Systemic Inflammation Is Associated with Pulmonary Hypertension in Isolated Giant Omphalocele: A Population-Based Study
Abstract
:1. Introduction
2. Population and Methods
2.1. Prenatal MRI and Echographic Acquisition
2.2. Surgical Care
2.3. Postnatal Management
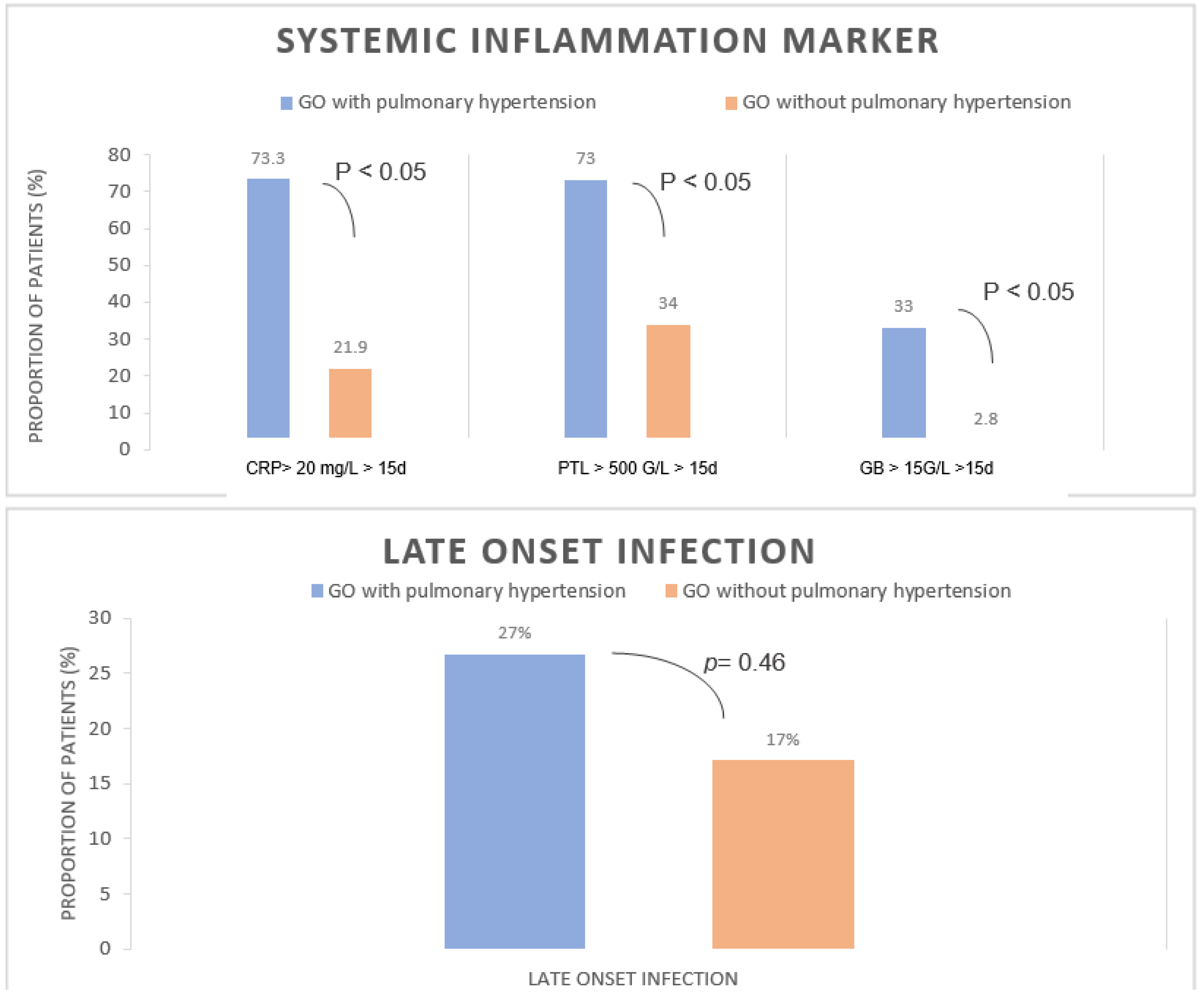
2.4. Late-Onset Infection
2.5. Prolonged and Short Inflammatory Syndrome
2.6. Primary Outcome
- -
- Right-to-left or bidirectional flow in DA;
- -
- Flattening or paradoxical septum if DA is not present;
- -
- Mean blood flow velocity less than 0.25 m/s in pulmonary arteries;
- -
- Pulmonary or tricuspid regurgitation gradient above 50% of systemic systolic pressure if DA is not present;
2.7. Statistical Analysis
3. Results
3.1. Population
3.2. Fetal Echographic and MRI Assessment
3.3. Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McNair, C.; Hawes, J.; Urquhart, H. Caring for the Newborn with an Omphalocele. Neonatal Netw. 2006, 25, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Danzer, E.; Gerdes, M.; D’Agostino, J.A.; Bernbaum, J.; Siegle, J.; Hoffman, C.; Rintoul, N.E.; Liechty, K.W.; Flake, A.W.; Adzick, N.S.; et al. Prospective, Interdisciplinary Follow-up of Children with Prenatally Diagnosed Giant Omphalocele: Short-Term Neurodevelopmental Outcome. J. Pediatr. Surg. 2010, 45, 718–723. [Google Scholar] [CrossRef] [PubMed]
- Ein, S.H.; Langer, J.C. Delayed Management of Giant Omphalocele Using Silver Sulfadiazine Cream: An 18-Year Experience. J. Pediatr. Surg. 2012, 47, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Danzer, E.; Victoria, T.; Bebbington, M.W.; Siegle, J.; Rintoul, N.E.; Johnson, M.P.; Flake, A.W.; Adzick, N.S.; Hedrick, H.L. Fetal MRI-Calculated Total Lung Volumes in the Prediction of Short-Term Outcome in Giant Omphalocele: Preliminary Findings. Fetal Diagn. Ther. 2012, 31, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Nolan, H.R.; Wagner, M.L.; Jenkins, T.; Lim, F.-Y. Outcomes in the Giant Omphalocele Population: A Single Center Comprehensive Experience. J. Pediatr. Surg. 2020, 55, 1866–1871. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.M.; Tatsuo, E.S.; Simões e Silva, A.C.; Guimarães, J.T.; Paixão, R.M.; Lanna, J.C.B.; Miranda, M.E. New Method of Surgical Delayed Closure of Giant Omphaloceles: Lazaro Da Silva’s Technique. J. Pediatr. Surg. 2004, 39, 1111–1115. [Google Scholar] [CrossRef]
