A Workcation Improves Cardiac Parasympathetic Function during Sleep to Decrease Arterial Stiffness in Workers
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
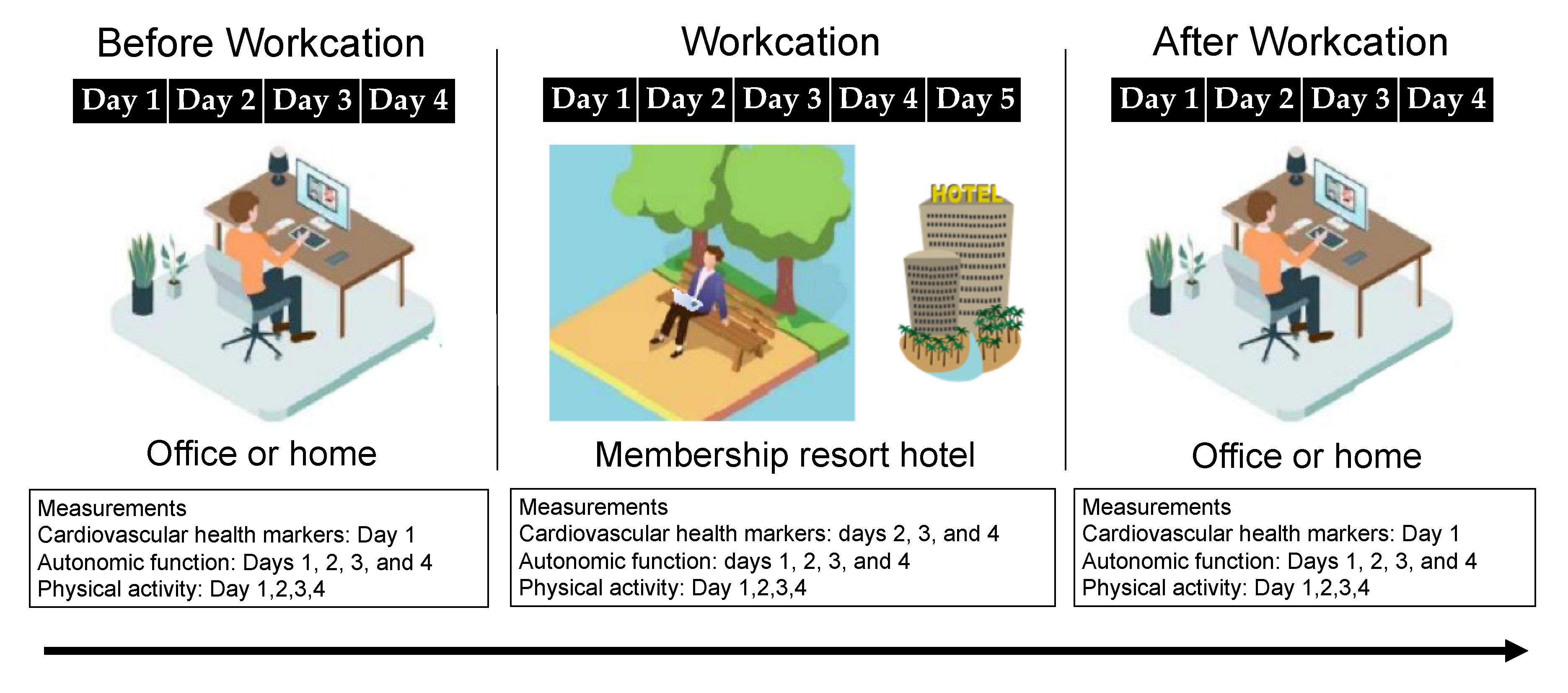
2.2. Study Design
2.3. Body Composition
2.4. Arterial Stiffness
2.5. BP and HR
2.6. Autonomic Nerve Activity
2.7. Physical Activity
2.8. Statistical Analysis
3. Results
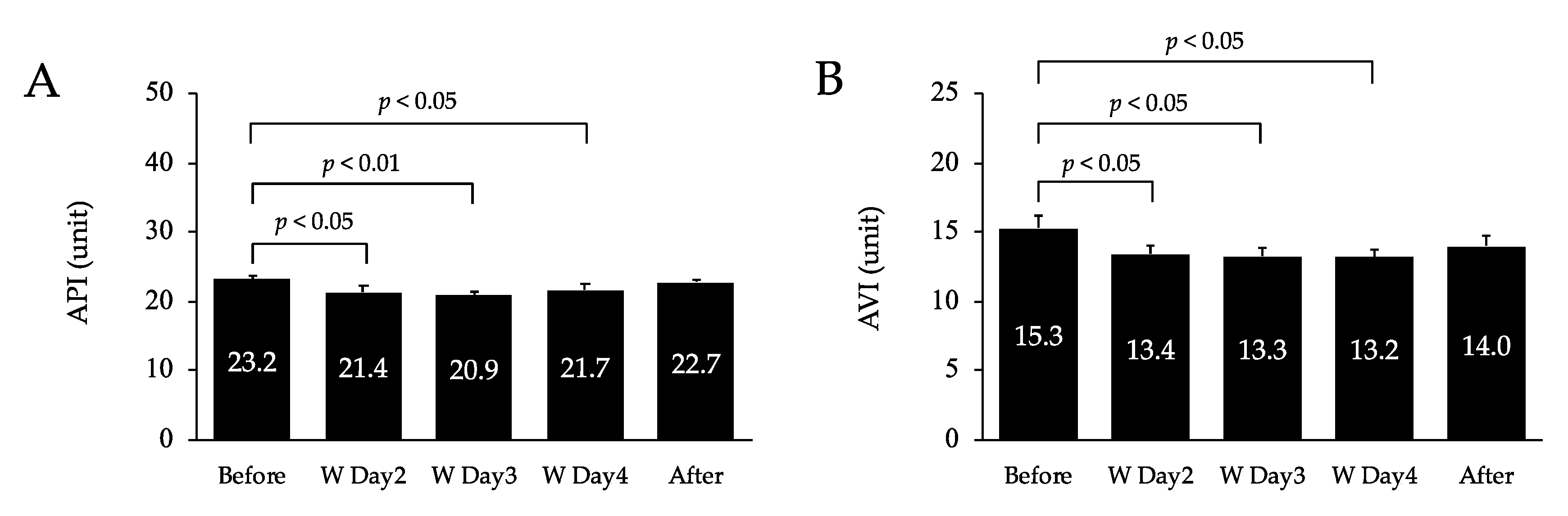
3.1. Arterial Stiffness
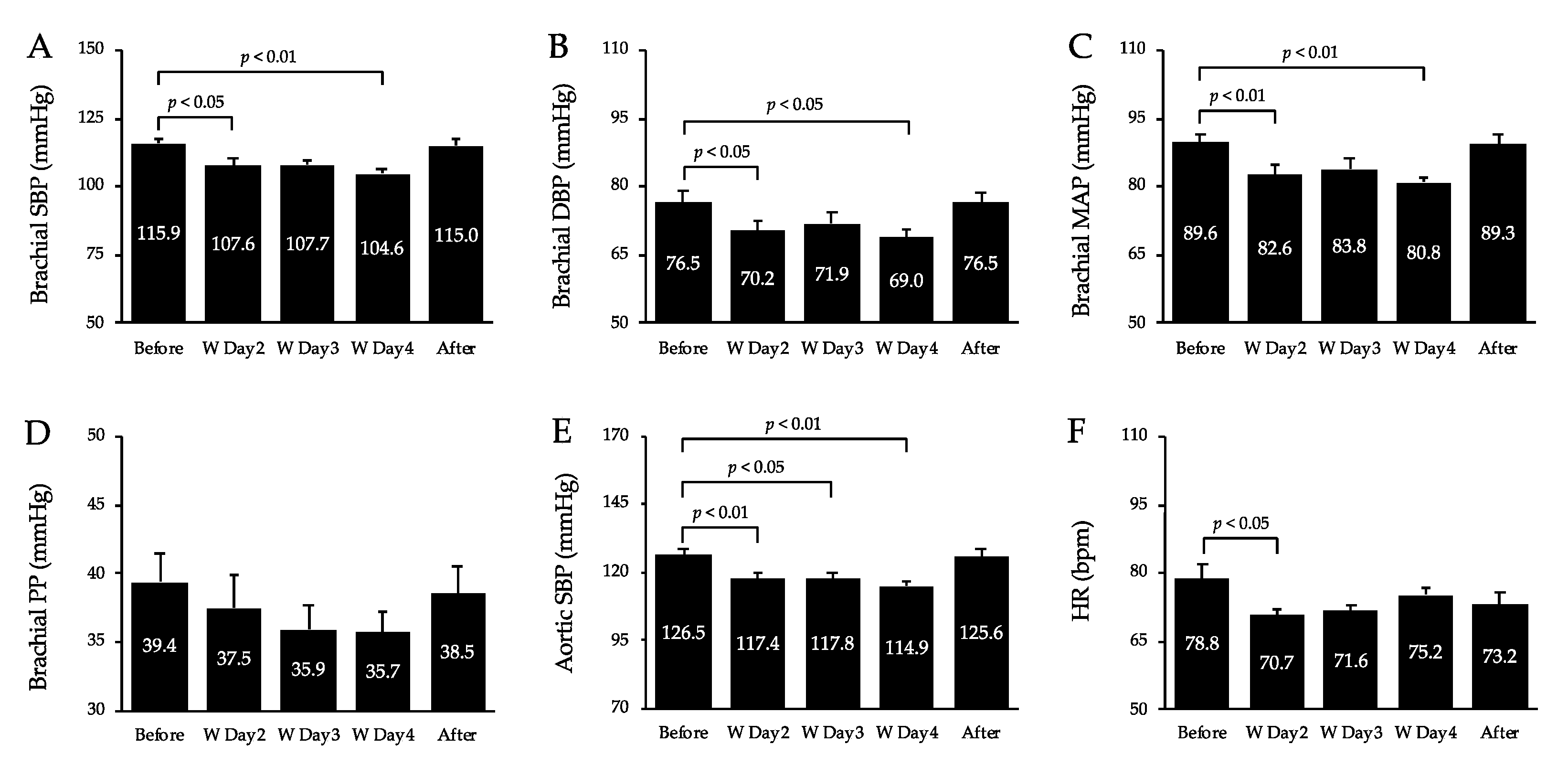
3.2. BP, HR, and Double Product (DP)
3.3. Autonomic Nerve Activity
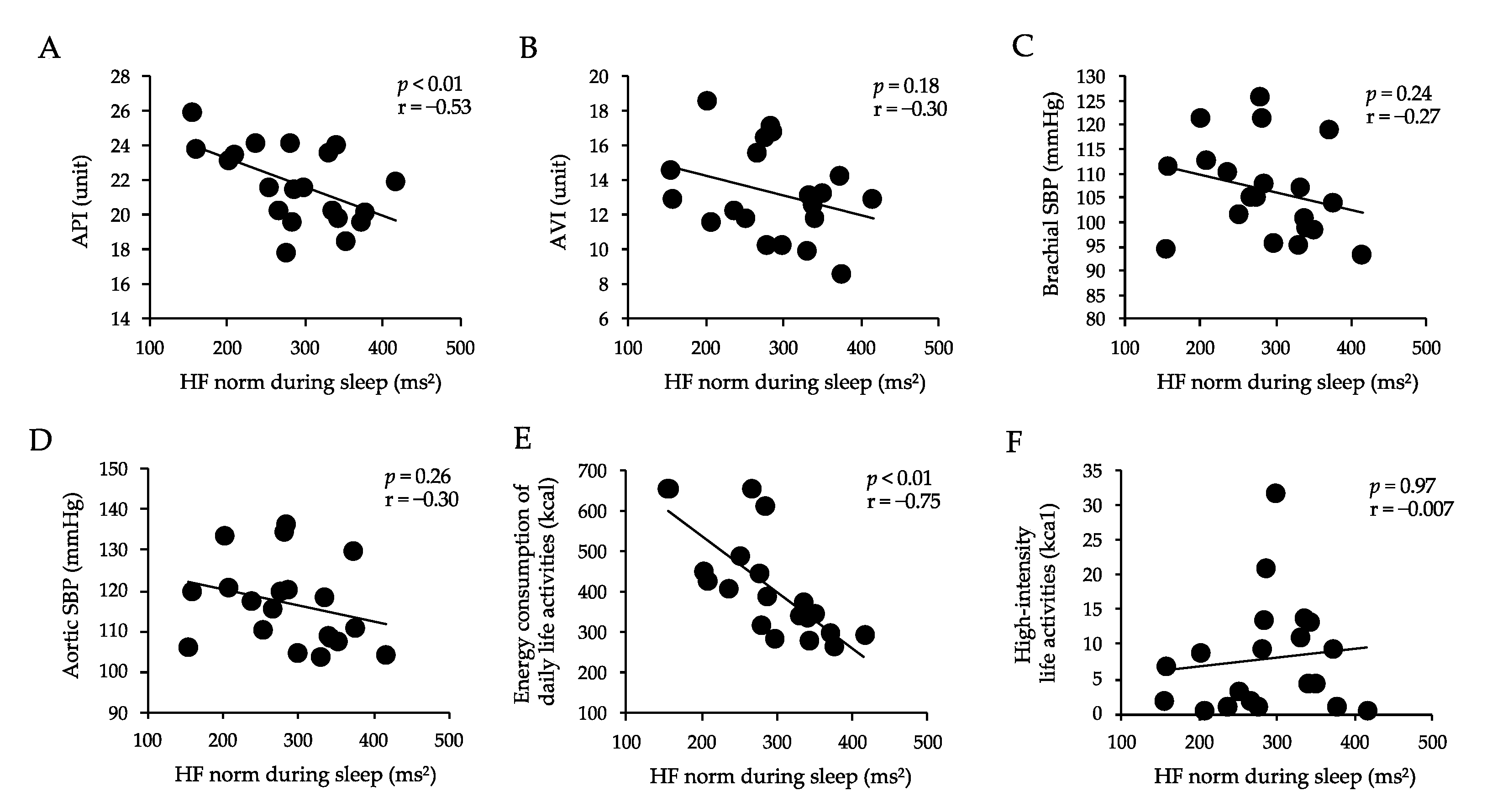
3.4. Correlations between Arterial Stiffness, Sbp, Energy Consumption of Daily Life Activities, High-Intensity Life Activities, and Hf Norm during Sleep
