Effects of Resistance Training on Skin Temperature and Its Relationship with Central Nervous System (CNS) Activation
Abstract
1. Introduction
2. Materials and Methods
2.1. Definition of the Sample
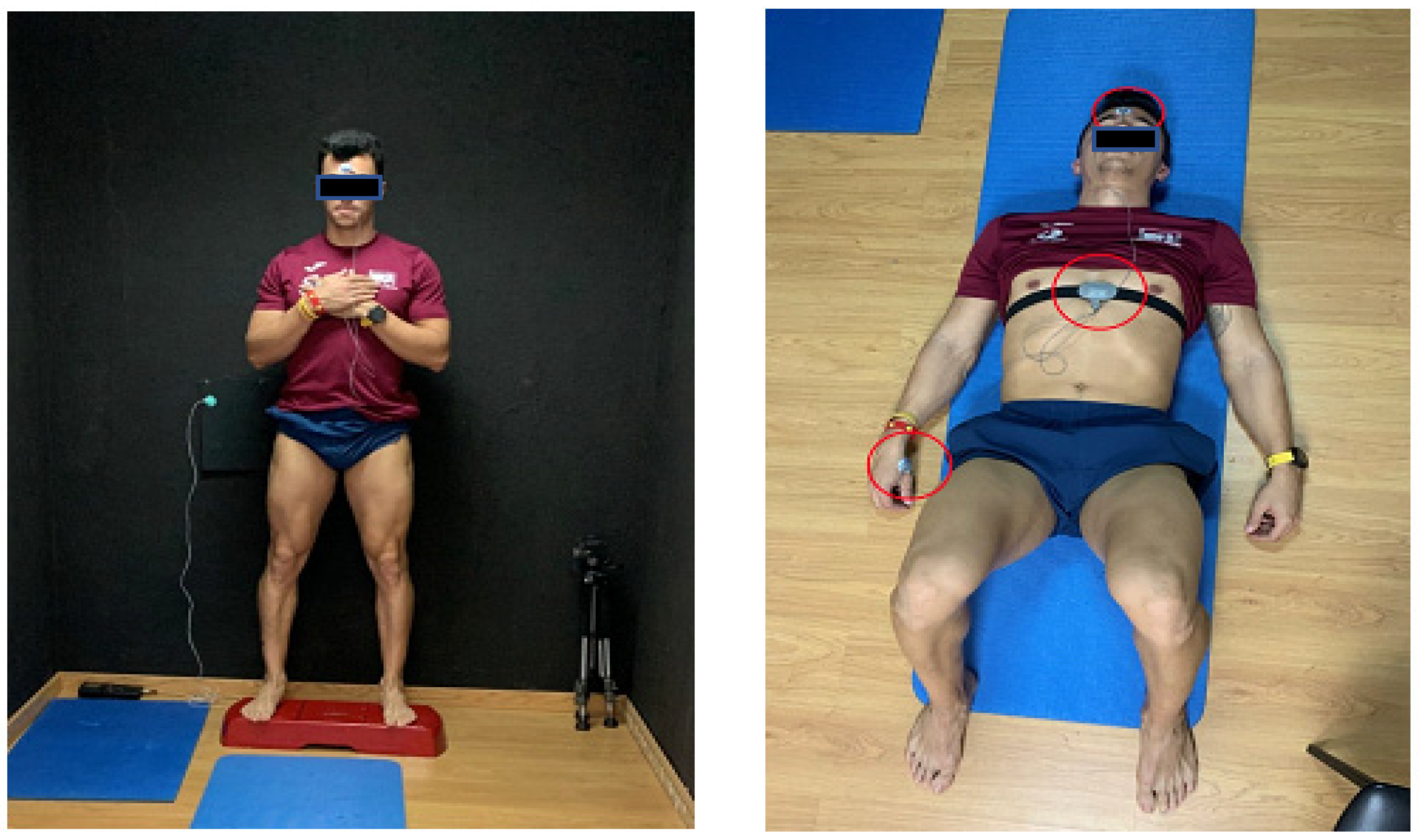
2.2. General Aspects of the Data Collection Procedure
2.3. Description of the CNS Activation Assessment
2.4. Description of the Skin Temperature Assessment
2.5. Description of the Resistance Training
2.6. Data Collection Procedure
2.7. Analysis of the Data
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gómez-Carmona, P.; Fernández-Cuevas, I.; Sillero-Quintana, M.; Arnaiz-Lastras, J.; Navandar, A. Infrared thermography protocol on reducing the incidence of soccer injuries. J. Sport Rehabil. 2020, 29, 1222–1227. [Google Scholar] [CrossRef]
- Marins, J.C.B.; Fernández-Cuevas, I.; Arnaiz-Lastras, J.; Fernandes, A.A.; Sillero-Quintana, M. Applications of infrared thermography in sports. A review. Rev. Int. Med. Cienc. Act. Fis. Dep. 2015, 15, 805–824. [Google Scholar]
- Ring, E.F.J.; Ammer, K. Infrared thermal imaging in medicine. Physiol. Meas. 2012, 33, R33. [Google Scholar] [CrossRef] [PubMed]
- Sillero-Quintana, M.; Gomez-Carmona, P.M.; Fernández-Cuevas, I. Infrared thermography as a means of monitoring and preventing sports injuries. In Innovative Research in Thermal Imaging for Biology and Medicine; Khosrow-Pour, M., Ed.; IGI Global, Information Resources Management Association: Hershey, PA, USA, 2017; pp. 165–198. [Google Scholar] [CrossRef]
- Abate, M.; Di Carlo, L.; Di Donato, L.; Romani, G.L.; Merla, A. Comparison of cutaneous termic response to a standardised warm up in trained and untrained individuals. J. Sports Med. Phys. Fit. 2013, 26, 18–37. [Google Scholar]
- Tauchmannova, H.; Gabrhel, J.; Cibak, M. Thermographic findings in different sports. their value in the prevention of soft tissue injuries. Thermol. Österr. 1993, 3, 91–95. [Google Scholar]
- Akimov, E.B.; Andreev, R.S.; Arkov, V.V.; Kirdin, A.A.; Saryanc, V.V.; Sonkin, V.D.; Tonevitsky, A.G. Thermal “portrait” of sportsmen with different aerobic capacity. Acta Kinesiol. Univ. Tartu. 2009, 14, 7–16. [Google Scholar] [CrossRef]
- Chudecka, M.; Lubkowska, A. Temperature changes of selected body’s surfaces of handball players in the course of training estimated by thermovision, and the study of the impact of physiological and morphological factors on the skin temperature. J. Therm. Biol. 2010, 35, 379–385. [Google Scholar] [CrossRef]
- Formenti, D.; Ludwig, N.; Gargano, M.; Gondola, M.; Dellerma, N.; Caumo, A.; Alberti, G. Thermal imaging of exercise-associated skin temperature changes in trained and untrained female subjects. Ann. Biomed. Eng. 2013, 41, 863–871. [Google Scholar] [CrossRef]