- Biard, J.-M.; Wilson, R.D.; Johnson, M.P.; Hedrick, H.L.; Schwarz, U.; Flake, A.W.; Crombleholme, T.M.; Adzick, N.S. Prenatally Diagnosed Giant Omphaloceles: Short- and Long-Term Outcomes. Prenat. Diagn. 2004, 24, 434–439. [Google Scholar] [CrossRef]
- Mitanchez, D.; Walter-Nicolet, E.; Humblot, A.; Rousseau, V.; Revillon, Y.; Hubert, P. Neonatal Care in Patients with Giant Ompholocele: Arduous Management but Favorable Outcomes. J. Pediatr. Surg. 2010, 45, 1727–1733. [Google Scholar] [CrossRef]
- Loder, R.T.; Guiboux, J.P. Musculoskeletal Involvement in Children with Gastroschisis and Omphalocele. J. Pediatr. Surg. 1993, 28, 584–590. [Google Scholar] [CrossRef] [Green Version]
- Paidas, M.J.; Crombleholme, T.M.; Robertson, F.M. Prenatal Diagnosis and Management of the Fetus with an Abdominal Wall Defect. Semin. Perinatol. 1994, 18, 196–214. [Google Scholar]
- Dal Col, A.K.; Bhombal, S.; Tacy, T.A.; Hintz, S.R.; Feinstein, J.; Altit, G. Comprehensive Echocardiographic Assessment of Ventricular Function and Pulmonary Pressure in the Neonatal Omphalocele Population. Am. J. Perinatol. 2020, 38, e109–e115. [Google Scholar] [CrossRef] [PubMed]
- Argyle, J.C. Pulmonary Hypoplasia in Infants with Giant Abdominal Wall Defects. Pediatr. Pathol. 1989, 9, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Baerg, J.E.; Munoz, A.N. Long Term Complications and Outcomes in Omphalocele. Semin. Pediatr. Surg. 2019, 28, 118–121. [Google Scholar] [CrossRef] [PubMed]
- Tamosiuniene, R.; Manouvakhova, O.; Mesange, P.; Saito, T.; Qian, J.; Sanyal, M.; Lin, Y.-C.; Nguyen, L.P.; Luria, A.; Tu, A.B.; et al. Dominant Role for Regulatory T Cells in Protecting Females Against Pulmonary Hypertension. Circ. Res. 2018, 122, 1689–1702. [Google Scholar] [CrossRef] [PubMed]
- Stacher, E.; Graham, B.B.; Hunt, J.M.; Gandjeva, A.; Groshong, S.D.; McLaughlin, V.V.; Jessup, M.; Grizzle, W.E.; Aldred, M.A.; Cool, C.D.; et al. Modern Age Pathology of Pulmonary Arterial Hypertension. Am. J. Respir. Crit. Care Med. 2012, 186, 261–272. [Google Scholar] [CrossRef] [Green Version]
- Danzer, E.; Edgar, J.C.; Eppley, E.; Goldshore, M.A.; Chotzoglou, E.; Herkert, L.M.; Oliver, E.R.; Rintoul, N.E.; Panitch, H.; Adzick, N.S.; et al. Predicting Neonatal Outcomes in Infants with Giant Omphalocele Using Prenatal Magnetic Resonance Imaging Calculated Observed-to-Expected Fetal Lung Volumes. Prenat. Diagn. 2021, 41, 1439–1448. [Google Scholar] [CrossRef]
