Orthostatic Intolerance in Long-Haul COVID after SARS-CoV-2: A Case-Control Comparison with Post-EBV and Insidious-Onset Myalgic Encephalomyelitis/Chronic Fatigue Syndrome Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Determination of the Severity of the Disease Using the ME Criteria [18]
2.3. Tilt Test Protocol
2.4. Doppler Echocardiography for Stroke Volume and Cardiac Index Measurements
2.5. Extracranial Doppler for Cerebral Blood Flow Measurements
2.6. End-Tidal pCO2 Measurements
2.7. Orthostatic Intolerance Questionnaire during Tilting
2.8. Statistics
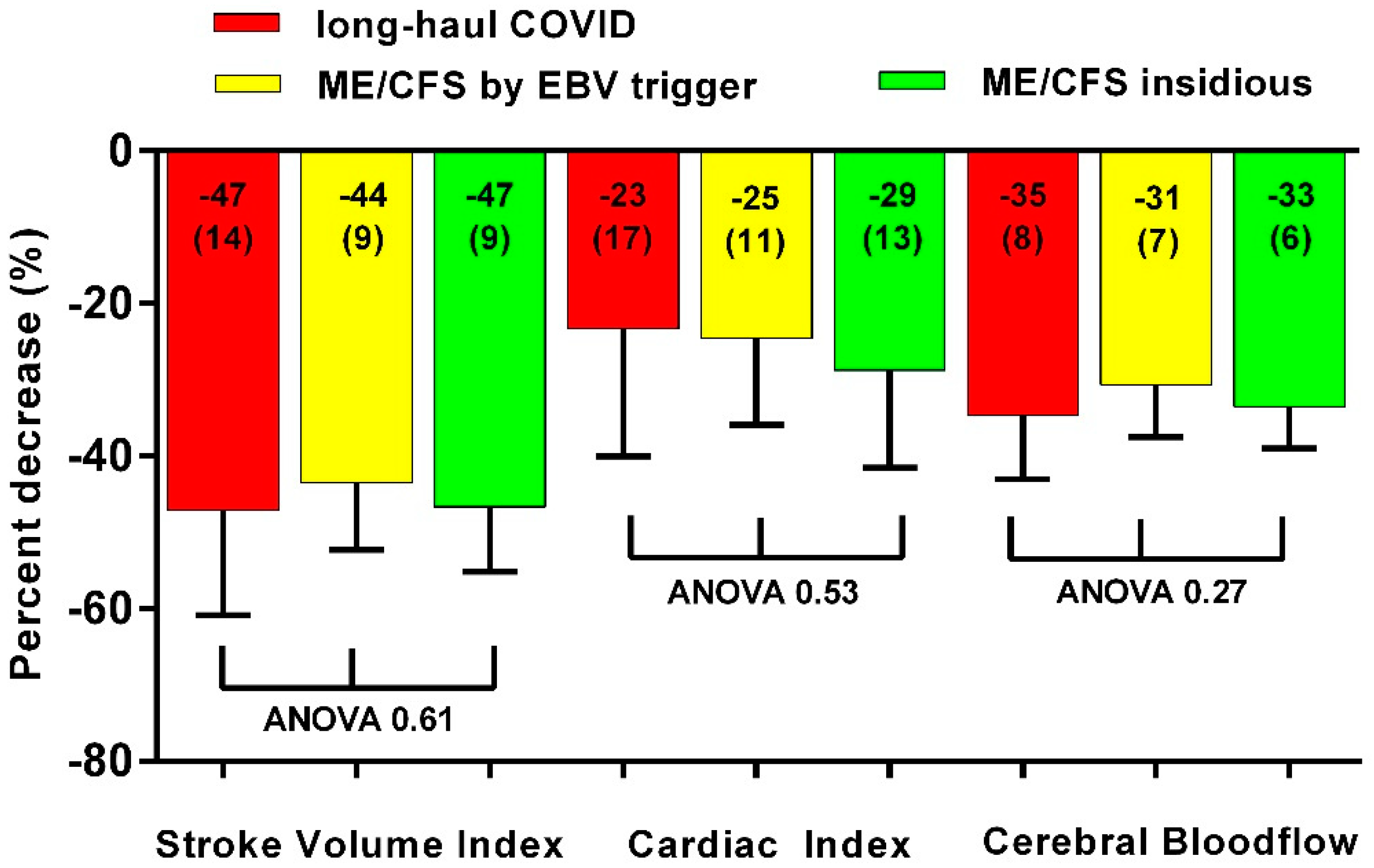
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
- Did you develop, after being tilted, complaints of dizziness or lightheadedness?