- Cheuvront, S.N.; Kenefick, R.W.; Montain, S.J.; Sawka, M.N. Mechanisms of aerobic performance impairment with heat stress and dehydration. J. Appl. Physiol. 2010, 109, 1989–1995. [Google Scholar] [CrossRef]
- Fernández-Cuevas, I.; Sillero-Quintana, M.; Garcia-Concepcion, M.A.; Serrano, J.R.; Gomez-Carmona, P.; Marins, J.B. Monitoring skin thermal response to training with infrared thermography. New Stud. Athl. 2014, 29, 57–71. [Google Scholar]
- Kenney, W.L.; Johnson, J.M. Control of skin blood flow during exercise. Med. Sci. Sports Exerc. 1992, 24, 303–312. [Google Scholar] [CrossRef]
- Laforgia, J.; Withers, R.T.; Gore, C.J. Effects of exercise intensity and duration on the excess post-exercise oxygen consumption. J. Sports Sci. 2006, 24, 1247–1264. [Google Scholar] [CrossRef]
- Peake, J.M.; Kerr, G.; Sullivan, J.P. A critical review of consumer wearables, mobile applications, and equipment for providing biofeedback, monitoring stress, and sleep in physically active populations. Front. Physiol. 2018, 9, 743. [Google Scholar] [CrossRef]
- Studer, P.; Kratz, O.; Gevensleben, H.; Rothenberger, A.; Moll, G.H.; Hautzinger, M.; Heinrich, H. Slow cortical potential and theta/beta neurofeedback training in adults: Effects on attentional processes and motor system excitability. Front. Hum. Neurosci. 2014, 8, 555. [Google Scholar] [CrossRef]
- Bauer, H.; Lauber, W. Operant conditioning of brain steady potential shifts in man. Biofeedback Self-Regul. 1979, 4, 145–154. [Google Scholar] [CrossRef]
- Karavaev, A.S.; Kiselev, A.R.; Runnova, A.E.; Zhuravlev, M.O.; Borovkova, E.I.; Prokhorov, M.D.; Ponomarenko, V.I.; Pchelintseva, S.V.; Efremova, T.U.; Koronovskii, A.A.; et al. Synchronization of infra-slow oscillations of brain potentials with respiration. Chaos 2018, 28, 081102. [Google Scholar] [CrossRef]
- Holman, A.J.; Ng, E. Heart rate variability predicts anti-tumor necrosis factor therapy response for inflammatory arthritis. Auton. Neurosci. Basic Clin. 2008, 143, 58–67. [Google Scholar] [CrossRef]
- Naranjo-Orellana, J.; Ruso-Álvarez, J.F.; Rojo-Álvarez, J.L. Comparison of Omegawave Device and an Ambulatory ECG for RR Interval Measurement at rest. Int. J. Sports Med. 2021, 42, 138–146. [Google Scholar] [CrossRef]
- Valenzuela, P.L.; Sánchez-Martínez, G.; Torrontegi, E.; Vázquez-Carrión, J.; Montalvo, Z.; Kara, O. Validity, Reliability, and Sensitivity to Exercise-Induced Fatigue of a Customer-Friendly Device for the Measurement of the Brain’s Direct Current Potential. J. Strength Cond. Res. 2020. [Google Scholar] [CrossRef]
- Heishman, A.D.; Curtis, M.A.; Saliba, E.N.; Hornett, R.J.; Malin, S.K.; Weltman, A.L. Comparing performance during morning vs. afternoon training sessions in intercollegiate basketball players. J. Strength Cond. Res. 2017, 31, 1557–1562. [Google Scholar] [CrossRef]