- Rypens, F.; Metens, T.; Rocourt, N.; Sonigo, P.; Brunelle, F.; Quere, M.P.; Guibaud, L.; Maugey-Laulom, B.; Durand, C.; Avni, F.E.; et al. Fetal Lung Volume: Estimation at MR Imaging-Initial Results. Radiology 2001, 219, 236–241. [Google Scholar] [CrossRef]
- Skarsgard, E.D. Immediate versus Staged Repair of Omphaloceles. Semin. Pediatr. Surg. 2019, 28, 89–94. [Google Scholar] [CrossRef]
- Pahwa, R.; Goyal, A.; Jialal, I. Chronic Inflammation. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Partridge, E.A.; Hanna, B.D.; Panitch, H.B.; Rintoul, N.E.; Peranteau, W.H.; Flake, A.W.; Scott Adzick, N.; Hedrick, H.L. Pulmonary Hypertension in Giant Omphalocele Infants. J. Pediatr. Surg. 2014, 49, 1767–1770. [Google Scholar] [CrossRef]
- Hutson, S.; Baerg, J.; Deming, D.; St Peter, S.D.; Hopper, A.; Goff, D.A. High Prevalence of Pulmonary Hypertension Complicates the Care of Infants with Omphalocele. Neonatology 2017, 112, 281–286. [Google Scholar] [CrossRef]
- Baerg, J.E.; Thorpe, D.L.; Sharp, N.E.; Ramlogan, S.R.; Hutson, S.M.; Goff, D.A.; Hopper, A.O.; St Peter, S.D. Pulmonary Hypertension Predicts Mortality in Infants with Omphalocele. J. Neonatal Perinat. Med. 2015, 8, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Pennaforte, T.; Rakza, T.; Sfeir, R.; Aubry, E.; Bonnevalle, M.; Fayoux, P.; Deschildre, A.; Thumerelle, C.; de Lagausie, P.; Benachi, A.; et al. Congenital diaphragmatic hernia: Respiratory and vascular outcomes. Rev. Mal. Respir. 2012, 29, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Jani, J.C.; Benachi, A.; Nicolaides, K.H.; Allegaert, K.; Gratacós, E.; Mazkereth, R.; Matis, J.; Tibboel, D.; Van Heijst, A.; Storme, L.; et al. Prenatal Prediction of Neonatal Morbidity in Survivors with Congenital Diaphragmatic Hernia: A Multicenter Study. Ultrasound Obstet. Gynecol. 2009, 33, 64–69. [Google Scholar] [CrossRef]
- de Lagausie, P.; de Buys-Roessingh, A.; Ferkdadji, L.; Saada, J.; Aisenfisz, S.; Martinez-Vinson, C.; Fund, X.; Cayuela, J.M.; Peuchmaur, M.; Mercier, J.C.; et al. Endothelin Receptor Expression in Human Lungs of Newborns with Congenital Diaphragmatic Hernia. J. Pathol. 2005, 205, 112–118. [Google Scholar] [CrossRef]
- Montero, F.J.; Simpson, L.L.; Brady, P.C.; Miller, R.S. Fetal Omphalocele Ratios Predict Outcomes in Prenatally Diagnosed Omphalocele. Am. J. Obstet. Gynecol. 2011, 205, 284.e1–284.e7. [Google Scholar] [CrossRef]
