Association between Body Weight and Body Mass Index and Patellar Tendinopathy in Elite Basketball and Volleyball Players, a Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Methods
2.1. The Methods Followed the PRISMA Guidelines [18]
2.2. Data Sources and Search Strategy
2.3. Selection and Data Extraction
2.4. Quality Appraisal
2.5. Effect Measures
2.6. Synthesis Methods
2.7. Certainty Assessment
3. Results
3.1. Search Strategy
3.2. Study Characteristics
3.3. Quality Assessment
3.4. Results of Individual Studies and Synthesis
3.5. Narrative Synthesis
3.6. Robustness of the Synthesis Assessment
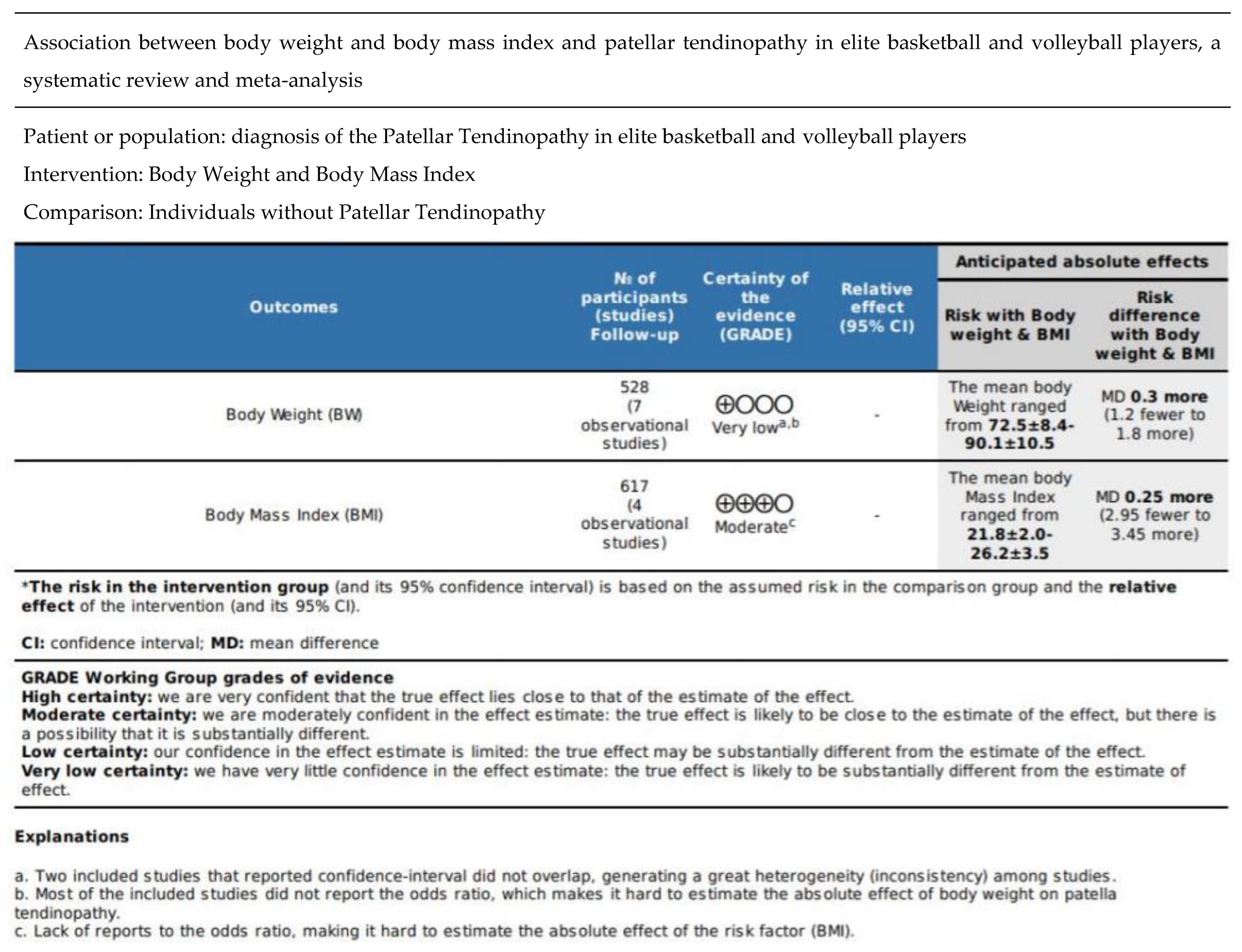
3.7. Certainty Assessment
4. Discussion
5. Limitations and Implications
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cong, G.-T.; Carballo, C.; Camp, C.L.; Album, Z.; Lebaschi, A.; Zong, J.; Rodeo, S.A. Platelet-Rich Plasma in Treating Patellar Tendinopathy. Oper. Tech. Orthop. 2016, 26, 110–116. [Google Scholar] [CrossRef]
- Khan, K.M.; Cook, J.L.; Kannus, P.; Maffulli, N.; Bonar, S.F. Time to abandon the “tendinitis” myth: Painful, overuse tendon conditions have a non-inflammatory pathology. BMJ 2002, 324, 626–627. [Google Scholar] [CrossRef] [PubMed]
- Peers, K.H.E.; Lysens, R.J.J. Patellar Tendinopathy in Athletes. Sports Med. 2005, 35, 71–87. [Google Scholar] [CrossRef]
- Blazina, M.E.; Kerlan, R.K.; Jobe, F.W.; Carter, V.S.; Carlson, G.J. Jumper’s knee. Orthop. Clin. N. Am. 1973, 4, 665–678. [Google Scholar] [CrossRef]
- Lian, Ø.B.; Engebretsen, L.; Bahr, R. Prevalence of jumper’s knee among elite athletes from different sports: A cross-sectional study. Am. J. Sports Med. 2005, 33, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Steinmann, S.; Pfeifer, C.G.; Brochhausen, C.; Docheva, D. Spectrum of tendon pathologies: Triggers, trails and end-state. Int. J. Mol. Sci. 2020, 21, 844. [Google Scholar] [CrossRef]
- Fairley, J.; Toppi, J.; Cicuttini, F.M.; Wluka, A.E.; Giles, G.G.; Cook, J.; O’Sullivan, R.; Wang, Y. Association between obesity and magnetic resonance imaging defined patellar tendinopathy in community-based adults: A cross-sectional study. BMC Musculoskelet. Disord. 2014, 15, 266. [Google Scholar] [CrossRef]
- Crossley, K.M.; Thancanamootoo, K.; Metcalf, B.R.; Cook, J.L.; Purdam, C.R.; Warden, S.J. Clinical features of patellar tendinopathy and their implications for rehabilitation. J. Orthop. Res. 2007, 25, 1164–1175. [Google Scholar] [CrossRef]
- Kregel, J.; van Wilgen, C.P.; Zwerver, J. Pain Assessment in Patellar Tendinopathy Using Pain Pressure Threshold Algometry: An Observational Study. Pain Med. 2013, 14, 1769–1775. [Google Scholar] [CrossRef]
- Williams, C.M.; Haines, T.P. An exploration of emergency department presentations related to high heel footwear in Victoria, Australia, 2006–2010. J. Foot Ankle Res. 2014, 7, 4. [Google Scholar] [CrossRef] [Green Version]
