Vertical Jump Tests: A Safe Instrument to Improve the Accuracy of the Functional Capacity Assessment in Robust Older Women
Abstract
:1. Introduction
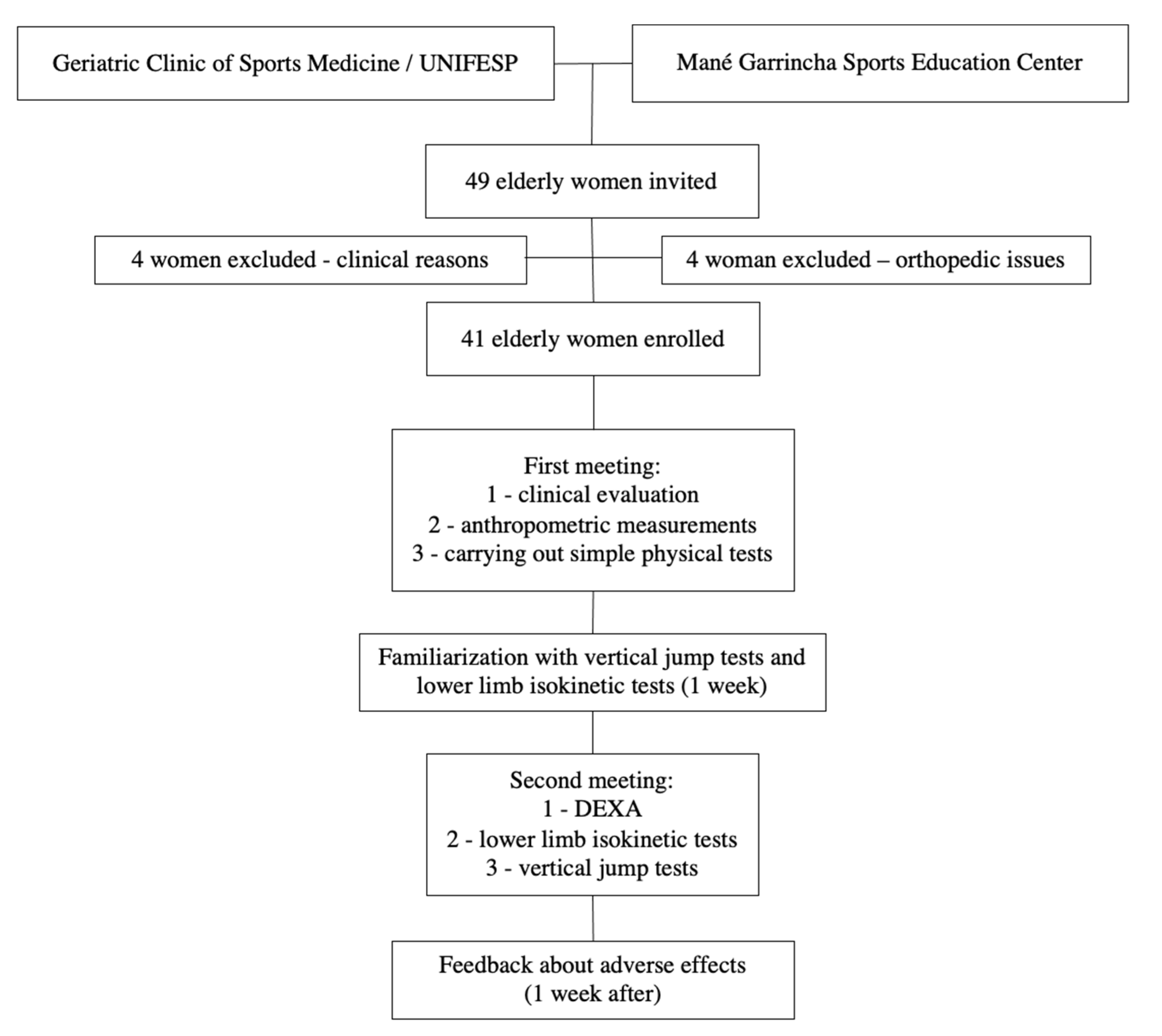
2. Materials and Methods
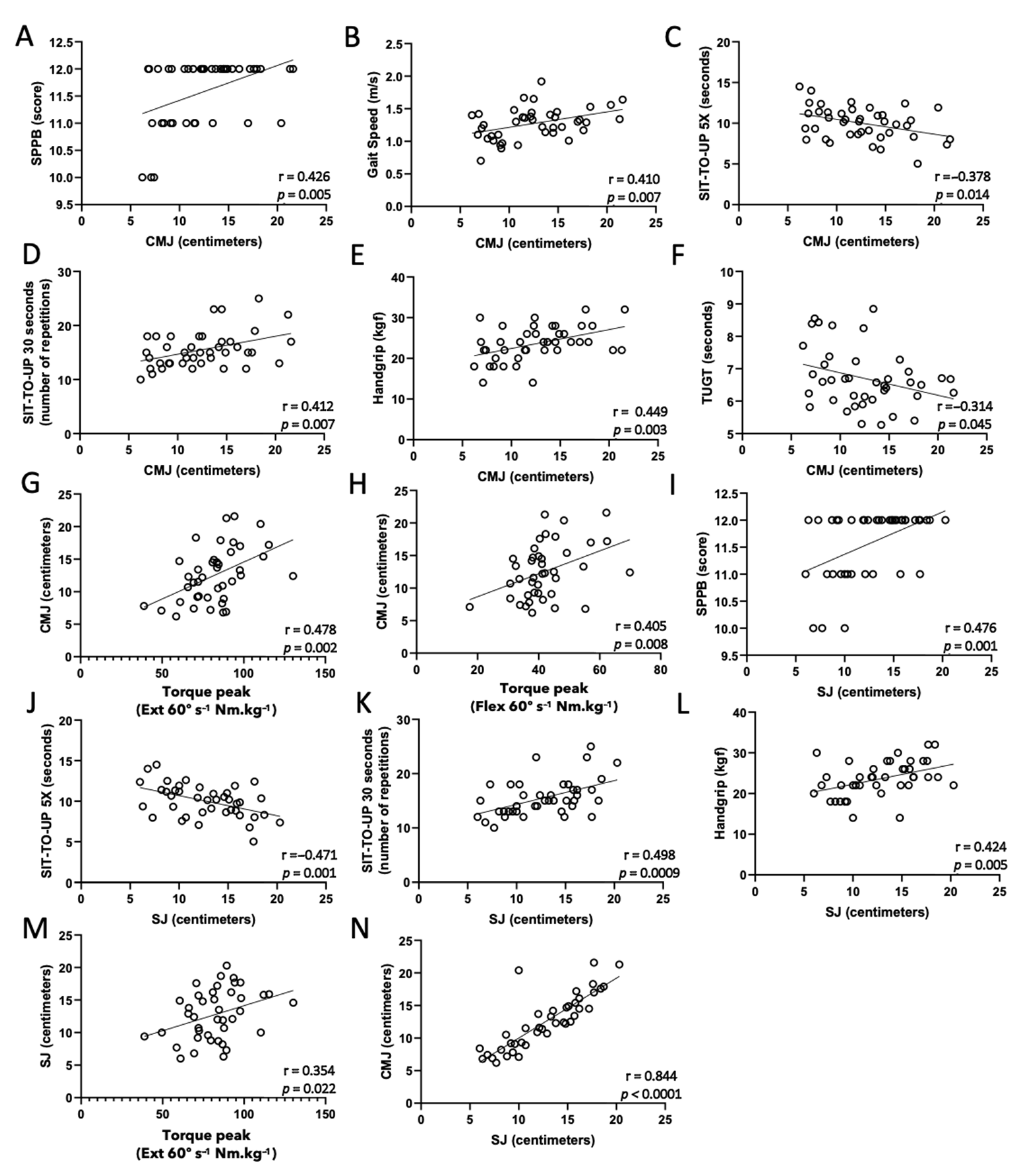
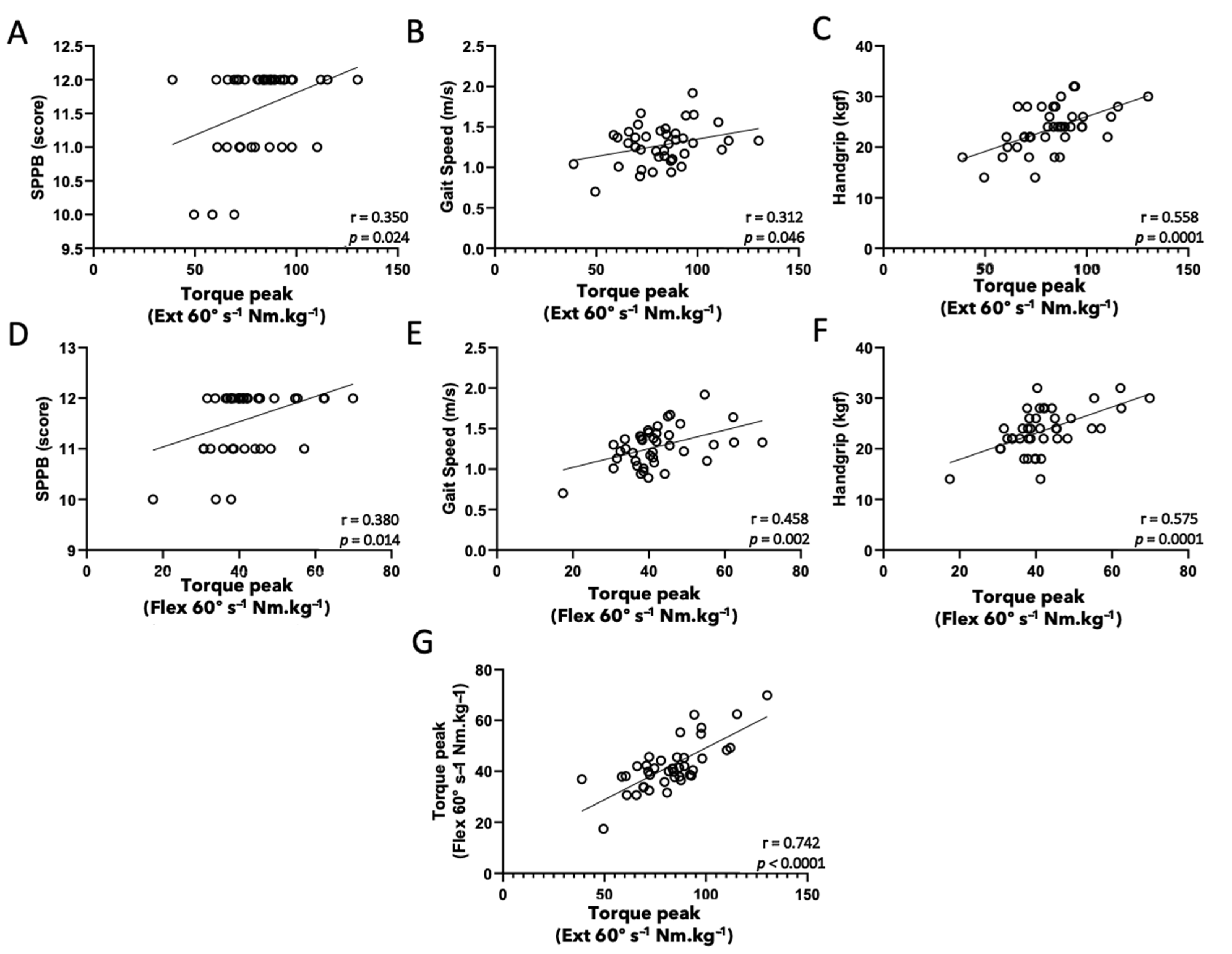
3. Results
4. Discussion
5. Limitations of the Study
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reid, K.F.; Fielding, R.A. Skeletal Muscle Power: A Critical Determinant of Physical Functioning in Older Adults. Exerc. Sport Sci. Rev. 2012, 40, 4. [Google Scholar] [CrossRef] [PubMed]
- Larsson, L.; Sjödin, B.; Karlsson, J. Histochemical and Biochemical Changes in Human Skeletal Muscle with Age in Sedentary Males, Age 22–65 Years. Acta Physiol. Scand. 1978, 103, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Lauretani, F.; Russo, C.R.; Bandinelli, S.; Bartali, B.; Cavazzini, C.; Di Iorio, A.; Ferrucci, L. Age-Associated Changes in Skeletal Muscles and Their Effect on Mobility: An Operational Diagnosis of Sarcopenia. J. Appl. Physiol. 2003, 95, 1851–1860. [Google Scholar] [CrossRef] [PubMed]
- Zampieri, S.; Pietrangelo, L.; Loefler, S.; Fruhmann, H.; Vogelauer, M.; Burggraf, S.; Kern, H. Lifelong Physical Exercise Delays Age-Associated Skeletal Muscle Decline. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2015, 70, 163–173. [Google Scholar] [CrossRef] [Green Version]
- Manini, T.M.; Clark, B.C. Dynapenia and Aging: An Update. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2012, 67, 28–40. [Google Scholar] [CrossRef] [Green Version]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J. Cardiovascular Health Study Collaborative Research Group. Frailty in Older Adults: Evidence for a Phenotype. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2001, 56, M146. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Landi, F.; Schneider, S.M.; Zúñiga, C.; Arai, H.; Boirie, Y.; Cederholm, T. Prevalence of and Interventions for Sarcopenia in Ageing Adults: A Systematic Review. Report of the International Sarcopenia Initiative (Ewgsop and Iwgs). Age Ageing 2014, 43, 748–759. [Google Scholar] [CrossRef]