- Coyne, J.O.; Coutts, A.J.; Fomin, R.; French, D.N.; Newton, R.U.; Haff, G.G. Heart rate variability and direct current measurement characteristics in professional mixed martial arts athletes. Sports 2020, 8, 109. [Google Scholar] [CrossRef]
- Weigert, M.; Nitzsche, N.; Kunert, F.; Lösch, C.; Baumgärtel, L.; Schulz, H. Acute exercise-associated skin surface temperature changes after resistance training with different exercise intensities. IJKSS 2018, 6, 12–18. [Google Scholar] [CrossRef]
- Fröhlich, M.; Ludwig, O.; Zeller, P.; Felder, H. Changes in skin surface temperature after a 10-minute warm-up on a bike ergometer. IJKSS 2018, 3, 13–17. [Google Scholar] [CrossRef][Green Version]
- World Medical Association. World Medical Association Declaration of Helsinki. Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- Shaffer, F.; Ginsberg, J.P. An overview of heart rate variability metrics and norms. Front. Public Health 2017, 5, 258. [Google Scholar] [CrossRef]
- Sillero-Quintana, M.; Fernández-Cuevas, I.; Arnáiz-Lastras, J.; Marins, J.C.B. Protocol for thermographic assessment in humans. In Pre-Congress XIII EAT Congress Course on Medical Applications of Human Thermography; Ammer, K., Ed.; INEF Madrid: Madrid, Spain, 2015; pp. 1–57. [Google Scholar]
- Moreira, D.G.; Costello, J.T.; Brito, C.J.; Adamczyk, J.G.; Ammer, K.; Bach, A.J.E.; Costa, C.M.A.; Eglin, C.; Fernandes, A.A.; Fernández-Cuevas, I.; et al. Thermographic imaging in sports and exercise medicine: A Delphi study and consensus statement on the measurement of human skin temperature. J. Therm. Biol. 2017, 69, 155–162. [Google Scholar] [CrossRef]
- Brzycki, M. Strength testing predicting a one-rep max from reps-to-fatigue. J. Phys. Educ. Recreat. Danc. 1993, 64, 88–90. [Google Scholar] [CrossRef]
- Fernández-Cuevas, I.; Marins, J.C.B.; Lastras, J.A.; Carmona, P.M.G.; Cano, S.P.; García-Concepción, M.A.; Sillero-Quintana, M. Classification of factors influencing the use of infrared thermography in humans: A review. Infrared Phys. Technol. 2015, 71, 28–55. [Google Scholar] [CrossRef]
- Borg, G.A. Psychophysical bases of perceived exertion. Med. Sci. Sports Exerc. 1982, 14, 377–381. [Google Scholar] [CrossRef]
- Joyner, M.J.; Casey, D.P. Regulation of increased blood flow (hyperemia) to muscles during exercise: A hierarchy of competing physiological needs. Physiol. Rev. 2015, 95, 549–601. [Google Scholar] [CrossRef] [PubMed]
- Krustrup, P.; González-Alonso, J.; Quistorff, B.; Bangsbo, J. Muscle heat production and anaerobic energy turnover during repeated intense dynamic exercise in humans. J. Physiol. 2001, 536, 947–956. [Google Scholar] [CrossRef] [PubMed]