- Hopkins, C.; Fu, S.C.; Chua, E.; Hu, X.; Rolf, C.; Mattila, V.M.; Qin, L.; Yung, P.S.; Chan, K.M. Critical review on the socio-economic impact of tendinopathy. Asia Pac. J. Sports Med. Arthrosc. Rehabil. Technol. 2016, 22, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Magra, M.; Maffulli, N. Genetic aspects of tendinopathy. J. Sci. Med. Sport 2008, 11, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Finch, C. A new framework for research leading to sports injury prevention. J. Sci. Med. Sport 2006, 9, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Van Der Worp, H.; Van Ark, M.; Roerink, S.; Pepping, G.-J.; Akker-Scheek, I.V.D.; Zwerver, J. Risk factors for patellar tendinopathy: A systematic review of the literature. Br. J. Sports Med. 2011, 45, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Sprague, A.L.; Smith, A.H.; Knox, P.; Pohlig, R.T.; Silbernagel, K.G. Modifiable risk factors for patellar tendinopathy in athletes: A systematic review and meta-analysis. Br. J. Sports Med. 2018, 52, 1575–1585. [Google Scholar] [CrossRef] [PubMed]
- Seaman, D.R. Body mass index and musculoskeletal pain: Is there a connection? Chiropr. Man. Ther. 2013, 21, 15. [Google Scholar] [CrossRef]
- Stovitz, S.D.; Pardee, P.E.; Vazquez, G.; Duval, S.; Schwimmer, J.B. Musculoskeletal pain in obese children and adolescents. Acta Paediatr. 2008, 97, 489–493. [Google Scholar] [CrossRef]
- Herzog, R.; Álvarez-Pasquin, M.J.; Díaz, C.; Del Barrio, J.L.; Estrada, J.M.; Gil, Á. Are healthcare workers’ intentions to vaccinate related to their knowledge, beliefs and attitudes? A systematic review. BMC Public Health 2013, 13, 1–17. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int. J. Surg. 2021, 88, 105906. [Google Scholar] [CrossRef]
- Visentini, P.J.; Khan, K.M.; Cook, J.L.; Kiss, Z.S.; Harcourt, P.R.; Wark, J.D. The VISA score: An index of severity of symptoms in patients with jumper’s knee (patellar tendinosis). Victorian Institute of Sport Tendon Study Group. J. Sci. Med. Sport 1998, 1, 22–28. [Google Scholar] [CrossRef]
- Warden, S.J.; Kiss, Z.S.; Malara, F.A.; Ooi, A.B.T.; Cook, J.L.; Crossley, K. Comparative Accuracy of Magnetic Resonance Imaging and Ultrasonography in Confirming Clinically Diagnosed Patellar Tendinopathy. Am. J. Sports Med. 2007, 35, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Crossley, K.M.; Bennell, K.L.; Cowan, S.M.; Green, S. Analysis of outcome measures for persons with patellofemoral pain: Which are reliable and valid? Arch. Phys. Med. Rehabil. 2004, 85, 815–822. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Ng, G.Y.F.; Lee, W.C.; Fu, A. Increase in passive muscle tension of the quadriceps muscle heads in jumping athletes with patellar tendinopathy. Scand. J. Med. Sci. Sports 2017, 27, 1099–1104. [Google Scholar] [CrossRef] [PubMed]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. 2000. Available online: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 20 March 2022).