- WHO. WHO Clinical Consortium on Healthy Ageing 2019. In Proceedings of the Report of Consortium Meeting, Geneva, Switzerland, 21–22 November 2019; Available online: https://www.who.int/PUBLICATIONS/I/ITEM/9789240009752 (accessed on 31 December 2021).
- Welch, C.; Greig, C.; Masud, T.; Wilson, D.; Jackson, T.A. Covid-19 and Acute Sarcopenia. Aging Dis. 2020, 11, 1345. [Google Scholar] [CrossRef]
- Morley, J.E.; Kalantar-Zadeh, K.; Anker, S.D. Covid-19: A Major Cause of Cachexia and Sarcopenia? J. Cachexia Sarcopenia Muscle 2020, 11, 863–865. [Google Scholar] [CrossRef]
- Mijnarends, D.M.; Meijers, J.M.; Halfens, R.J.; Ter Borg, S.; Luiking, Y.C.; Verlaan, S.; Schols, J.M. Validity and Reliability of Tools to Measure Muscle Mass, Strength, and Physical Performance in Community-Dwelling Older People: A Systematic Review. J. Am. Med. Dir. Assoc. 2013, 14, 170–178. [Google Scholar] [CrossRef]
- Guralnik, J.M.; Simonsick, E.M.; Ferrucci, L.; Glynn, R.J.; Berkman, L.F.; Blazer, D.G.; Wallace, R.B. A Short Physical Performance Battery Assessing Lower Extremity Function: Association with Self-Reported Disability and Prediction of Mortality and Nursing Home Admission. J. Gerontol. 1994, 49, M85. [Google Scholar] [CrossRef]
- Rikli, R.E.Y.; Jones, C.J. Development and Validation of a Functional Fitness Test For Community-Residing Older Adults. J. Aging Phys. Act. 1999, 7, 29–161. [Google Scholar] [CrossRef]
- Santana, M.G.; De Lira, C.A.; Passos, G.S.; Santos, C.A.; Silva, A.H.; Yoshida, C.H.; De Mello, M.T. Is the Six-Minute Walk Test Appropriate for Detecting Changes in Cardiorespiratory Fitness in Healthy Elderly Men? J. Sci. Med. Sport 2012, 15, 259–265. [Google Scholar] [CrossRef]
