Long-Haul COVID Patients: Prevalence of POTS Are Reduced but Cerebral Blood Flow Abnormalities Remain Abnormal with Longer Disease Duration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Tilt Test Protocol to Quantify Orthostatic Intolerance
2.3. Extracranial Doppler Measurements for Determination of Cerebral Blood Flow
2.4. Statistics
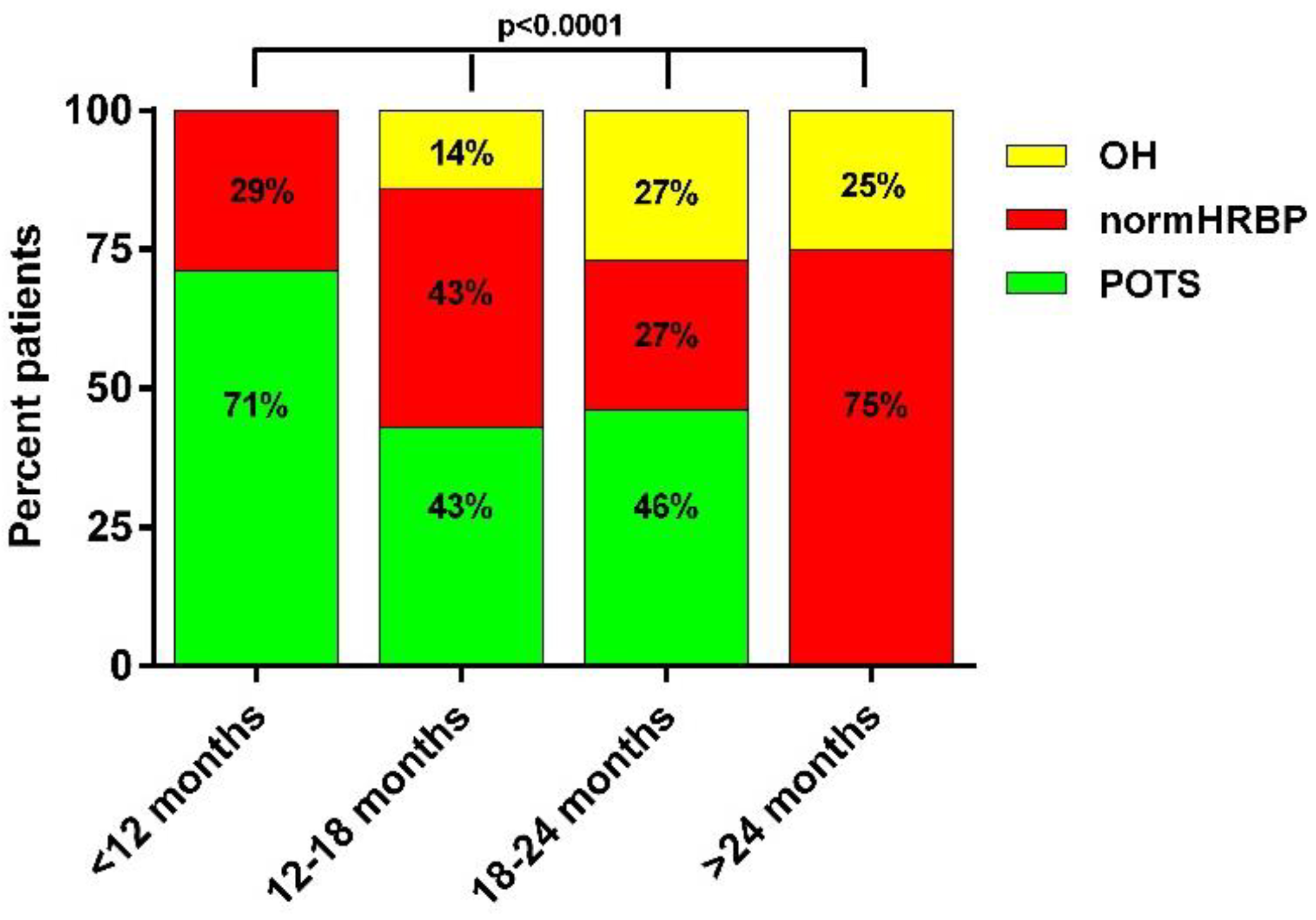
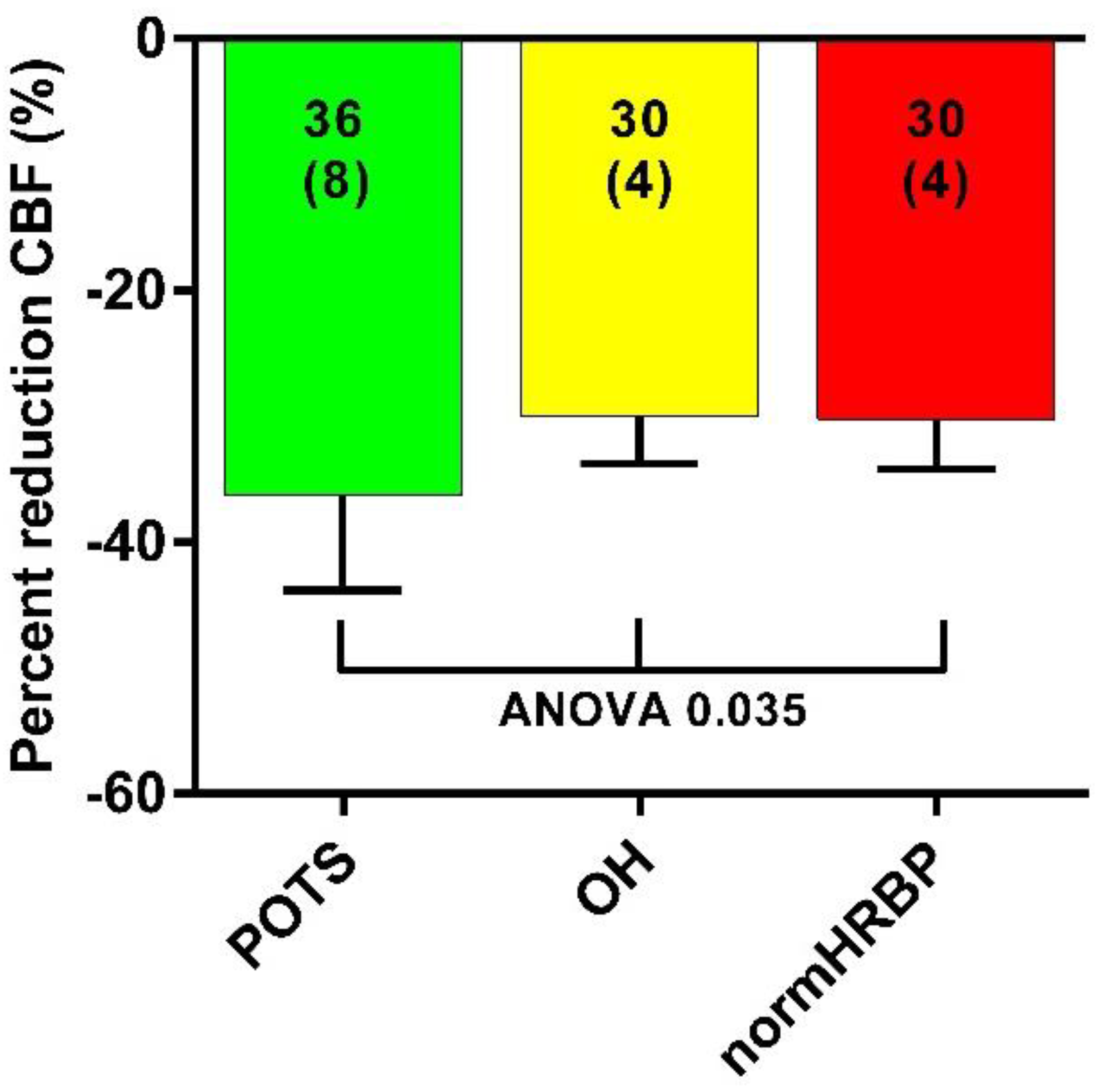
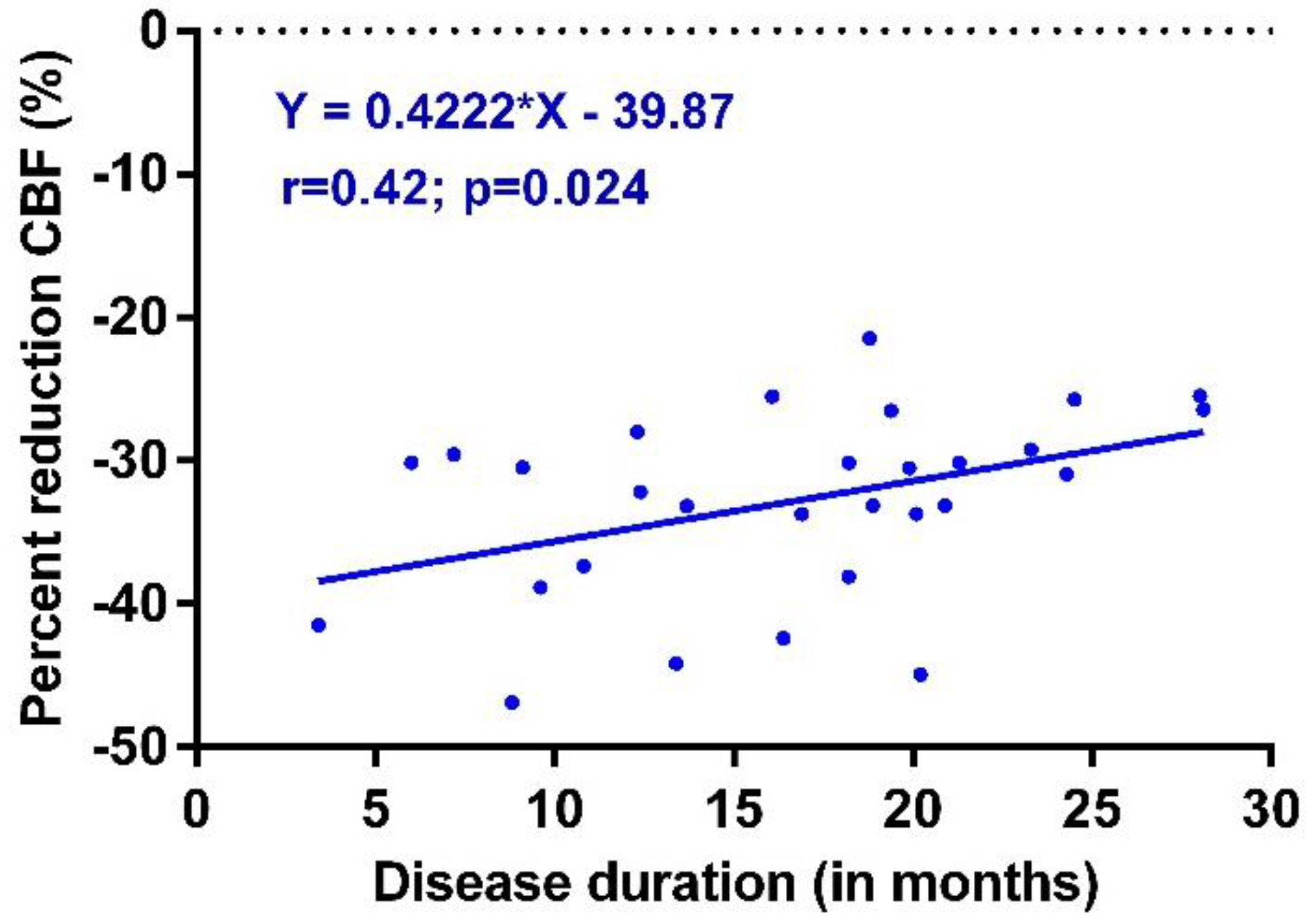
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
Appendix A.1. Tilt Test Protocol to Quantify Orthostatic Intolerance
Appendix A.2. Extracranial Doppler Measurements for Determination of Cerebral Blood Flow
References
- Blitshteyn, S.; Whitelaw, S. Postural orthostatic tachycardia syndrome (POTS) and other autonomic disorders after COVID-19 infection: A case series of 20 patients. Immunol. Res. 2021, 69, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Dani, M.; Dirksen, A.; Taraborrelli, P.; Torocastro, M.; Panagopoulos, D.; Sutton, R.; Lim, P.B. Autonomic dysfunction in ‘long COVID’: Rationale, physiology and management strategies. Clin. Med. 2021, 21, e63–e67. [Google Scholar] [CrossRef] [PubMed]
- Gall, N.P.; James, S.; Kavi, L. Observational case series of postural tachycardia syndrome (PoTS) in post-COVID-19 patients. Br. J. Cardiol. 2022, 29, 3. [Google Scholar] [CrossRef] [PubMed]
- Jamal, S.M.; Landers, D.B.; Hollenberg, S.M.; Turi, Z.G.; Glotzer, T.V.; Tancredi, J.; Parrillo, J.E. Prospective Evaluation of Autonomic Dysfunction in Post-Acute Sequela of COVID-19. J. Am. Coll. Cardiol. 2022, 79, 2325–2330. [Google Scholar] [CrossRef]
- Johansson, M.; Stahlberg, M.; Runold, M.; Nygren-Bonnier, M.; Nilsson, J.; Olshansky, B.; Bruchfeld, J.; Fedorowski, A. Long-Haul Post-COVID-19 Symptoms Presenting as a Variant of Postural Orthostatic Tachycardia Syndrome: The Swedish Experience. JACC Case Rep. 2021, 3, 573–580. [Google Scholar] [CrossRef]