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mojtahedzadeh, N.; Rohwer, E.; Lengen, J.; Harth, V.; Mache, S. Health-promoting work design for telework in the context of the COVID-19 pandemic. Zent. Arb. Arb. Ergon. 2021, 71, 69–74. [Google Scholar] [CrossRef]
- Toniolo-Barrios, M.; Pitt, L. Mindfulness and the Challenges of Working from Home in Times of Crisis. Bus. Horiz. 2021, 64, 189–197. [Google Scholar] [CrossRef] [PubMed]
- James, S.M.; Honn, K.A.; Gaddameedhi, S.; Van Dongen, H.P.A. Shift Work: Disrupted Circadian Rhythms and Sleep-Implications for Health and Well-Being. Curr. Sleep Med. Rep. 2017, 3, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Li, H.; Huang, X.; Bao, H.; Song, Y.; Wang, B.; Liu, C.; Xu, R.; Liu, L.; Wang, X.; et al. Association of Self-Reported Sleep Duration and Quality with BaPWV Levels in Hypertensive Patients. Hypertens. Res. 2020, 43, 1392–1402. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, F.D.; Whelton, P.K. High Blood Pressure and Cardiovascular Disease. Hypertension 2020, 75, 285–292. [Google Scholar] [CrossRef]
- Okamoto, M.; Nakamura, F.; Musha, T.; Kobayashi, Y. Association between Novel Arterial Stiffness Indices and Risk Factors of Cardiovascular Disease. BMC Cardiovasc. Disord. 2016, 16, 211. [Google Scholar] [CrossRef]
- Okui, T. Age-Period-Cohort Analysis of Cardiovascular Disease Mortality in Japan, 1995–2018. J. Prev. Med. Public Health 2020, 53, 198–204. [Google Scholar] [CrossRef]
- Pearson, T.A.; Blair, S.N.; Daniels, S.R.; Eckel, R.H.; Fair, J.M.; Fortmann, S.P.; Franklin, B.A.; Goldstein, L.B.; Greenland, P.; Grundy, S.M.; et al. AHA Guidelines for Primary Prevention of Cardiovascular Disease and Stroke: 2002 Update: Consensus Panel Guide to Comprehensive Risk Reduction for Adult Patients Without Coronary or Other Atherosclerotic Vascular Diseases. American Heart Association Science Advisory and Coordinating Committee. Circulation 2002, 106, 388–391. [Google Scholar] [CrossRef]
- Ministry of the Environment. Efforts to Attract Workers and Travelers Seeking High Quality Services; Ministry of the Environment: Tokyo, Japan, 2020.