- Higgins, J.P.T.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1. 0 [Updated March 2011]. The Cochrane Collaboration. 2011. Available online: www.cochrane-handbook.Org (accessed on 21 August 2022).
- Lovakov, A.; Agadullina, E.R. Empirically derived guidelines for effect size interpretation in social psychology. Eur. J. Soc. Psychol. 2021, 51, 485–504. [Google Scholar] [CrossRef]
- Lang, J.M.; Rothman, K.; Cann, C.I. That Confounded P-Value. Epidemiology 1998, 9, 7–8. [Google Scholar] [CrossRef]
- Yu, C.H.; Ds, P. Meta-analysis and effect size. Small 2015, 500, 20. [Google Scholar]
- Szumilas, M. Explaining odds ratios. J. Can. Acad. Child Adolesc. Psychiatry 2010, 19, 227–229. [Google Scholar]
- Guyatt, G.; Oxman, A.D.; Akl, E.A.; Kunz, R.; Vist, G.; Brozek, J.; Schünemann, H.J. GRADE guidelines: 1. Introduction—GRADE evidence profiles and summary of findings tables. J. Clin. Epidemiol. 2011, 64, 383–394. [Google Scholar] [CrossRef]
- De Vries, A.J.; van der Worp, H.; Diercks, R.L.; van den Akker-Scheek, I.; Zwerver, J. Risk factors for patellar tendinopathy in volleyball and basketball players: A survey-based prospective cohort study. Scand. J. Med. Sci. Sports 2015, 25, 678–684. [Google Scholar] [CrossRef]
- Gaida, J.E.; Cook, J.L.; Bass, S.L.; Austen, S.; Kiss, Z.S. Are unilateral and bilateral patellar tendinopathy distinguished by differences in anthropometry, body composition, or muscle strength in elite female basketball players? Br. J. Sports Med. 2004, 38, 581–585. [Google Scholar] [CrossRef]
- Lian, Ø.; Refsnes, P.-E.; Engebretsen, L.; Bahr, R. Performance Characteristics of Volleyball Players with Patellar Tendinopathy. Am. J. Sports Med. 2003, 31, 408–413. [Google Scholar] [CrossRef] [PubMed]
- Malliaras, P.; Cook, J.L.; Kent, P.M.; Alfredson, H. Anthropometric risk factors for patellar tendon injury among volleyball players. Br. J. Sports Med. 2007, 41, 259–263. [Google Scholar] [CrossRef] [Green Version]
- Visnes, H.; Bahr, R. Training volume and body composition as risk factors for developing jumper’s knee among young elite volleyball players. Scand. J. Med. Sci. Sports 2013, 23, 607–613. [Google Scholar] [CrossRef]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1–9. [Google Scholar] [CrossRef]
- GRADEpro GDT: GRADEpro Guideline Development Tool [Software, GTP]; McMaster University and Evidence Prime: Hamilton, ON, Canada, 2022; Available online: https://www.gradepro.org/ (accessed on 10 June 2022).