- Carter, R., III; Wilson, T.E.; Watenpaugh, D.E.; Smith, M.L.; Crandall, C.G. Effects of mode of exercise recovery on thermoregulatory and cardiovascular responses. J. Appl. Physiol. 2002, 93, 1918–1924. [Google Scholar] [CrossRef] [PubMed]
- Kingsley, J.D.; Figueroa, A. Acute and training effects of resistance exercise on heart rate variability. Clin. Physiol. Funct. Imaging 2016, 36, 179–187. [Google Scholar] [CrossRef]
- Brito, C.J.; Moreira, D.G.; Ferreira, J.J.; Díaz-de-Durana, A.L.; Miarka, B.; Marins, J.C.B.; Sillero-Quintana, M. Immune response related with skin thermal pattern in judokas: A new application for infrared thermography? J. Strength Cond. Res. 2020, 34, 2886–2894. [Google Scholar] [CrossRef]




| Skin Temperatures (°C) | PRE | POST | POST-20 | F(2,36) | p | η²p |
|---|---|---|---|---|---|---|
| Anterior Thigh | 31.4 ± 0.9 | 31.3 ± 1.4 | 32.3 ± 0.8 | 11.976 | <0.001 | 0.400 |
| Posterior Thigh | 31.3 ± 1.0 | 30.8 ± 1.3 | 31.5 ± 0.7 | 6.972 | 0.003 | 0.279 |
| Anterior Knee | 29.5 ± 1.2 | 29.0 ± 1.4 | 30.1 ± 1.0 | 11.248 | <0.001 | 0.385 |
| Posterior Knee | 31.9 ± 0.9 | 31.1 ± 1.1 | 31.8 ± 0.9 | 19.018 | <0.001 | 0.514 |
| Anterior Leg | 30.9 ± 1.0 | 29.7 ± 1.2 | 30.3 ± 0.7 | 21.197 | <0.001 | 0.541 |
| Posterior Leg | 31.1 ± 1.0 | 29.5 ± 1.2 | 29.9 ± 0.8 | 40.803 | <0.001 | 0.694 |
| Activation levels (0–1) | ||||||
| Sympathetic | 0.56 ± 0.14 | 0.62 ± 0.20 | 0.59 ± 0.20 | 0.970 | 0.389 | 0.051 |
| Parasympathetic | 0.41 ± 0.14 | 0.36 ± 0.14 | 0.39 ± 0.19 | 0.535 | 0.590 | 0.029 |
| Sympathetic PRE Mean (0.55 ± 0.14) | Sympathetic POST Mean (0.61 ± 0.19) | Sympathetic POST-20 Mean (0.59 ± 0.20) | |
|---|---|---|---|
| Parasympathetic PRE Mean (0.42 ± 0.19) | −0.880 (<0.001) * | −0.372 (0.177) | −0.436 (0.062) |
| Parasympathetic POST Mean (0.36 ± 0.14) | −0.342 (0.152) | −0.914 (<0.001) * | −0.606 (0.006) * |
| Parasympathetic POST-20 Mean (0.39 ± 0.19) | −0.268 (0.268) | −0.626 (0.004) * | −0.936 (<0.001) * |
| Lower Activity (°C) | Higher Activity (°C) | Dif (°C) | Test Statistic | p | Location Parameter | 95% CI | Effect Size | 95% CI | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Lower | Upper | Lower | Upper | ||||||||
| Δ-exercise ant_thigh | 0.29 (0.90) | −0.29 (1.31) | 0.58 | 1.430 a | 0.171 | 0.769 a | −0.366 | 1.903 | 0.657 a | −0.279 | 1.575 |
| Δ-exercise ant_knee | −0.52 (1.57) | −0.36 (0.89) | 0.16 | 50.00 b | 0.720 | 0.178 b | −0.785 | 1.380 | 0.111 b | −0.398 | 0.568 |
| Δ-exercise ant_leg | −1.19 (0.57) | −1.32 (0.68) | 0.13 | 0.426 a | 0.675 | 0.181 a | −0.715 | 1.077 | 0.196 a | −0.710 | 1.096 |
| Δ-exercise post_thigh | −0.09 (1.03) | −0.61 (0.73) | 0.52 | 1.497 a | 0.153 | 0.558 a | −0.229 | 1.345 | 0.688 a | −0.251 | 1.608 |