- Brigitta Pecsek, B. Working on Holiday: The Theory and Practice of Workcation. Balk. J. Emerg. Trends Soc. Sci. 2018, 1, 1–13. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A Flexible Statistical Power Analysis Program for the Social, Behavioral, and Biomedical Sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Sasaki-Nakashima, R.; Kino, T.; Chen, L.; Doi, H.; Minegishi, S.; Abe, K.; Sugano, T.; Taguri, M.; Ishigami, T. Successful Prediction of Cardiovascular Risk by New Non-Invasive Vascular Indexes Using Suprasystolic Cuff Oscillometric Waveform Analysis. J. Cardiol. 2017, 69, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Billman, G.E.; Hoskins, R.S. Time-Series Analysis of Heart Rate Variability during Submaximal Exercise. Evidence for Reduced Cardiac Vagal Tone in Animals Susceptible to Ventricular Fibrillation. Circulation 1989, 80, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Thayer, J.F.; Yamamoto, S.S.; Brosschot, J.F. The Relationship of Autonomic Imbalance, Heart Rate Variability and Cardiovascular Disease Risk Factors. Int. J. Cardiol. 2010, 141, 122–131. [Google Scholar] [CrossRef]
- Fujino, H.; Kondo, H.; Hirayama, Y.; Maezawa, T.; Tanaka, M.; Tsutou, A.; Shinozaki, R.; Nagatomo, F.; Ishihara, A. Heart Rate Variability Analyses Using Wearable Sensing System in Response to Parabolic Flight (LB799). FASEB J. 2014, 28, LB799. [Google Scholar] [CrossRef]
- Kobayashi, R.; Sato, K.; Takahashi, T.; Asaki, K.; Iwanuma, S.; Ohashi, N.; Hashiguchi, T. Arterial Stiffness during Hyperglycemia in Older Adults with High Physical Activity vs Low Physical Activity. J. Clin. Biochem. Nutr. 2019, 65, 146–152. [Google Scholar] [CrossRef]
- Albakri, U.; Drotos, E.; Meertens, R. Sleep Health Promotion Interventions and Their Effectiveness: An Umbrella Review. Int. J. Environ. Res. Public Health 2021, 18, 5533. [Google Scholar] [CrossRef]
- Liu, X.; Uchiyama, M.; Kim, K.; Okawa, M.; Shibui, K.; Kudo, Y.; Doi, Y.; Minowa, M.; Ogihara, R. Sleep Loss and Daytime Sleepiness in the General Adult Population of Japan. Psychiatry Res. 2000, 93, 1–11. [Google Scholar] [CrossRef]
- Vanoli, E.; Adamson, P.B.; Ba-Lin; Pinna, G.D.; Lazzara, R.; Orr, W.C. Heart Rate Variability during Specific Sleep Stages. A Comparison of Healthy Subjects with Patients after Myocardial Infarction. Circulation 1995, 91, 1918–1922. [Google Scholar] [CrossRef]
- Fantozzi, M.P.T.; Artoni, F.; Faraguna, U. Heart Rate Variability at Bedtime Predicts Subsequent Sleep Features. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2019, 2019, 6784–6788. [Google Scholar] [CrossRef]
- Busek, P.; Vanková, J.; Opavský, J.; Salinger, J.; Nevsímalová, S. Heart rate variability during sleep. Cas Lek Cesk 2005, 144, 685–688. [Google Scholar]