- Shaffer, N.C.; Ferrucci, L.; Shardell, M.; Simonsick, E.M.; Studenski, S. Agreement and Predictive Validity Using Less-Conservative Foundation for the National Institutes of Health Sarcopenia Project Weakness Cutpoints. J. Am. Geriatr. Soc. 2017, 65, 574–579. [Google Scholar] [CrossRef] [Green Version]
- Grober, E.; Mowrey, W.; Katz, M.; Derby, C.; Lipton, R.B. Conventional and Robust Norming in Identifying Preclinical Dementia. J. Clin. Exp. Neuropsychol. 2015, 37, 1098–1106. [Google Scholar] [CrossRef] [PubMed]
- Tsutsumimoto, K.; Doi, T.; Makizako, H.; Hotta, R.; Nakakubo, S.; Makino, K.; Shimada, H. Aging-Related Anorexia and Its Association with Disability and Frailty. J. Cachexia Sarcopenia Muscle 2018, 9, 834–843. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Zamboni, M. Sarcopenia: Revised European Consensus on Definition and Diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simonsick, E.M.; Newman, A.B.; Nevitt, M.C.; Kritchevsky, S.B.; Ferrucci, L.; Guralnik, J.M.; Harris, T. Measuring Higher Level Physical Function in Well-Functioning Older Adults: Expanding Familiar Approaches in the Health ABC Study. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2001, 56, M644–M649. [Google Scholar] [CrossRef] [PubMed]
- Neder, J.A.; Nery, L.E.; Shinzato, G.T.; Andrade, M.S.; Peres, C.; Silva, A.C. Reference Values for Concentric Knee Isokinetic Strength and Power in Nonathletic Men and Women from 20 to 80 Years Old. J. Orthop. Sports Phys. Ther. 1999, 29, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Loturco, I.; Pereira, L.A.; Kobal, R.; Abad, C.C.C.; Komatsu, W.; Cunha, R.; Cohen, M. Functional Screening Tests: Interrelationships and Ability to Predict Vertical Jump Performance. Int. J. Sports Med. 2018, 39, 189–197. [Google Scholar] [CrossRef]
- Hartmann, A.; Knols, R.; Murer, K.; De Bruin, E.D. Reproducibility of an Isokinetic Strength-Testing Protocol of the Knee and Ankle in Older Adults. Gerontology 2009, 55, 259–268. [Google Scholar] [CrossRef]