- Cook, R.; Cowan, C. The Adipose Tissue. Int. J. Biomed. Health Sci. 2021, 9. Available online: https://en.wikipedia.org/wiki/Adipose_tissue (accessed on 12 September 2022).
- Wearing, S.C.; Hennig, E.M.; Byrne, N.M.; Steele, J.R.; Hills, A.P. Musculoskeletal disorders associated with obesity: A biomechanical perspective. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2006, 7, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Rees, J.D.; Maffulli, N.; Cook, J. Management of tendinopathy. Am. J. Sports Med. 2009, 37, 1855–1867. [Google Scholar] [CrossRef]
- Curwin, S. The Aetiology and Treatment of Tendinitis. In Oxford Textbook of Sports Medicine; Oxford University Press: Oxford, UK, 1994. [Google Scholar]
- Galloway, M.T.; Lalley, A.L.; Shearn, J.T. The Role of Mechanical Loading in Tendon Development, Maintenance, Injury, and Repair. J. Bone Jt. Surg. Am. Vol. 2013, 95, 1620–1628. [Google Scholar] [CrossRef] [PubMed]
- Snedeker, J.G.; Foolen, J. Tendon injury and repair–A perspective on the basic mechanisms of tendon disease and future clinical therapy. Acta Biomater. 2017, 63, 18–36. [Google Scholar] [CrossRef]
- Florit, D.; Pedret, C.; Casals, M.; Malliaras, P.; Sugimoto, D.; Rodas, G. Incidence of Tendinopathy in Team Sports in a Multidisciplinary Sports Club Over 8 Seasons. J. Sports Sci. Med. 2019, 18, 780–788. [Google Scholar]
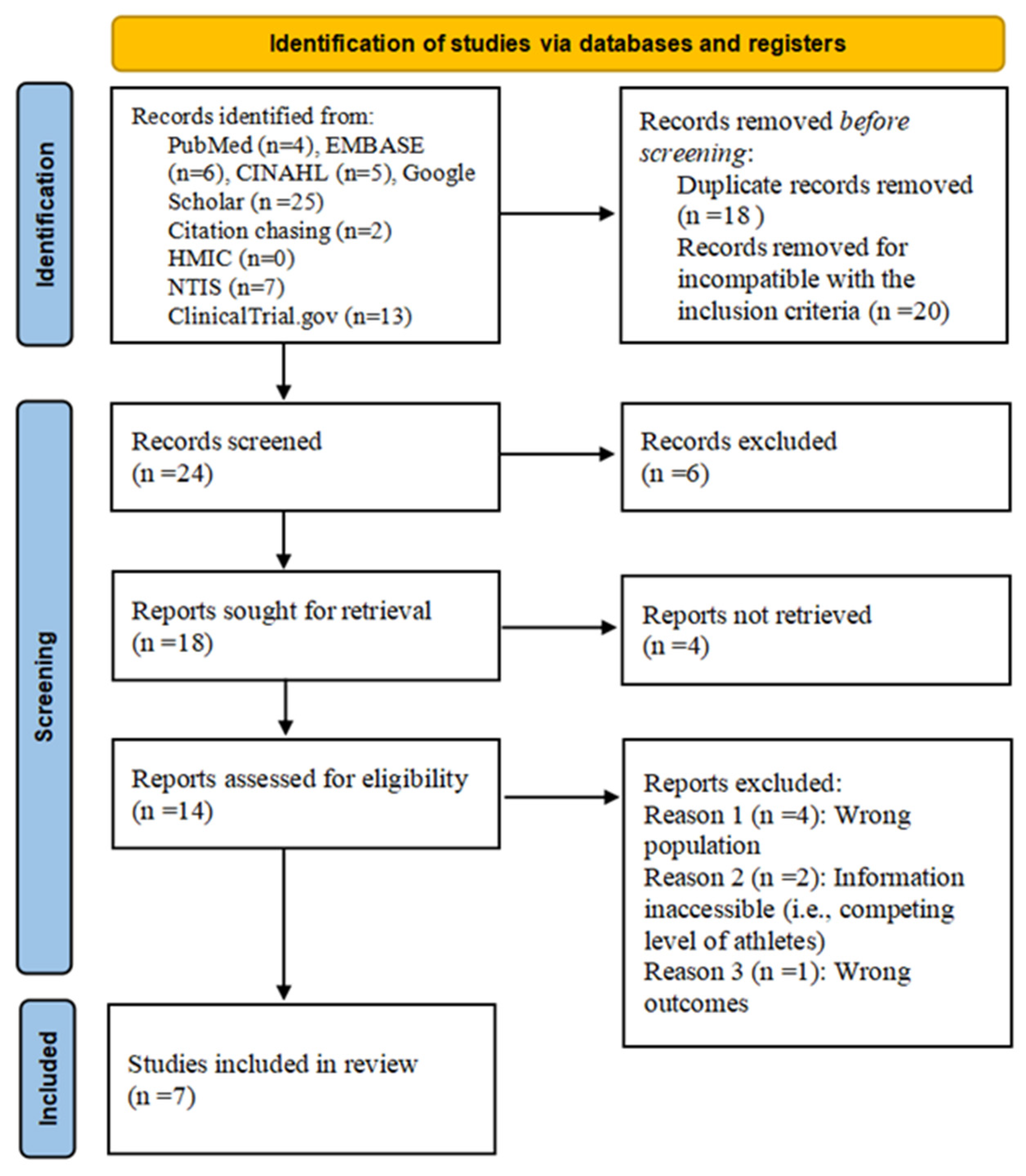
| Study (Study Design) | Potential Risk Factors | Diagnostic Criteria | Sample Size (PT%) | Sports Competition Level | Age | Nationality |
|---|---|---|---|---|---|---|
| de Vries et al. (2015) [31] (Prospective study) | BMI and BW Gender, physical demand | (1): Indicate pain in the inferior pole of patellar tendon (2): Diagnose by physician | 381 (13%) Male/female = 142/243 | Basketball and volleyball players competing at the elite (regional or national) or non-elite level | 25.3 ± 4.5 (2008); 28.3 ± 4.5 (2011) | Dutch |
| Visnes & Bahr (2013) [35] (Prospective study) | Training volume and body composition | (1): History of pain in patellar tendon (2): Tenderness of palpation corresponding to the painful area | 141 (28/141) 69 males 72 females | Volleyball players competing at elite level | 16–18 | Norway |
| Lian et al. (2003) (cross-sectional study) [33] | BW, activity volume, capable of jumping | (1): History of pain localize to the lower patellar pole or insertion of the quadriceps tendon (2): Distinct palpation tenderness corresponding to the painful area | 47 (24/47) All male | Volleyball players competing at elite level | 22.4 ± 2.5 (PT) 22.0 ± 4.0 (healthy) | Norway |