- Are you, after being tilted, more fatigued in comparison to when you were lying down?
- Did you develop, after being tilted, muscle weakness of your legs?
- Did you develop, after being tilted, a feeling of dyspnea or breathlessness?
- Do you see less sharp after being tilted?
- Do you hear me differently, after being tilted, in comparison to when you were lying down?
- Are you less concentrated while standing, compared to when you were lying down?
- Did you develop, after being tilted, pain in the muscles of your neck or shoulders?
- Did you develop, after being tilted, a feeling of nausea?
- Did you develop, after being tilted, a tingling feeling in your right hand *?
- Did you develop, after being tilted, a feeling of chest pain or pressure on your chest?
- Did you develop, after being tilted, low back pain?
- Did you start to sweat after being tilted?
- Did you develop, after being tilted, palpitations?
- Did you develop, after being tilted a feeling of a pressure in your head or head ache?
References
- Wong, T.L.; Weitzer, D.J. Long COVID and Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS)-A Systemic Review and Comparison of Clinical Presentation and Symptomatology. Medicina 2021, 57, 418. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Yuki, K.; Fujiogi, M.; Koutsogiannaki, S. COVID-19 pathophysiology: A review. Clin. Immunol. 2020, 215, 108427. [Google Scholar] [CrossRef] [PubMed]
- Somani, S.; Agnihotri, S.P. Emerging Neurology of COVID-19. Neurohospitalist 2020, 10, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Carfi, A.; Bernabei, R.; Landi, F. Persistent Symptoms in Patients after Acute COVID-19. JAMA 2020, 324, 603–605. [Google Scholar] [CrossRef]
- Davis, H.E.; Assaf, G.S.; McCorkell, L.; Wei, H.; Low, R.J.; Re’Em, Y.; Redfield, S.; Austin, J.P.; Akrami, A. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine 2021, 38, 101019. [Google Scholar] [CrossRef]
- Franco, J.V.; Garegnani, L.I.; Oltra, G.V.; Metzendorf, M.-I.; Trivisonno, L.F.; Sgarbossa, N.; Ducks, D.; Heldt, K.; Mumm, R.; Barnes, B.; et al. Long-Term Health Symptoms and Sequelae Following SARS-CoV-2 Infection: An Evidence Map. Int. J. Environ. Res. Public Health 2022, 19, 9915. [Google Scholar] [CrossRef]
- Townsend, L.; Dowds, J.; O’Brien, K.; Sheill, G.; Dyer, A.H.; O’Kelly, B.; Hynes, J.P.; Mooney, A.; Dunne, J.; Ni Cheallaigh, C.; et al. Persistent Poor Health after COVID-19 Is Not Associated with Respiratory Complications or Initial Disease Severity. Ann. Am. Thorac. Soc. 2021, 18, 997–1003. [Google Scholar] [CrossRef]
- Celi, E.; Espinoza, C.; Paredes, A.; Montenegro, M.; Velin, D. COVID-19 Post-Acute and Chronic Disease. Health Sci. J. 2022, 16, 1–14. [Google Scholar] [CrossRef]
- Morrow, A.K.; Malone, L.A.; Kokorelis, C.; Petracek, L.S.; Eastin, E.F.; Lobner, K.L.; Neuendorff, L.; Rowe, P.C. Long-Term COVID 19 Sequelae in Adolescents: The Overlap with Orthostatic Intolerance and ME/CFS. Curr. Pediatr. Rep. 2022, 10, 31–44. [Google Scholar] [CrossRef]