- Buehring, B.; Krueger, D.; Fidler, E.; Gangnon, R.; Heiderscheit, B.; Binkley, N. Reproducibility of Jumping Mechanography and Traditional Measures of Physical and Muscle Function in Older Adults. Osteoporos. Int. 2015, 26, 819–825. [Google Scholar] [CrossRef]
- Singh, H.; Kim, D.; Kim, E.; Bemben, M.G.; Anderson, M.; Seo, D.I.; Bemben, D.A. Jump Test Performance and Sarcopenia Status in Men and Women, 55 to 75 Years of Age. J. Geriatr. Phys. Ther. 2014, 37, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Kim, D.; Bemben, M.G.; Bemben, D.A. Relationship between Muscle Performance and DXA-Derived Bone Parameters in Community-Dwelling Older Adults. J. Musculoskelet. Neuronal Interact. 2017, 17, 50. [Google Scholar] [PubMed]
- Buehring, B.; Krueger, D.; Binkley, N. Jumping Mechanography: A Potential Tool for Sarcopenia Evaluation in Older Individuals. J. Clin. Densitom. 2010, 13, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Alley, D.E.; Shardell, M.D.; Peters, K.W.; Mclean, R.R.; Dam, T.T.L.; Kenny, A.M.; Cawthon, P.M. Grip Strength Cutpoints for the Identification of Clinically Relevant Weakness. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2014, 69, 559–566. [Google Scholar] [CrossRef]
- Hicks, G.E.; Shardell, M.; Alley, D.E.; Miller, R.R.; Bandinelli, S.; Guralnik, J.; Ferrucci, L. Absolute Strength and Loss of Strength as Predictors of Mobility Decline in Older Adults: The Inchianti Study. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2012, 67, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Langhammer, B.; Stanghelle, J.K. Functional Fitness in Elderly Norwegians Measured with the Senior Fitness Test. Adv. Physiother. 2011, 13, 137–144. [Google Scholar] [CrossRef]
- Westman, A.W.; Combs-Miller, S.; Moore, J.; Ehrlich-Jones, L. Measurement Characteristics and Clinical Utility of the Short Physical Performance Battery among Community-Dwelling Older Adults. Arch. Phys. Med. Rehabil. 2019, 100, 185–187. [Google Scholar] [CrossRef]