| Δ-exercise post_knee | −0.65 (0.55) | −1.18 (0.64) | 0.53 | 1.608 a | 0.126 | 0.477 a | −0.149 | 1.102 | 0.739 a | −0.208 | 1.662 |
| Δ-exercise post_leg | −1.47 (0.49) | −1.73 (1.29) | 0.26 | 1.330 a | 0.201 | 0.443 a | −0.260 | 1.146 | 0.611 a | −0.321 | 1.526 |
| Δ-recovery ant_thigh | 1.07 (0.99) | 0.52 (1.02) | 0.55 | 56.00 b | 0.400 | 0.339 b | −0.516 | 1.178 | 0.244 b | −0.277 | 0.654 |
| Δ-recovery ant_knee | 1.38 (0.81) | 0.54 (0.66) | 0.84 | 67.00 b | 0.079 | 0.666 b | −0.110 | 1.290 | 0.489 b | 0.001 | 0.789 |
| Δ-recovery ant_leg | 0.87 (1.02) | 0.30 (0.29) | 0.57 | 56.00 b | 0.400 | 0.497 b | −0.308 | 1.136 | 0.244 b | −0.277 | 0.654 |
| Δ-recovery post_thigh | 0.59 (0.61) | 0.48 (0.65) | 0.13 | −0.050 a | 0.960 | −0.021 a | −0.893 | 0.852 | −0.023 a | −0.923 | 0.878 |
| Δ-recovery post_knee | 0.91 (0.75) | 0.59 (0.52) | 0.32 | 54.00 b | 0.497 | 0.238 b | −0.630 | 0.820 | 0.200 b | −0.319 | 0.627 |
| Δ-recovery post_leg | 0.48 (0.71) | 0.48 (0.65) | 0.00 | 51.00 b | 0.661 | 0.146 b | −0.599 | 0.725 | 0.133 b | −0.379 | 0.583 |
| Δ-total ant_thigh | 1.45 (0.75) | 0.26 (0.96) | 1.19 * | 3.466 a | 0.003 * | 1.039 a | 0.406 | 1.671 | 1.593 a | 0.530 | 2.621 |
| Δ-total post_thigh | 0.33 (0.27) | −0.10 (0.62) | 0.44 * | 2.107 a | 0.050 * | 0.538 a | −0.001 | 1.076 | 0.968 a | −0.001 | 1.912 |
| Δ-total ant_knee | 0.90 (0.71) | 0.15 (0.15) | 0.75 * | 79.00 b | 0.004 * | 0.765 b | 0.235 | 1.340 | 0.756 b | 0.424 | 0.909 |
| Δ-total post_knee | 0.09 (0.79) | −0.67 (0.89) | 0.76 | 1.867 a | 0.079 | 0.541 a | −0.070 | 1.153 | 0.858 a | −0.098 | 1.791 |
| Δ-total ant_leg | −0.23 (0.81) | −1.01 (0.50) | 0.78 | 1.473 a | 0.159 | 0.447 a | −0.193 | 1.087 | 0.677 a | −0.261 | 1.596 |
| Δ-total post_leg | −1.05 (0.69) | −1.59 (1.05) | 0.54 | 0.995 a | 0.353 | 0.336 a | −0.406 | 1.078 | 0.439 a | −0.480 | 1.345 |
| Model | R2 | Adjusted R2 | RMSE | F | df1 | df2 | p | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Anterior Thigh | 0.677 | 0.637 | 0.499 | 16.777 * | 2 | 16 | <0.001 | |||
| Unstandardized | SE | Standardized | T | p | [95% CI] | [95% CI] | ||||
| Anterior Thigh initial | −0.550 | 0.125 | −0.628 | −4.419 * | <0.001 | −0.814 | −0.286 | |||
| Sympathetic Activity | −3.182 | 0.837 | −0.540 | −3.802 * | 0.002 | −4.957 | −1.408 | |||
| Model | R2 | Adjusted R2 | RMSE | F | df1 | df2 | p | |||
| Posterior Thigh | 0.683 | 0.644 | 0.362 | 17.271 * | 2 | 16 | <0.001 | |||
| Unstandardized | SE | Standardized | T | P | [95% CI] | [95% CI] | ||||
| Posterior Thigh initial | −0.451 | 0.084 | −0.752 | −5.343 * | <0.001 | −0.814 | −0.286 | |||
| Sympathetic Activity | −1.358 | 0.607 | −0.315 | −2.237 * | 0.040 | −2.645 | −0.071 | |||