- Gerrity, T.R.; Bates, J.; Bell, D.S.; Chrousos, G.; Furst, G.; Hedrick, T.; Hurwitz, B.; Kula, R.W.; Levine, S.M.; Moore, R.C.; et al. Chronic fatigue syndrome: What role does the autonomic nervous system play in the pathophysiology of this complex illness? Neuroimmunomodulation 2002, 10, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Institute Of Medicine (IOM) (Ed.) Beyond Mayalgic Encephalomyelitis/Chronic Fatigue Syndrome: Redefining An Illness; The National Academies Press: Washington, DC, USA, 2015. [Google Scholar]
- Raj, S.R.; Arnold, A.C.; Barboi, A.; Claydon, V.E.; Limberg, J.K.; Lucci, V.-E.M.; Numan, M.; Peltier, A.; Snapper, H.; Vernino, S.; et al. Long-COVID postural tachycardia syndrome: An American Autonomic Society statement. Clin. Auton. Res. 2021, 31, 365–368. [Google Scholar] [CrossRef]
- van Campen, C.M.C.; Verheugt, F.W.A.; Rowe, P.C.; Visser, F.C. Cerebral blood flow is reduced in ME/CFS during head-up tilt testing even in the absence of hypotension or tachycardia: A quantitative, controlled study using Doppler echography. Clin. Neurophysiol. Pract. 2020, 5, 50–58. [Google Scholar] [CrossRef] [PubMed]
- van Campen, C.M.C.; Rowe, P.C.; Visser, F.C. Orthostatic Symptoms and Reductions in Cerebral Blood Flow in Long-Haul COVID-19 Patients: Similarities with Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Medicina 2021, 58, 28. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.F.; Cotler, J.; Jason, L.A. Post-viral fatigue and COVID-19: Lessons from past epidemics. Fatigue Biomed. Health Behav. 2020, 8, 61–69. [Google Scholar] [CrossRef]
- Chu, L.; Valencia, I.J.; Garvert, D.W.; Montoya, J.G. Onset Patterns and Course of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Front. Pediatr. 2019, 7, 12. [Google Scholar] [CrossRef]
- Carruthers, B.M.; van de Sande, M.I.; De Meirleir, K.L.; Klimas, N.G.; Broderick, G.; Mitchell, T.; Staines, D.; Powles, A.C.; Speight, N.; Vallings, R.; et al. Myalgic encephalomyelitis: International Consensus Criteria. J. Intern. Med. 2011, 270, 327–338. [Google Scholar] [CrossRef] [Green Version]
- Fukuda, K.; Straus, S.E.; Hickie, I.; Sharpe, M.C.; Dobbins, J.G.; Komaroff, A. The chronic fatigue syndrome: A comprehensive approach to its definition and study. International Chronic Fatigue Syndrome Study Group. Ann. Intern. Med. 1994, 121, 953–959. [Google Scholar] [CrossRef]
- van Campen, C.M.C.; Verheugt, F.W.A.; Visser, F.C. Cerebral blood flow changes during tilt table testing in healthy volunteers, as assessed by Doppler imaging of the carotid and vertebral arteries. Clin. Neurophysiol. Pract. 2018, 3, 91–95. [Google Scholar] [CrossRef]
- Eeftinck Schattenkerk, D.W.; van Lieshout, J.J.; van den Meiracker, A.H.; Wesseling, K.R.; Blanc, S.; Wieling, W.; Van Montfrans, G.A.; Settels, J.J.; Westerhof, B.E. Nexfin noninvasive continuous blood pressure validated against Riva-Rocci/Korotkoff. Am. J. Hypertens. 2009, 22, 378–383. [Google Scholar] [CrossRef]
