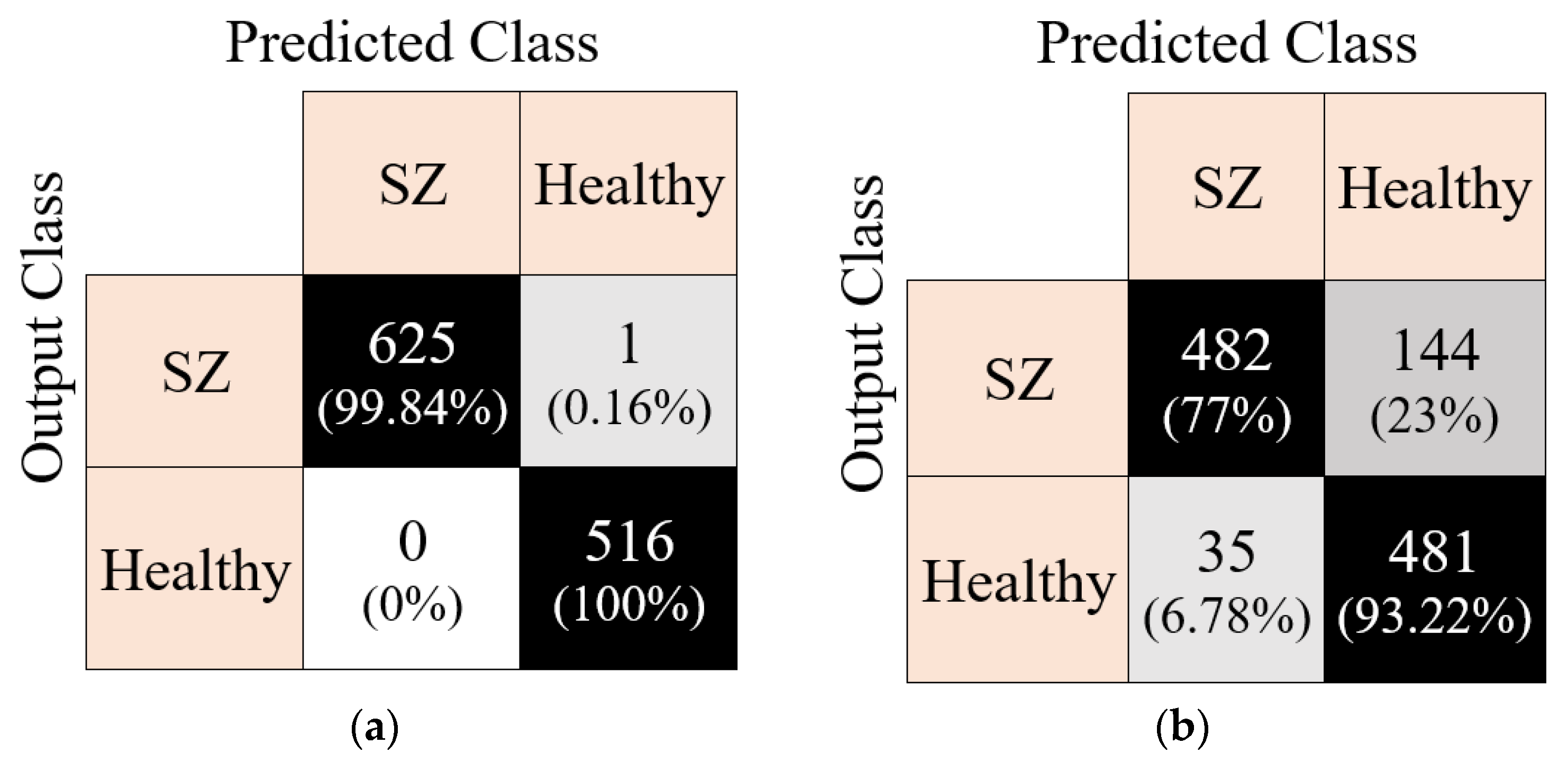
Figure 7 using confusion matrices.
To denote the success of the presented model, we compared our results with other machine learning models for the automatic detection of schizophrenia reported from 2019 to 2021 as tabulated in
Table 8.
As observed from
Table 8, Shoeibi et al. [
49] attained 99.25% accuracy using five-fold cross-validation when CNN and LSTM were used. Oh et al. [
21] presented a CNN-based model, where they calculated both subject-wise and non-subject-wise results. An accuracy of 98.07% was obtained using 10-fold cross-validation and 81.26% with LOSO cross-validation. Shoeibi et al. [
49] and Oh et al. [
21] used deep learning to attain high classification accuracies. Our CGP17Pat-based model, on the other hand, achieved the highest classification accuracy of 99.91% for 10-fold cross-validation and 84.33% with LOSO validation. Furthermore, our presented model is a lightweight EEG classification method, where the time complexity of the CGP17Pat is
and the time burden of the presented multilevel CGP17Pat-based feature extraction method is
. Since a hand-modeled classification method was used here, parameter tuning was not required. Baygin [
46] presented a statistical model to automatically detect SZ and attained over 99% classification accuracy deploying 10-fold cross-validation. However, there were no results on LOSO cross-validation in the paper. In summary, the presented CGP17Pat-based EEG signal classification model attained the best classification accuracy among the available machine learning methods with 10-fold cross-validation.
The benefits and limitations of the work are discussed below.
Benefits:
A cryptographic model (CGP) was used, where CGP was used for its feature extraction ability;
A simple machine learning model was presented using the presented CGP17Pat;
A hand-modeled EEG signal classification model was proposed with low time complexity. Only CGP17Pat was used to extract the salient features, and the time complexity of this function is O(nlong) according to Big O notation;
LOSO and 10-fold cross-validations were used to depict the robustness of this model;
To denote the feature generation capability of the CGP17Pat, a shallow classifier was used, and high accuracy values were obtained.
Limitations:
Using LOSO validation, the presented model (i.e., CGP17Pat-based classification model) attained unsatisfactory results for several channels (especially Fz, F4, C3, and Cz);
We used a hand-modeled learning technique, but INCA had high time complexity;
The hyperparameters of the kNN can be optimized.
The proposed model can be used in psychiatry clinics to detect schizophrenia using EEG signals, and we also intend to use this model to detect different types of schizophrenia to help clinicians in their treatment.