- Castro-Diehl, C.; Diez Roux, A.V.; Redline, S.; Seeman, T.; McKinley, P.; Sloan, R.; Shea, S. Sleep Duration and Quality in Relation to Autonomic Nervous System Measures: The Multi-Ethnic Study of Atherosclerosis (MESA). Sleep 2016, 39, 1927–1940. [Google Scholar] [CrossRef] [PubMed]
- Farina, B.; Dittoni, S.; Colicchio, S.; Testani, E.; Losurdo, A.; Gnoni, V.; Di Blasi, C.; Brunetti, R.; Contardi, A.; Mazza, S.; et al. Heart Rate and Heart Rate Variability Modification in Chronic Insomnia Patients. Behav. Sleep Med. 2014, 12, 290–306. [Google Scholar] [CrossRef] [PubMed]
- Chien, L.-W.; Cheng, S.L.; Liu, C.F. The Effect of Lavender Aromatherapy on Autonomic Nervous System in Midlife Women with Insomnia. Evid. Based Complement. Alternat. Med. 2012, 2012, 740813. [Google Scholar] [CrossRef] [PubMed]
- Lipman, R.D.; Grossman, P.; Bridges, S.E.; Hamner, J.W.; Taylor, J.A. Mental Stress Response, Arterial Stiffness, and Baroreflex Sensitivity in Healthy Aging. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2002, 57, B279–B284. [Google Scholar] [CrossRef]
- Mattson, M.P. Hormesis and Disease Resistance: Activation of Cellular Stress Response Pathways. Hum. Exp. Toxicol. 2008, 27, 155–162. [Google Scholar] [CrossRef]
- Sugawara, J.; Komine, H.; Hayashi, K.; Yoshizawa, M.; Yokoi, T.; Otsuki, T.; Shimojo, N.; Miyauchi, T.; Maeda, S.; Tanaka, H. Effect of Systemic Nitric Oxide Synthase Inhibition on Arterial Stiffness in Humans. Hypertens. Res. 2007, 30, 411–415. [Google Scholar] [CrossRef]
- Nakao, M.; Nomura, K.; Karita, K.; Nishikitani, M.; Yano, E. Relationship between Brachial-Ankle Pulse Wave Velocity and Heart Rate Variability in Young Japanese Men. Hypertens. Res. 2004, 27, 925–931. [Google Scholar] [CrossRef]
- Yeragani, V.K.; Tancer, M.; Seema, K.P.; Josyula, K.; Desai, N. Increased Pulse-Wave Velocity in Patients with Anxiety: Implications for Autonomic Dysfunction. J. Psychosom. Res. 2006, 61, 25–31. [Google Scholar] [CrossRef]
- Logan, J.G.; Teachman, B.A.; Liu, X.; Farber, C.R.; Liu, Z.; Annex, B.H. Acute Psychological Stress, Autonomic Function, and Arterial Stiffness among Women. Int. J. Psychophysiol. 2020, 155, 219–226. [Google Scholar] [CrossRef]
- Mäki-Petäjä, K.M.; Barrett, S.M.L.; Evans, S.V.; Cheriyan, J.; McEniery, C.M.; Wilkinson, I.B. The Role of the Autonomic Nervous System in the Regulation of Aortic Stiffness. Hypertension 2016, 68, 1290–1297. [Google Scholar] [CrossRef]
- Tucker, W.D.; Arora, Y.; Mahajan, K. Anatomy, blood vessels. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Schmidt, F.P.; Basner, M.; Kröger, G.; Weck, S.; Schnorbus, B.; Muttray, A.; Sariyar, M.; Binder, H.; Gori, T.; Warnholtz, A.; et al. Effect of Nighttime Aircraft Noise Exposure on Endothelial Function and Stress Hormone Release in Healthy Adults. Eur. Heart J. 2013, 34, 3508–3514a. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Health Risks: Mortality and Burden of Disease Attributable to Selected Major Risks; World Health Organization: Geneva, Switzerland, 2009.