- Kanjwal, K.; Jamal, S.; Kichloo, A.; Grubb, B.P. New-onset Postural Orthostatic Tachycardia Syndrome Following Coronavirus Disease 2019 Infection. J. Innov. Card. Rhythm Manag. 2020, 11, 4302–4304. [Google Scholar] [CrossRef]
- Miglis, M.G.; Prieto, T.; Shaik, R.; Muppidi, S.; Sinn, D.I.; Jaradeh, S. A case report of postural tachycardia syndrome after COVID-19. Clin. Auton. Res. 2020, 30, 449–451. [Google Scholar] [CrossRef]
- Miglis, M.G.; Seliger, J.; Shaik, R.; Gibbons, C.H. A case series of cutaneous phosphorylated α-synuclein in Long-COVID POTS. Clin. Auton. Res. 2022, 32, 209–212. [Google Scholar] [CrossRef]
- Monaghan, A.; Jennings, G.; Xue, F.; Byrne, L.; Duggan, E.; Romero-Ortuno, R. Orthostatic Intolerance in Adults Reporting Long COVID Symptoms Was Not Associated With Postural Orthostatic Tachycardia Syndrome. Front. Physiol. 2022, 13, 833650. [Google Scholar] [CrossRef]
- Shouman, K.; Vanichkachorn, G.; Cheshire, W.P.; Suarez, M.D.; Shelly, S.; Lamotte, G.J.; Sandroni, P.; Benarroch, E.E.; Berini, S.E.; Cutsforth-Gregory, J.K.; et al. Autonomic dysfunction following COVID-19 infection: An early experience. Clin. Auton. Res. 2021, 31, 385–394. [Google Scholar] [CrossRef]
- Sullivan, J.S.; Lyne, A.; Vaughan, C.J. COVID-19-induced postural orthostatic tachycardia syndrome treated with ivabradine. BMJ Case Rep. 2021, 14, e243585. [Google Scholar] [CrossRef]
- Umapathi, T.; Poh, M.Q.W.; Fan, B.E.; Li, K.F.C.; George, J.; Tan, J.Y.L. Acute hyperhidrosis and postural tachycardia in a COVID-19 patient. Clin. Auton. Res. 2020, 30, 571–573. [Google Scholar] [CrossRef]