- Akinkuotu, A.C.; Sheikh, F.; Cass, D.L.; Zamora, I.J.; Lee, T.C.; Cassady, C.I.; Mehollin-Ray, A.R.; Williams, J.L.; Ruano, R.; Welty, S.E.; et al. Are All Pulmonary Hypoplasias the Same? A Comparison of Pulmonary Outcomes in Neonates with Congenital Diaphragmatic Hernia, Omphalocele and Congenital Lung Malformation. J. Pediatr. Surg. 2015, 50, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto-Kataoka, T.; Hosen, N.; Sonobe, T.; Arita, Y.; Yasui, T.; Masaki, T.; Minami, M.; Inagaki, T.; Miyagawa, S.; Sawa, Y.; et al. Interleukin-6/Interleukin-21 Signaling Axis Is Critical in the Pathogenesis of Pulmonary Arterial Hypertension. Proc. Natl. Acad. Sci. USA 2015, 112, E2677–E2686. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.-M.; Haghighat, L.; Spiekerkoetter, E.; Sawada, H.; Alvira, C.M.; Wang, L.; Acharya, S.; Rodriguez-Colon, G.; Orton, A.; Zhao, M.; et al. Neutrophil Elastase Is Produced by Pulmonary Artery Smooth Muscle Cells and Is Linked to Neointimal Lesions. Am. J. Pathol. 2011, 179, 1560–1572. [Google Scholar] [CrossRef]
- Zaidi, S.H.E.; You, X.-M.; Ciura, S.; Husain, M.; Rabinovitch, M. Overexpression of the Serine Elastase Inhibitor Elafin Protects Transgenic Mice from Hypoxic Pulmonary Hypertension. Circulation 2002, 105, 516–521. [Google Scholar] [CrossRef] [Green Version]
- Cowan, K.N.; Heilbut, A.; Humpl, T.; Lam, C.; Ito, S.; Rabinovitch, M. Complete Reversal of Fatal Pulmonary Hypertension in Rats by a Serine Elastase Inhibitor. Nat. Med. 2000, 6, 698–702. [Google Scholar] [CrossRef]
- Yoo, H.H.B.; Marin, F.L. Treating Inflammation Associated with Pulmonary Hypertension: An Overview of the Literature. Int. J. Gen. Med. 2022, 15, 1075–1083. [Google Scholar] [CrossRef] [PubMed]

| GO with PH (n = 15) | GO without PH (n = 35) | p-Value | |
|---|---|---|---|
| Pulmonary hypoplasia (Rypens) (n = 28) | 3/7 (43) | 6/21 (27) | 0.64 |
| Ratio CO/CA at T2 (n = 10) | 1.01 [0.97–1.05] | 0.82 [0.74–0.93] | |
| Ratio CO/CA at T3 (n = 8) | 0.95 [0.89–1.02] | 0.72 [0.65–0.75] | |
| Ratio CO/CC at T2 (n = 10) | 0.67 [0.66–0.80] | 0.67 [0.58–0.71] | |
| Ratio CO/CC at T3 (n = 10) | 0.64 [0.63–0.73] | 0.64 [0.58–0.67] | |
| GO collar at T2 mm (n = 30) | 25 [22–30] | 24 [20–30] | 0.76 |
| GO collar at T3 mm (n = 24) | 39 [37–45] | 47 [29–52] | 0.83 |
| GO collar at MRI evaluation mm (n = 18) | 38 [35–44] | 40 [30–50] | 0.93 |
| Variable | GO with PH (n = 15) | GO without PH (n = 35) | p-Value |
|---|---|---|---|
| Male, n (%) | 5 (33) | 17 (48) | 0.5 |