| Zhang et al. (2017) [23] (Cross-sectional study) | BW, BMI, Passive muscle tension | (1): Pain in the inferior pole of the proximal part of the patellar tendon (2): Pain aggregation during single leg squatting and jumping (3): Pain duration longer than 3 months (4): Maximum intensity of pain in the previous week >3 on the visual analog scale. (5): VISA-P score < 80 points 6) Thickening of proximal part of patellar tendon with area of hypoechoic signal on ultrasound imaging. | 66 (36/66) All male | Volleyball and basketball players | 21.1 ± 4.4 | Hong Kong |
| Crossley et al. (2007) [8] (Cross-sectional study) | BW, BMI, training volume, thigh flexibility and strength | (1): Functional measure (VISA scale) Symptom measures (NPS-W and NPS-U) | 58 (27/58) Female: Male = 19:39 | Participants in competitive basketball, netball volleyball or tennis | 24 ± 6 | Dutch |
| Gaida et al. (2004) [32] (Cross-sectional study) | BW, height, tibial length to stature ratio (UL vs. control), waist-to-hip ratio (UL vs. control) | Ultrasound examination | 39 (15/39) All female | Elite basketball players | Unilateral (20 ± 2) Bilateral&Control (21 ± 3) | Australia |
| Malliaras et al. (2007) [34] (cross-sectional) | BMI, BW, gender, height, waist girth, hip girth, waist-to-hip ratio | Female and male tendon Normal imaging Abnormal imaging UL Abnormal imaging BL | 113 (73 male, 40 female) | Competitive volleyball player | Unknown | Australia |
| Author (Year) | Potential Risk Factors | Diagnostic Criteria | Sample Size | Sports Competition Level | Selection | Comparability | Exposure/ Outcome | Total Stars | Study Quality |
|---|---|---|---|---|---|---|---|---|---|
| Prospective cohort studies | |||||||||
| de Vries et al. (2015) [31] | BMI, BW | (1): Indicate pain in the inferior pole of patella tendon (2): Diagnose by physician | 381 | Basketball and volleyball players competing at the elite (regional or national) or non-elite level | 4 | 2 | 3 | 9 | Very good study |
| Visnes & Bahr (2013) [35] | Training volume and body composition | (1): History of pain in patella tendon (2): Tenderness of palpation corresponding to the painful area | 141 | Volleyball players competing at elite level | 3 | 2 | 3 | 8 | Good study |
| Cross-sectional studies | |||||||||
| Malliaras et al. (2007) [34] | BMI, BW, height, waist girth, hip girth, waist-to-hip ratio | Female and male tendon Normal imaging Abnormal imaging UL Abnormal imaging BL | 113 | Competitive volleyball player | 4 | 2 | 3 | 9 | Very good study |
| Gaida et al. (2004) [32] | BW, height, tibial length to stature ratio (UL vs. control), waist-to-hip ratio (UL vs. control) | Ultrasound examination | 39 | Elite basketball players | 3 (included sample not representative) | 0 (poor control of confronting factors) | 3 | 6 | Satisfactory study |