- van Campen, C.L.M.C.; Rowe, P.C.; Visser, F.C. Orthostatic Symptoms and Reductions in Cerebral Blood Flow in Long-Haul COVID-19 Patients: Similarities with Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Medicina 2021, 58, 28. [Google Scholar] [CrossRef]
- Institute Of Medicine (IOM). Beyond Mayalgic Encephalomyelitis/Chronic Fatigue Syndrome: Redefining an Illness; The National Academies Press: Washington, DC, USA, 2015; p. 304. [Google Scholar]
- van Campen, C.L.M.C.; Verheugt, F.W.A.; Visser, F.C. Cerebral blood flow changes during tilt table testing in healthy volunteers, as assessed by Doppler imaging of the carotid and vertebral arteries. Clin. Neurophysiol. Pr. 2018, 3, 91–95. [Google Scholar] [CrossRef]
- van Campen, C.L.M.C.; Verheugt, F.W.A.; Rowe, P.C.; Visser, F.C. Cerebral blood flow is reduced in ME/CFS during head-up tilt testing even in the absence of hypotension or tachycardia: A quantitative, controlled study using Doppler echography. Clin. Neurophysiol. Pr. 2020, 5, 50–58. [Google Scholar] [CrossRef]
- Bosco, J.; Titano, R. Severe Post-COVID-19 dysautonomia: A case report. BMC Infect. Dis. 2022, 22, 214. [Google Scholar] [CrossRef]
- Kokorelis, C.; Malone, L.; Byrne, K.; Morrow, A.; Rowe, P.C. Onset of Postural Orthostatic Tachycardia Syndrome (POTS) Following COVID-19 Infection: A Pediatric Case Report. Clin. Pediatr. 2022. [Google Scholar] [CrossRef]
- Petracek, L.S.; Suskauer, S.J.; Vickers, R.F.; Patel, N.R.; Violand, R.L.; Swope, R.L.; Rowe, P.C. Adolescent and Young Adult ME/CFS After Confirmed or Probable COVID-19. Front. Med. 2021, 8, 668944. [Google Scholar] [CrossRef]
- Varanasi, S.; Sathyamoorthy, M.; Chamakura, S.; Shah, S.A. Management of Long-COVID Postural Orthostatic Tachycardia Syndrome With Enhanced External Counterpulsation. Cureus 2021, 13, e18398. [Google Scholar] [CrossRef]