| Model | R2 | Adjusted R2 | RMSE | F | df1 | df2 | p | |||
| Anterior Knee | 0.522 | 0.463 | 0.521 | 8.750 * | 2 | 16 | 0.003 | |||
| Unstandardized | SE | Standardized | T | p | [95% CI] | [95% CI] | ||||
| Anterior Knee initial | −0.281 | 0.105 | −0.464 | −2.685 * | 0.016 | −0.502 | −0.059 | |||
| Sympathetic Activity | −2.688 | 0.875 | −0.531 | −3.071 * | 0.007 | −4.543 | −0.832 | |||
| Model | R2 | Adjusted R2 | RMSE | F | df1 | df2 | p | |||
| Posterior Knee | 0.315 | 0.230 | 0.591 | 3.682 * | 2 | 16 | 0.048 | |||
| Unstandardized | SE | Standardized | T | p | [95% CI] | [95% CI] | ||||
| Posterior Knee initial | −0.288 | 0.155 | −0.386 | −1.859 | 0.082 | −0.615 | 0.040 | |||
| Sympathetic Activity | −1.779 | 0.996 | −0.371 | −1.787 | 0.093 | −3.889 | 0.332 | |||
| Model | R2 | Adjusted R2 | RMSE | F | df1 | df2 | p | |||
| Anterior Leg | 0.537 | 0.479 | 0.492 | 9.281 * | 2 | 16 | 0.002 | |||
| Unstandardized | SE | Standardized | T | p | [95% CI] | [95% CI] | ||||
| Anterior Leg initial | −0.494 | 0.120 | −0.705 | −4.134 * | <0.001 | −0.748 | −0.241 | |||
| Sympathetic Activity | −0.726 | 0.828 | −0.150 | −0.878 | 0.393 | −2.481 | −1.028 | |||
| Model | R2 | Adjusted R2 | RMSE | F | df1 | df2 | p | |||
| Posterior Leg | 0.402 | 0.328 | 0.626 | 5.384 * | 2 | 16 | 0.016 | |||
| Unstandardized | SE | Standardized | T | p | [95% CI] | [95% CI] | ||||
| Posterior Leg initial | −0.466 | 0.150 | −0.609 | −3.111 * | 0.007 | −0.784 | −0.149 | |||
| Sympathetic Activity | −0.571 | 1.064 | −0.105 | −0.537 | 0.599 | −2.826 | 1.683 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sillero-Quintana, M.; Jones-Rando, J.; Refoyo, I.; Marins, J.C.B.; Seixas, A. Effects of Resistance Training on Skin Temperature and Its Relationship with Central Nervous System (CNS) Activation. Healthcare 2022, 10, 207. https://doi.org/10.3390/healthcare10020207
Sillero-Quintana M, Jones-Rando J, Refoyo I, Marins JCB, Seixas A. Effects of Resistance Training on Skin Temperature and Its Relationship with Central Nervous System (CNS) Activation. Healthcare. 2022; 10(2):207. https://doi.org/10.3390/healthcare10020207
Chicago/Turabian StyleSillero-Quintana, Manuel, Jacob Jones-Rando, Ignacio Refoyo, João Carlos Bouzas Marins, and Adérito Seixas. 2022. "Effects of Resistance Training on Skin Temperature and Its Relationship with Central Nervous System (CNS) Activation" Healthcare 10, no. 2: 207. https://doi.org/10.3390/healthcare10020207
APA StyleSillero-Quintana, M., Jones-Rando, J., Refoyo, I., Marins, J. C. B., & Seixas, A. (2022). Effects of Resistance Training on Skin Temperature and Its Relationship with Central Nervous System (CNS) Activation. Healthcare, 10(2), 207. https://doi.org/10.3390/healthcare10020207