| Zhang et al. (2017) [23] | BMI, BW | (1): Pain in the inferior pole of the proximal part of the patella tendon (2): Pain aggregation during single leg squatting and jumping (3): Pain duration longer than 3 months (4): Maximum intensity of pain in the previous week >3 on the visual analog scale. (5): VISA-P score < 80 points (6): Thickening of proximal part of patellar tendon with area of hypoechoic signal on ultrasound imaging. | 66 | Volleyball and basketball players | 4 | 2 | 3 | 9 | Very good study |
| Crossley et al. (2007) [8] | BW, BMI, arch height during maximal weight bearing, leg length difference | (1): Functional measure (VISA scale) Symptom measures (NPS-W and NPS-U) | 58 | Participants in competitive basketball, netball volleyball or tennis | 3 (the recruited sample does not represent the whole population) | 2 | 3 | 8 | Good study |
| Lian et al. (2003) [33] | BW, activity volume | (1): History of pain localize to the lower patella pole or insertion of the quadriceps tendon (2): Distinct palpation tenderness corresponding to the painful area | 47 | Volleyball players competing at elite level | 4 | 2 | 3 | 9 | Very good study |
| Study (Study Design) | Potential Risk Factors | Value of Risk Factor (BW; BMI) Mean ± SD Unit: Kg; Kg/m2 | Sample Size (PT%) | Conclusion | Secondary Findings |
|---|---|---|---|---|---|
| Statistical significance of main findings (p < 0.05) | |||||
| de Vries et al. (2015) [31] (Prospective cohort study) Dutch | BMI and BW Gender, physical demand | BW: 76.1 ± 12.6 BMI: 23.6 ± 3.1 | 381 (13%) Male/female = 142/243 | Weight [OR 1.2 95% (1.0–1.3) p < 0.05]. | Male gender (p < 0.05) [odds ratio (OR) 2.0, 95% confidence interval (CI) 1.1–3.5] Physical demand work (OR 2.3, 95% CI 0.9–6.3) |
| Lian et al. (2003) (cross-sectional study) [33] Norway | BW, activity volume, capable of jumping | 86.7 ± 7.9(PT) 81.9 ± 8.1(healthy) | 47 (24/47) All male | Weight is associated with PT (p < 0.05) | Weight training (p < 0.05) Composite jumping score (p < 0.05) |
| Zhang et al. (2017) [23] (Cross-sectional study) Hong Kong | BW, BMI, Passive muscle tension | BW: 74.1 ± 6.6(PT) 72.5 ± 8.4(control) BMI: 22.9 ± 1.9(PT) 21.8 ± 2.0(control) | 66 (36/66) All male | BMI (p < 0.05) | Tension of vastus lateralis is associated with PT (r = 0.38; p < 0.05) |