- Ocher, R.A.; Padilla, E.; Hsu, J.C.; Taub, P.R. Clinical and Laboratory Improvement in Hyperadrenergic Postural Orthostatic Tachycardia Syndrome (POTS) after COVID-19 Infection. Case Rep. Cardiol. 2021, 2021, 7809231. [Google Scholar] [CrossRef]
- Raj, S.R.; Bourne, K.M.; Stiles, L.E.; Miglis, M.G.; Cortez, M.M.; Miller, A.J.; Freeman, R.; Biaggioni, I.; Rowe, P.C.; Sheldon, R.S.; et al. Postural orthostatic tachycardia syndrome (POTS): Priorities for POTS care and research from a 2019 National Institutes of Health Expert Consensus Meeting—Part 2. Auton. Neurosci. Basic Clin. 2021, 235, 102836. [Google Scholar] [CrossRef]
- Vernino, S.; Bourne, K.M.; Stiles, L.E.; Grubb, B.P.; Fedorowski, A.; Stewart, J.M.; Arnold, A.C.; Pace, L.A.; Axelsson, J.; Boris, J.R.; et al. Postural orthostatic tachycardia syndrome (POTS): State of the science and clinical care from a 2019 National Institutes of Health Expert Consensus Meeting—Part 1. Auton. Neurosci. Basic Clin. 2021, 235, 102828. [Google Scholar] [CrossRef]
- Barnes, M.A.; Carson, M.J.; Nair, M.G. Non-traditional cytokines: How catecholamines and adipokines influence macrophages in immunity, metabolism and the central nervous system. Cytokine 2015, 72, 210–219. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Kem, D.C.; Reim, S.; Khan, M.; Vanderlinde-Wood, M.; Zillner, C.; Collier, D.; Liles, C.; Hill, M.A.; Cunningham, M.W.; et al. Agonistic Autoantibodies as Vasodilators in Orthostatic Hypotension. Hypertension 2012, 59, 402–408. [Google Scholar] [CrossRef] [Green Version]
- van Campen, C.L.M.C.; Visser, F.C. Orthostatic Intolerance in Long-Haul COVID after SARS-CoV-2: A Case-Control Comparison with Post-EBV and Insidious-Onset Myalgic Encephalomyelitis/Chronic Fatigue Syndrome Patients. Healthcare 2022, 10, 2058. [Google Scholar] [CrossRef]
- Camm, A.J.; Fei, L. Chronotropic incompetence—Part I: Normal regulation of the heart rate. Clin. Cardiol. 1996, 19, 424–428. [Google Scholar] [CrossRef]