- Kim, K.; Choi, S.; Hwang, S.E.; Son, J.S.; Lee, J.-K.; Oh, J.; Park, S.M. Changes in Exercise Frequency and Cardiovascular Outcomes in Older Adults. Eur. Heart J. 2020, 41, 1490–1499. [Google Scholar] [CrossRef] [PubMed]
- Kredlow, M.A.; Capozzoli, M.C.; Hearon, B.A.; Calkins, A.W.; Otto, M.W. The Effects of Physical Activity on Sleep: A Meta-Analytic Review. J. Behav. Med. 2015, 38, 427–449. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.O. Understanding and Addressing the Epidemic of Obesity: An Energy Balance Perspective. Endocr. Rev. 2006, 27, 750–761. [Google Scholar] [CrossRef] [PubMed]
- Sullivan Bisson, A.N.; Robinson, S.A.; Lachman, M.E. Walk to a Better Night of Sleep: Testing the Relationship between Physical Activity and Sleep. Sleep Health 2019, 5, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Lopes, S.; Afreixo, V.; Teixeira, M.; Garcia, C.; Leitão, C.; Gouveia, M.; Figueiredo, D.; Alves, A.J.; Polonia, J.; Oliveira, J.; et al. Exercise Training Reduces Arterial Stiffness in Adults with Hypertension: A Systematic Review and Meta-Analysis. J. Hypertens. 2021, 39, 214–222. [Google Scholar] [CrossRef]

| Time | Activity |
|---|---|
| 7:00 am | Wake up time. |
| Participants drank a glass of water. | |
| 7:00 am–8:00 am | Participants had breakfast within an hour of waking up. |
| Participants ate breakfast, especially foods that contained carbohydrates (rice, bread, etc.) and proteins (meat, fish, etc.), and took a hot shower or had a lymphatic massage to expel waste. | |
| 9:00 am–10:00 am | Participants performed intellectual work that required calm judgment. |
| 10:00 am–11:30 am | Participants made important decisions and performed tasks that did not allow for errors in judgment (intensive work was completed in 90-min cycles.) |
| 11:30 am | Participants performed exercises with a steady rhythm, such as walking or radio calisthenics. |
| 12:00 pm | Lunch at a specific time. After lunch, nap time for no more than 15 min. |
| 2:00 pm | Participants performed tasks that required creativity, such as planning, and tasks that required a good memory. |
| 4:00 pm | Discussions, information exchanges, paperwork, and other calm tasks to finish up the day. |
| 6:00 pm–7:00 pm | Exercise, such as strength training and walking. |
| 8:00 pm | Participants ate a moderate number of calories and vegetables for dinner. |
| 9:00 pm | Participants turned off cell phones, smart phones, and computers. |
| 10:00 pm | Half-body bath time at a lukewarm temperature (38–41 °C). |
| 11:00 pm | Only water, herbal tea, or milk before going to bed. |
| 12:00 am | Bedtime. |
| Variable | Before | W | After |
|---|---|---|---|
| Energy consumption of walking (kcal) | 241.2 ± 13.5 | 255.3 ± 22.2 | 263.9 ± 18.6 |
| Energy consumption of daily life activities (kca1) | 319.1 ± 21.3 | 381.1 ± 25.5 * | 326.7 ± 34.6 |
| Total energy consumption (kcal) | 560.3 ± 28.1 | 636.3 ± 33.8 | 590.6 ± 48.9 |
| Walking exercises (EX) | 3.7 ± 0.2 | 3.7 ± 0.5 | 4.0 ± 0.3 |
| Activities of daily living exercises (EX) | 1.1 ± 0.1 | 1.7 ± 0.1 | 1.0 ± 0.1 |
| Exercise total (EX) | 4.8 ± 0.2 | 5.4 ± 0.5 | 5.0 ± 0.4 |
| Walking time (minutes) | 77.8 ± 4.7 | 79.9 ± 5.4 | 82.7 ± 5.6 |
| Duration of low-intensity activity (minutes) | 575.1 ± 16.0 | 636.3 ± 19.4 | 588.3 ± 31.6 |
| Duration of medium intensity activity (minutes) | 66.5 ± 2.5 | 65.5 ± 4.2 | 69.4 ± 4.7 |
| High-intensity activity time (minutes) | 3.1 ± 1.0 | 8.0 ± 2.2 * | 3.4 ± 4.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Negoro, H.; Kobayashi, R. A Workcation Improves Cardiac Parasympathetic Function during Sleep to Decrease Arterial Stiffness in Workers. Healthcare 2022, 10, 2037. https://doi.org/10.3390/healthcare10102037
Negoro H, Kobayashi R. A Workcation Improves Cardiac Parasympathetic Function during Sleep to Decrease Arterial Stiffness in Workers. Healthcare. 2022; 10(10):2037. https://doi.org/10.3390/healthcare10102037
Chicago/Turabian StyleNegoro, Hideyuki, and Ryota Kobayashi. 2022. "A Workcation Improves Cardiac Parasympathetic Function during Sleep to Decrease Arterial Stiffness in Workers" Healthcare 10, no. 10: 2037. https://doi.org/10.3390/healthcare10102037
APA StyleNegoro, H., & Kobayashi, R. (2022). A Workcation Improves Cardiac Parasympathetic Function during Sleep to Decrease Arterial Stiffness in Workers. Healthcare, 10(10), 2037. https://doi.org/10.3390/healthcare10102037