- Martina, J.R.; Westerhof, B.E.; van Goudoever, J.; de Beaumont, E.M.F.H.; Truijen, J.; Kim, Y.-S.; Immink, R.V.; Jöbsis, D.A.; Hollmann, M.W.; Lahpor, J.R.; et al. Noninvasive continuous arterial blood pressure monitoring with Nexfin (R). Anesthesiology 2012, 116, 1092–1103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freeman, R.; Wieling, W.; Axelrod, F.B.; Benditt, D.G.; Benarroch, E.; Biaggioni, I.; Cheshire, W.; Chelimsky, T.; Cortelli, P.; Gibbons, C.H.; et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Auton. Neurosci. 2011, 161, 46–48. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.S.; Grubb, B.P., 2nd; Olshansky, B.; Shen, W.-K.; Calkins, H.; Brignole, M.; Raj, S.R.; Krahn, A.D.; Morillo, C.A.; Stewart, J.M.; et al. 2015 heart rhythm society expert consensus statement on the diagnosis and treatment of postural tachycardia syndrome, inappropriate sinus tachycardia, and vasovagal syncope. Heart Rhythm. 2015, 12, e41–e63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fedorowski, A.; Burri, P.; Melander, O. Orthostatic hypotension in genetically related hypertensive and normotensive individuals. J. Hypertens. 2009, 27, 976–982. [Google Scholar] [CrossRef] [PubMed]
- van Campen, C.M.C.; Visser, F.C. Validation of Stroke volume measured with suprasternal aortic Doppler imaging: Comparison to transthoracic Stroke Volume measurements. J. Thromb. Circ. 2018, 2018, 1–5. [Google Scholar] [CrossRef]
- Kusumoto, F.; Venet, T.; Schiller, N.B.; Sebastian, A.; Foster, E. Measurement of aortic blood flow by Doppler echocardiography: Temporal, technician, and reader variability in normal subjects and the application of generalizability theory in clinical research. J. Am. Soc. Echocardiogr. 1995, 8, 647–653. [Google Scholar] [CrossRef]
- van Campen, C.M.C.; Visser, F.C.; de Cock, C.C.; Vos, H.S.; Kamp, O.; Visser, C.A. Comparison of the haemodynamics of different pacing sites in patients undergoing resynchronisation treatment: Need for individualisation of lead localisation. Heart 2006, 92, 1795–1800. [Google Scholar] [CrossRef]
- Shin, K.J.; Kim, S.E.; Park, K.M.; Park, J.; Ha, S.Y.; Kwon, O.-Y. Cerebral hemodynamics in orthostatic intolerance with normal head-up tilt test. Acta Neurol. Scand. 2016, 134, 108–115. [Google Scholar] [CrossRef]
- Novak, P. Hypocapnic cerebral hypoperfusion: A biomarker of orthostatic intolerance. PLoS ONE 2018, 13, e0204419. [Google Scholar] [CrossRef] [Green Version]
- Novak, P. Post COVID-19 syndrome associated with orthostatic cerebral hypoperfusion syndrome, small fiber neuropathy and benefit of immunotherapy: A case report. Eneurological. Sci. 2020, 21, 100276. [Google Scholar] [CrossRef]
- van Campen, C.M.C.; Visser, F.C. The abnormal Cardiac Index and Stroke Volume Index changes during a normal Tilt Table Test in ME/CFS patients compared to healthy volunteers, are not related to deconditioning. J. Thromb. Circ. 2018, 2018, 1–8. [Google Scholar] [CrossRef]
- Timmers, H.J.; Wieling, W.; Soetekouw, P.M.; Bleijenberg, G.; Van Der Meer, J.W.; Lenders, J.W. Hemodynamic and neurohumoral responses to head-up tilt in patients with chronic fatigue syndrome. Clin. Auton. Res. 2002, 12, 273–280. [Google Scholar] [CrossRef] [PubMed]
- van Campen, C.M.C.; Visser, F.C. The higher resting heart rate in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) patients compared to healthy controls: Relation with stroke volumes. Med. Res. Arch. 2022, 10. [Google Scholar] [CrossRef]