- Camm, A.J.; Fei, L. Chronotropic incompetence—Part II: Clinical implications. Clin. Cardiol. 1996, 19, 503–508. [Google Scholar] [CrossRef]
- McRitchie, R.J.; Vatner, S.F.; Boettcher, D.; Heyndrickx, G.R.; Patrick, T.A.; Braunwald, E. Role of arterial baroreceptors in mediating cardiovascular response to exercise. Am. J. Physiol.-Leg. Content 1976, 230, 85–89. [Google Scholar] [CrossRef] [Green Version]
- Bristow, M.R.; Ginsburg, R.; Minobe, W.; Cubicciotti, R.S.; Sageman, W.S.; Lurie, K.; Billingham, M.E.; Harrison, D.C.; Stinson, E.B. Decreased Catecholamine Sensitivity and β-Adrenergic-Receptor Density in Failing Human Hearts. N. Engl. J. Med. 1982, 307, 205–211. [Google Scholar] [CrossRef]
- van Campen, C.L.M.C.; Rowe, P.C.; Visser, F.C. Cerebral Blood Flow Is Reduced in Severe Myalgic Encephalomyelitis/Chronic Fatigue Syndrome Patients During Mild Orthostatic Stress Testing: An Exploratory Study at 20 Degrees of Head-Up Tilt Testing. Healthcare 2020, 8, 169. [Google Scholar] [CrossRef]
- Aponte-Becerra, L.; Novak, P. Tilt Test: A Review. J. Clin. Neurophysiol. 2021, 38, 279–286. [Google Scholar] [CrossRef]
- Shin, K.J.; Kim, S.E.; Park, K.M.; Park, J.; Ha, S.Y.; Kim, S.E.; Kwon, O.Y. Cerebral hemodynamics in orthostatic intolerance with normal head-up tilt test. Acta Neurol. Scand. 2016, 134, 108–115. [Google Scholar] [CrossRef]
- Park, J.; Kim, H.T.; Park, K.M.; Ha, S.Y.; Kim, S.E.; Shin, K.J.; Kim, S.E.; Jang, W.; Kim, J.S.; Youn, J.; et al. Orthostatic dizziness in Parkinson’s disease is attributed to cerebral hypoperfusion: A transcranial doppler study. J. Clin. Ultrasound 2017, 45, 337–342. [Google Scholar] [CrossRef]
- Novak, P. Hypocapnic cerebral hypoperfusion: A biomarker of orthostatic intolerance. PLoS ONE 2018, 13, e0204419. [Google Scholar] [CrossRef] [Green Version]