- Guralnik, J.M.; Ferrucci, L.; Simonsick, E.M.; Salive, M.E.; Wallace, R.B. Lower-Extremity Function in Persons over the Age of 70 Years as a Predictor of Subsequent Disability. N. Engl. J. Med. 1995, 332, 556–561. [Google Scholar] [CrossRef] [Green Version]
- Studenski, S.A.; Peters, K.W.; Alley, D.E.; Cawthon, P.M.; Mclean, R.R.; Harris, T.B.; Vassileva, M.T. The Fnih Sarcopenia Project: Rationale, Study Description, Conference Recommendations, and Final Estimates. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2014, 69, 547–558. [Google Scholar] [CrossRef]
- Ditroilo, M.; Forte, R.; Mckeown, D.; Boreham, C.; De Vito, G. Intra-And Inter-Session Reliability of Vertical Jump Performance in Healthy Middle-Aged and Older Men and Women. J. Sports Sci. 2011, 29, 1675–1682. [Google Scholar] [CrossRef]
- Argaud, S.; De Fontenay, B.P.; Blache, Y.; Monteil, K. Explosive Movement in the Older Men: Analysis and Comparative Study of Vertical Jump. Aging Clin. Exp. Res. 2017, 29, 985–992. [Google Scholar] [CrossRef] [PubMed]
- Palmer, T.B.; Followay, B.N.; Thompson, B.J. Age-Related Effects on Maximal and Rapid Hamstrings/Quadriceps Strength Capacities and Vertical Jump Power in Young and Older Females. Aging Clin. Exp. Res. 2017, 29, 1231–1239. [Google Scholar] [CrossRef]
- De Lira, C.; Vargas, V.; Silva, W.; Bachi, A.; Vancini, R.; Andrade, M. Relative strength, but not absolute muscle strength, is higher in exercising compared to non-exercising older women. Sports 2019, 7, 19. [Google Scholar] [CrossRef] [Green Version]
- Bohannon, R.W. Hand-Grip Dynamometry Predicts Future Outcomes in Aging Adults. J. Geriatr. Phys. Ther. 2008, 31, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Alexandre, T.; Duarte, Y.D.O.; Santos, J.F.; Wong, R.; Lebrão, M.L. Sarcopenia According to the European Working Group on Sarcopenia in Older People (Ewgsop) Versus Dynapenia as a Risk Factor for Disability in the Elderly. J. Nutr. Health Aging 2014, 18, 547–553. [Google Scholar] [CrossRef]