- Ocher, R.A.; Padilla, E.; Hsu, J.C.; Taub, P.R. Clinical and Laboratory Improvement in Hyperadrenergic Postural Orthostatic Tachycardia Syndrome (POTS) after COVID-19 Infection. Case Rep. Cardiol. 2021, 2021, 7809231. [Google Scholar] [CrossRef] [PubMed]
- Fedorowski, A. Postural orthostatic tachycardia syndrome: Clinical presentation, aetiology and management. J. Intern. Med. 2019, 285, 352–366. [Google Scholar] [CrossRef] [PubMed]
- Bryarly, M.; Phillips, L.T.; Fu, Q.; Vernino, S.; Levine, B.D. Postural Orthostatic Tachycardia Syndrome: JACC Focus Seminar. J. Am. Coll. Cardiol. 2019, 73, 1207–1228. [Google Scholar] [CrossRef]
- Garland, E.M.; Celedonio, J.E.; Raj, S.R. Postural Tachycardia Syndrome: Beyond Orthostatic Intolerance. Curr. Neurol. Neurosci. Rep. 2015, 15, 60. [Google Scholar] [CrossRef] [Green Version]
- Miglis, M.G.; Prieto, T.; Shaik, R.; Muppidi, S.; Sinn, D.-I.; Jaradeh, S. A case report of postural tachycardia syndrome after COVID-19. Clin. Auton. Res. 2020, 30, 449–451. [Google Scholar] [CrossRef]
- Petracek, L.S.; Suskauer, S.J.; Vickers, R.F.; Patel, N.R.; Violand, R.L.; Swope, R.L.; Rowe, P.C. Adolescent and Young Adult ME/CFS after Confirmed or Probable COVID-19. Front Med. 2021, 8, 668944. [Google Scholar] [CrossRef]
- Shouman, K.; Vanichkachorn, G.; Cheshire, W.P.; Suarez, M.D.; Shelly, S.; Lamotte, G.J.; Sandroni, P.; Benarroch, E.E.; Berini, S.E.; Cutsforth-Gregory, J.K.; et al. Autonomic dysfunction following COVID-19 infection: An early experience. Clin. Auton. Res. 2021, 31, 385–394. [Google Scholar] [CrossRef]
- Parsaik, A.; Allison, T.G.; Singer, W.; Sletten, D.M.; Joyner, M.J.; Benarroch, E.E.; Low, P.A.; Sandroni, P. Deconditioning in patients with orthostatic intolerance. Neurology 2012, 79, 1435–1439. [Google Scholar] [CrossRef] [Green Version]
- van Campen, C.M.C.; Rowe, P.C.; Visser, F.C. Deconditioning does not explain orthostatic intolerance in ME/CFS (myalgic encephalomyelitis/chronic fatigue syndrome. J. Transl. Med. 2021, 19, 193–203. [Google Scholar] [CrossRef] [PubMed]
- van Campen, C.M.C.; Visser, F.C. Comparison of the Degree of Deconditioning in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) Patients with and without Orthostatic Intolerance. Med. Res. Arch. 2022, 10. [Google Scholar] [CrossRef]
- Kindlon, T. Do graded activity therapies cause harm in chronic fatigue syndrome? J. Health Psychol. 2017, 22, 1146–1154. [Google Scholar] [CrossRef] [PubMed]
- Turner-Stokes, L.; Wade, D.T. Updated NICE guidance on chronic fatigue syndrome. BMJ 2020, 371, m4774. [Google Scholar] [CrossRef]
- Vink, M.; Vink-Niese, A. The Updated NICE Guidance Exposed the Serious Flaws in CBT and Graded Exercise Therapy Trials for ME/CFS. Healthcare 2022, 10, 898. [Google Scholar] [CrossRef]




| Group 1 (n = 14) | Group 2 (n = 14) | Group 3 (n = 14) | p-Value | |
|---|---|---|---|---|
| Male/female * | 2/12 (14/86%) | 2/12 (14/86%) | 2/12 (14/86%) | 1.0 |
| Age (years) | 34 (10) | 34 (10) | 34 (10) | F (2, 42) = 0.0006; p = 0.99 |
| Height (cm) | 175 (10) | 174 (10) | 175 (8) | F (2, 42) = 0.11; p = 0.89 |
| Weight (kg) | 72 (15) | 68 (17) | 76 (23) | F (2, 42) = 0.70; p = 0.50 |