- Castelli, L.; De Giglio, L.; Haggiag, S.; Traini, A.; De Luca, F.; Ruggieri, S.; Prosperini, L. Premorbid functional reserve modulates the effect of rehabilitation in multiple sclerosis. Neurol. Sci. 2020, 41, 1251–1257. [Google Scholar] [CrossRef]
- Freeman, R.; Wieling, W.; Axelrod, F.B.; Benditt, D.G.; Benarroch, E.; Biaggioni, I.; Cheshire, W.P.; Chelimsky, T.; Cortelli, P.; Gibbons, C.H.; et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Auton. Neurosci. 2011, 161, 46–48. [Google Scholar] [CrossRef]
- Sheldon, R.S.; Grubb, B.P., 2nd; Olshansky, B.; Shen, W.K.; Calkins, H.; Brignole, M.; Raj, S.R.; Krahn, A.D.; Morillo, C.A.; Stewart, J.M.; et al. 2015 heart rhythm society expert consensus statement on the diagnosis and treatment of postural tachycardia syndrome, inappropriate sinus tachycardia, and vasovagal syncope. Heart Rhythm 2015, 12, e41–e63. [Google Scholar] [CrossRef] [Green Version]
- Fedorowski, A.; Burri, P.; Melander, O. Orthostatic hypotension in genetically related hypertensive and normotensive individuals. J. Hypertens. 2009, 27, 976–982. [Google Scholar] [CrossRef]
| POTS (n = 13) | OH (n = 5) | normHRBP (n = 11) | p-Value | |
|---|---|---|---|---|
| Male/female * | 4/9 (31/69%) | 1/4 (20/80%) | 2/9 (18/82%) | 0.75 |
| Age (years) | 36 (10) | 43 (7) | 42 (16) | F (2, 26) = 1.88; p = 0.17 |
| Height (cm) | 176 (10) | 177 (6) | 172 (7) | F (2, 26) = 0.86; p = 0.44 |
| Weight (kg) | 71 (14) | 84(11) | 71 (17) | F (2, 26) = 1.58; p = 0.22 |
| BMI (kg/m2) | 22.8 (3.6) | 26.9 (4.2) | 23.9 (5.6) | F (2, 26) = 1.43; p = 0.26 |
| BSA (m2) | 1.86 (0.20) | 2.01 (0.11) | 1.83 (0.20) | F (2, 26) = 1.54; p = 0.23 |
| Disease duration (years) # | 16.4 (9.0–18.5) | 19.4 (17.5–22.2) | 20.9 (12.3–24.5) | X2 (33) = 4.58; p = 0.10 |