- Delinocente, M.L.B.; De Carvalho, D.H.T.; De Oliveira Máximo, R.; Chagas, M.H.N.; Santos, J.L.F.; De Oliveira Duarte, Y.A.; Da Silva Alexandre, T. Accuracy of Different Handgrip Values to Identify Mobility Limitation in Older Adults. Arch. Gerontol. Geriatr. 2021, 94, 104347. [Google Scholar] [CrossRef] [PubMed]
- Hong, N.; Kim, C.O.; Youm, Y.; Kim, H.C.; Rhee, Y. Low Peak Jump Power is Associated with Elevated Odds of Dysmobility Syndrome in Community-Dwelling Elderly Individuals: The Korean Urban Rural Elderly (Kure) Study. Osteoporos. Int. 2018, 29, 1427–1436. [Google Scholar] [CrossRef]
- Macfarlane, D.J.; Chou, K.L.; Cheng, Y.H.; Chi, I. Validity and Normative Data for Thirty-Second Chair Stand Test in Elderly Community-Dwelling Hong Kong Chinese. Am. J. Hum. Biol. Off. J. Hum. Biol. Assoc. 2006, 18, 418–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Netz, Y.; Ayalon, M.; Dunsky, A.; Alexander, N. ‘The Multiple-Sit-To-Stand’ Field Test for Older Adults: What Does It Measure? Gerontology 2004, 50, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Glenn, J.M.; Gray, M.; Vincenzo, J.; Paulson, S.; Powers, M. An Evaluation of Functional Sit-To-Stand Power in Cohorts of Healthy Adults Aged 18–97 Years. J. Aging Phys. Act. 2017, 25, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Moore, B.A.; Bemben, D.A.; Lein, D.H.; Bemben, M.G.; Singh, H. Fat Mass is Negatively Associated with Muscle Strength and Jump Test Performance. J. Frailty Aging 2020, 9, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Hao, Q.; Yue, J.; Hou, L.; Xia, X.; Zhao, W.; Dong, B. Sarcopenia, Obesity and Sarcopenia Obesity in Comparison: Prevalence, Metabolic Profile, and Key Differences: Results from Wchat Study. J. Nutr. Health Aging 2020, 24, 429–437. [Google Scholar] [CrossRef]