| BMI (kg/m2) | 23.5 (4.8) | 22.3 (4.5) | 24.7 (6.4) | F (2, 42) = 0.76; p = 0.47 |
| BSA (m2) | 1.87 (0.20) | 1.81 (0.24) | 1.90 (0.28) | F (2, 42) = 0.52; p = 0.60 |
| Disease duration (years) # | 1 (1–2) | 16 (9–23) | 11 (4–16) | X2(3) = 29.25; p < 0.0001. Post-hoc tests: 1 vs. 2 p < 0.0001; 1 vs. 3 p = 0.0001 |
| Disease severity * & (mild/moderate/severe) | 0/11/3 (0/85/15%) | 2/10/2 (14/82/14%) | 2/7/5 (14/50/36%) | 0.36 |
| OI in daily life yes/no * | 14/0 (0/100%) | 14/0 (0/100%) | 14/0 (0/100%) | 1.0 |
| Group 1 (n = 14) | Group 2 (n = 14) | Group 3 (n = 14) | p-Value | |
|---|---|---|---|---|
| Hemodynamics: (normHRBP/POTS) | 0/14 (0/100%) | 8/6 (57/43%) | 7/7 (50/50%) | 0.002 |
| HR supine (bpm) | 74 (14) | 69 (11) | 73 (8) | F (2, 42) = 0.93; p = 0.40 |
| HR end-tilt (bpm) | 108 (13) | 95 (20) | 103 (20) | F (2, 42) = 1.96; p = 0.15 |
| SBP supine (mmHg) | 131 (17) | 133 (15) | 133 (16) | F (2, 42) = 0.059; p = 0.94 |
| SBP end-tilt (mmHg) | 127 (21) | 126 (15) | 127 (19) | F (2, 42) = 0.04; p = 0.96 |
| DBP supine (mmHg) | 80 (13) | 77 (9) | 82 (18) | F (2, 42) = 0.59; p = 0.56 |
| DBP end-tilt (mmHg) | 88 (17) | 82 (10) | 90 (21) | F (2, 42) = 0.85; p = 0.43 |
| SVI supine (ml/m2) | 40 (6) | 42 (8) | 40 (7) | F (2, 42) = 0.54; p = 0.58 |
| SVI end-tilt (ml/m2) | 21 (5) | 23 (5) | 21 (7) | F (2, 42) = 0.96; p = 0.39 |
| CI supine (L/min/m2) | 2.91 (0.51) | 2.84 (0.52) | 2.74 (0.44) | F (2, 42) = 0.44; p = 0.65 |
| CI end-tilt (L/min/m2) | 2.27 (0.75) | 2.13 (0.42) | 1.94 (0.36) | F (2, 42) = 1.43; p = 0.25 |
| PETCO2 supine (mmHg) | 39 (3) | 38 (3) | 37 (2) | F (2, 42) = 3.08; p = 0.06 |
| PETCO2 end-tilt (mmHg) | 28 (4) | 29 (5) | 27 (4) | F (2, 42) = 1.05; p = 0.36 |
| CBF supine (ml/min) | 620 (68) | 618 (75) | 631 (87) | F (2, 42) = 0.12; p = 0.89 |
| CBF end-tilt (ml/min) | 405 (70) | 428 (66) | 419 (67) | F (2, 42) = 0.47; p = 0.63 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Campen, C.M.C.; Visser, F.C. Orthostatic Intolerance in Long-Haul COVID after SARS-CoV-2: A Case-Control Comparison with Post-EBV and Insidious-Onset Myalgic Encephalomyelitis/Chronic Fatigue Syndrome Patients. Healthcare 2022, 10, 2058. https://doi.org/10.3390/healthcare10102058
van Campen CMC, Visser FC. Orthostatic Intolerance in Long-Haul COVID after SARS-CoV-2: A Case-Control Comparison with Post-EBV and Insidious-Onset Myalgic Encephalomyelitis/Chronic Fatigue Syndrome Patients. Healthcare. 2022; 10(10):2058. https://doi.org/10.3390/healthcare10102058
Chicago/Turabian Stylevan Campen, C. (Linda) M. C., and Frans C. Visser. 2022. "Orthostatic Intolerance in Long-Haul COVID after SARS-CoV-2: A Case-Control Comparison with Post-EBV and Insidious-Onset Myalgic Encephalomyelitis/Chronic Fatigue Syndrome Patients" Healthcare 10, no. 10: 2058. https://doi.org/10.3390/healthcare10102058
APA Stylevan Campen, C. M. C., & Visser, F. C. (2022). Orthostatic Intolerance in Long-Haul COVID after SARS-CoV-2: A Case-Control Comparison with Post-EBV and Insidious-Onset Myalgic Encephalomyelitis/Chronic Fatigue Syndrome Patients. Healthcare, 10(10), 2058. https://doi.org/10.3390/healthcare10102058