| OI in daily life yes/no * | 13/0 (0/100%) | 5/0 (0/100%) | 11/0 (0/100%) | 1.0 |
| POTS (n = 13) | OH (n = 5) | normHRBP (n = 11) | p-Value | |
|---|---|---|---|---|
| HR supine (bpm) | 75 (15) | 69 (13) | 71 (9) | F (2, 26) = 0.47; p = 0.63 |
| HR end-tilt (bpm) | 110 (21) | 95 (14) | 86 (12) | F (2, 26) = 6.03; p = 0.007; gr 1 vs 3 p = 0.006 |
| SBP supine (mmHg) | 131 (18) | 141 (19) | 141 (26) | F (2, 26) = 0.68; p = 0.52 |
| SBP end-tilt (mmHg) | 126 (22) | 109 (15) | 139 (28) | F (2, 26) = 2.89; p = 0.07 |
| DBP supine (mmHg) | 79 (13) | 81 (11) | 81 (12) | F (2, 26) = 0.07; p = 0.93 |
| DBP end-tilt (mmHg) | 86 (17) | 72 (11) | 85 (14) | F (2, 26) = 0.63; p = 0.22 |
| PETCO2 supine (mmHg) | 40 (3) | 37 (2) | 39 (2) | F (2, 26) = 2.80; p = 0.08 |
| PETCO2 end-tilt (mmHg) | 28 (5) | 27 (3) | 29 (3) | F (2, 26) = 0.88; p = 0.43 |
| CBF supine (mL/min) | 616 (73) | 657 (58) | 632 (69) | F (2, 26) = 0.64; p = 0.54 |
| CBF end-tilt (mL/min) | 396 (70) | 459 (39) | 441 (61) | F (2, 26) = 2.53; p = 0.10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campen, C.M.C.v.; Visser, F.C. Long-Haul COVID Patients: Prevalence of POTS Are Reduced but Cerebral Blood Flow Abnormalities Remain Abnormal with Longer Disease Duration. Healthcare 2022, 10, 2105. https://doi.org/10.3390/healthcare10102105
Campen CMCv, Visser FC. Long-Haul COVID Patients: Prevalence of POTS Are Reduced but Cerebral Blood Flow Abnormalities Remain Abnormal with Longer Disease Duration. Healthcare. 2022; 10(10):2105. https://doi.org/10.3390/healthcare10102105
Chicago/Turabian StyleCampen, C. (Linda) M. C. van, and Frans C. Visser. 2022. "Long-Haul COVID Patients: Prevalence of POTS Are Reduced but Cerebral Blood Flow Abnormalities Remain Abnormal with Longer Disease Duration" Healthcare 10, no. 10: 2105. https://doi.org/10.3390/healthcare10102105