| Birth weight (g), mean ± SD | 2586 ± 792 | 2838 ± 1000 | 0.44 |
| GA at delivery (wks), mean ± SD | 36 ± 3 | 37 ± 2 | 0.1 |
| Apgar at 1 min, median [IQR] | 9 [4–10] | 10 [6–10] | <0.05 |
| Apgar at 5 min, median [IQR] | 10 [8–10] | 10 [8–10] | 0.2 |
| Venous umbilical cord pH1, mean ± SD | 7.32 ± 0.04 | 7.33 ± 0.06 | 0.6 |
| Variable | GO with PH (n = 15) | GO without PH (n = 35) | p-Value |
|---|---|---|---|
| DOL at final abdominal closure (d) | 425 [13–790] | 296 [1–1125] | 0.51 |
| Duration of parenteral feeding (d) | 43 [5–381] | 9 [0–40] | 0.09 |
| Enteral nutrition DOL at initial feedings (d) DOL on full goal volume feedings (d) | 5.5 [0–30] 43 [5–375] | 1 [0–15] 13 [0–40] | 0.16 <0.05 |
| Need of gastrostomy tube, n (%) | 7 (46) | 3 (8) | <0.05 |
| Length of supplemental O2 > 30% (d) | 8 [0–60] | 0 [0–5] | <0.05 |
| Need for GERD medication, n (%) | 13 (86) | 15 (43) | <0.05 |
| Need for tracheostomy, n (%) | 2 (13) | 1 (2.8) | 0.2 |
| Duration of mechanical ventilation (d) | 15 [0–56] | 0 [0–8] | <0.05 |
| Mechanical ventilation > 14 d, n (%) | 9 (60) | 0 (0) | <0.05 |
| Death, n (%) | 2 (13) | 0 (0) | <0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teillet, B.; Boukhris, M.R.; Sfeir, R.; Mur, S.; Cailliau, E.; Sharma, D.; Vaast, P.; Storme, L.; Le Duc, K. Systemic Inflammation Is Associated with Pulmonary Hypertension in Isolated Giant Omphalocele: A Population-Based Study. Healthcare 2022, 10, 1998. https://doi.org/10.3390/healthcare10101998
Teillet B, Boukhris MR, Sfeir R, Mur S, Cailliau E, Sharma D, Vaast P, Storme L, Le Duc K. Systemic Inflammation Is Associated with Pulmonary Hypertension in Isolated Giant Omphalocele: A Population-Based Study. Healthcare. 2022; 10(10):1998. https://doi.org/10.3390/healthcare10101998
Chicago/Turabian StyleTeillet, Baptiste, Mohamed Riadh Boukhris, Rony Sfeir, Sébastien Mur, Emeline Cailliau, Dyuti Sharma, Pascal Vaast, Laurent Storme, and Kévin Le Duc. 2022. "Systemic Inflammation Is Associated with Pulmonary Hypertension in Isolated Giant Omphalocele: A Population-Based Study" Healthcare 10, no. 10: 1998. https://doi.org/10.3390/healthcare10101998
APA StyleTeillet, B., Boukhris, M. R., Sfeir, R., Mur, S., Cailliau, E., Sharma, D., Vaast, P., Storme, L., & Le Duc, K. (2022). Systemic Inflammation Is Associated with Pulmonary Hypertension in Isolated Giant Omphalocele: A Population-Based Study. Healthcare, 10(10), 1998. https://doi.org/10.3390/healthcare10101998