| Parameter | Mean ± SD | BMI | CALF | % FAT | ASMi | ||||
|---|---|---|---|---|---|---|---|---|---|
| r | p Value | r | p Value | r | p Value | r | p Value | ||
| Weight (kg) | 60.7 ± 10.9 | 1 | n.a. | 1 | n.a. | 1 | n.a. | 1 | n.a. |
| Height (m) | 1.55 ± 0,1 | 1 | n.a. | 1 | n.a. | 1 | n.a. | 1 | n.a. |
| BMI (kg/m2) | 25.3 ± 3.9 | 1 | n.a. | 1 | n.a. | 1 | n.a. | 1 | n.a. |
| CALF (cm) | 34.3 ± 2.9 | 0.551 | <0.0001 | 1 | n.a. | 1 | n.a. | 1 | n.a. |
| % FAT | 38.9 ± 7.7 | 0.789 | <0.0001 | 0.453 | 0.003 | 1 | n.a. | 1 | n.a. |
| ASMi (g/m2) | 6.5 ± 0.6 | 0.315 | 0.045 | 0.163 | 0.310 | −0.125 | 0.438 | 1 | n.a. |
| SIT-UP 5X (s) | 10.0 ± 2.0 | 0.232 | 0.144 | 0.275 | 0.082 | 0.256 | 0.106 | 0.051 | 0.752 |
| GS (m/s) | 1.5 ± 0.2 | −0.221 | 0.165 | −0.063 | 0.695 | −0.267 | 0.092 | −0.017 | 0.915 |
| SPPB (score) | 11.6 ± 0.6 | −0.184 | 0.249 | −0.204 | 0.200 | −0.229 | 0.149 | −0.112 | 0.485 |
| SIT-UP 30” (repetitions) | 16.0 ± 3.3 | −0.273 | 0.084 | −0.303 | 0.054 | −0.282 | 0.074 | −0.006 | 0.970 |
| HG (kgf) | 23.6 ± 4.3 | 0.139 | 0.385 | 0.026 | 0.870 | 0.064 | 0.692 | −0.001 | 0.993 |
| TUGT (s) | 6.7 ± 0.9 | 0.053 | 0.741 | −0.089 | 0.581 | 0.028 | 0.864 | 0.157 | 0.328 |
| CMJ (cm) | 12.5 ± 6.2 | −0.236 | 0.138 | −0.253 | 0.110 | −0.349 | 0.025 | 0.185 | 0.247 |
| SJ (cm) | 12.5 ± 6.2 | −0.313 | 0.046 | −0.373 | 0.016 | −0.425 | 0.006 | 0.258 | 0.103 |
| Ext 60°·s−1 (Nm·kg−1) | 82.4 ± 17.4 | 0.361 | 0.021 | 0.191 | 0.232 | 0.199 | 0.213 | 0.242 | 0.127 |
| Flex 60°·s−1 (Nm·kg−1) | 42.0 ± 9.5 | 0.178 | 0.265 | 0.096 | 0.549 | −0.059 | 0.715 | 0.156 | 0.329 |
| Ext 180°·s−1 (Nm·kg−1) | 87.1 ± 14.6 | 0.112 | 0.497 | −0.285 | 0.078 | 0.023 | 0.888 | 0.194 | 0.237 |
| Flex 180°·s−1 (Nm·kg−1) | 49.8 ± 14.7 | 0.139 | 0.398 | −0.254 | 0.119 | 0.098 | 0.551 | 0.033 | 0.844 |
| Parameter | SIT-UP 5X | GS | SPPB | SIT-UP 30” | ||||
|---|---|---|---|---|---|---|---|---|
| r | p Value | r | p Value | r | p Value | r | p Value | |
| GS | −0.072 | 0.654 | 1 | n.a. | 1 | n.a. | 1 | n.a. |
| SPPB | −0.690 | <0.0001 | 0.222 | 0.163 | 1 | n.a. | 1 | n.a. |
| SIT-UP 30” | −0.915 | <0.0001 | 0.074 | 0.647 | 0.541 | <0.0001 | 1 | n.a. |
| HG | −0.298 | 0.058 | 0.261 | 0.100 | 0.432 | 0.005 | 0.236 | 0.137 |
| TUGT | 0.387 | 0.013 | −0.403 | 0.009 | −0.423 | 0.006 | −0.311 | 0.048 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, C.A.F.; Amirato, G.R.; Jacinto, A.F.; Pedrosa, A.V.; Caldo-Silva, A.; Sampaio, A.R.; Pimenta, N.; Santos, J.M.B.; Pochini, A.; Bachi, A.L.L. Vertical Jump Tests: A Safe Instrument to Improve the Accuracy of the Functional Capacity Assessment in Robust Older Women. Healthcare 2022, 10, 323. https://doi.org/10.3390/healthcare10020323
Santos CAF, Amirato GR, Jacinto AF, Pedrosa AV, Caldo-Silva A, Sampaio AR, Pimenta N, Santos JMB, Pochini A, Bachi ALL. Vertical Jump Tests: A Safe Instrument to Improve the Accuracy of the Functional Capacity Assessment in Robust Older Women. Healthcare. 2022; 10(2):323. https://doi.org/10.3390/healthcare10020323
Chicago/Turabian StyleSantos, Carlos A. F., Gislene R. Amirato, Alessandro F. Jacinto, Ana V. Pedrosa, Adriana Caldo-Silva, António R. Sampaio, Nuno Pimenta, Juliana M. B. Santos, Alberto Pochini, and André L. L. Bachi. 2022. "Vertical Jump Tests: A Safe Instrument to Improve the Accuracy of the Functional Capacity Assessment in Robust Older Women" Healthcare 10, no. 2: 323. https://doi.org/10.3390/healthcare10020323
APA StyleSantos, C. A. F., Amirato, G. R., Jacinto, A. F., Pedrosa, A. V., Caldo-Silva, A., Sampaio, A. R., Pimenta, N., Santos, J. M. B., Pochini, A., & Bachi, A. L. L. (2022). Vertical Jump Tests: A Safe Instrument to Improve the Accuracy of the Functional Capacity Assessment in Robust Older Women. Healthcare, 10(2), 323. https://doi.org/10.3390/healthcare10020323