| Crossley et al. (2007) [8] (Cross-sectional study) Dutch | BW, BMI, training volume, thigh flexibility and strength | BW: 80 ± 16 (unilateral PT); 82 ± 14 (bilateral PT) BMI: 25.2 ± 4 26.2 ± 3.5 | 58 (27/58) F:M = 19:39 | Weight (p < 0.05) BMI (p < 0.05) | Training volume (p < 0.05) Thigh flexibility (greater in bilateral PT) p < 0.05 Thigh strength (bilateral PT has greater force production) p < 0.05 |
| Gaida et al. (2004) [32] (Cross-sectional study) Australia | BW, height, tibial length to stature ratio (UL vs. control), waist-to-hip ratio (UL vs. control) | BW: 74 ± 13 | 39 (15/39) All female | Weight (p < 0.05) | Tibial length to stature ratio was 1.3 above zero in unilateral group. (p < 0.05) Waist-to-hip ratio was 0.66 SD above zero in unilateral group. (p > 0.05) Leg is weaker in the path |
| Malliaras et al. (2007) [34] (cross-sectional) Australia | BMI, BW, gender, height, waist girth, hip girth, waist-to-hip ratio | Male: BW:87.2 ± 12. BMI 24.8 ± 2(unilateral) 90.1 ± 10.5. BMI 25.7 ± 2.6(bilateral) | 113 (73 male, 40 female) | Male BW and BMI (p < 0.05) | Waist-to -hip ratio, waist and hip girth in male (p < 0.05) |
| Statistical significance of main findings (p > 0.05) | |||||
| Visnes & Bahr (2013) [35] (Prospective cohort study) Norway | Training volume and body composition | BW: 75.3± 7.8(healthy) 76.3 ± 8.5(PT) | 141 (28/141) 69 males 72 females | Weight is not associated with PT (p > 0.05) OR: 3.2 (−0.9,3.7) | Training volume (increase every hour): (OR) 1.72 (1.18–2.53) |
| de Vries et al. (2015) [31] (Prospective cohort study) Dutch | BMI and BW Gender, physical demand | BW: 76.1 ± 12.6 BMI: 23.6 ± 3.1 | 381 (13%) Male/female = 142/243 | BMI is not associated with PT. [OR 1.1 (1.0–1.2) (p > 0.05)] | Male gender (p < 0.05) [odds ratio (OR) 2.0, 95% confidence interval (CI) 1.1–3.5] Physical demand work (OR 2.3, 95% CI 0.9–6.3) |
| Zhang et al. (2017) [23] (Cross-sectional study) Hong Kong | BW, BMI, Passive muscle tension | BW: 74.1 ± 6.6 (PT) 72.5 ± 8.4 (control) BMI: 22.9 ± 1.9 (PT) 21.8 ± 2.0(control) | 66 (36/66) All male | Weight (p > 0.05) | Tension of vastus lateralis is associated with PT (r = 0.38; p < 0.05) |
| Malliaras et al. (2007) [34] (cross-sectional) Australia | BMI, BW, gender, height, waist girth, hip girth, waist-to-hip ratio | Male: BW:87.2 ± 12. BMI 24.8 ± 2(unilateral) 90.1 ± 10.5. BMI 25.7 ± 2.6(bilateral) | 113 (73 male, 40 female) | Female BW and BMI (p > 0.05) | Waist-to-hip ratio, waist and hip girth in male (p < 0.05) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, M.; Mansfield, M. Association between Body Weight and Body Mass Index and Patellar Tendinopathy in Elite Basketball and Volleyball Players, a Systematic Review and Meta-Analysis. Healthcare 2022, 10, 1928. https://doi.org/10.3390/healthcare10101928
Deng M, Mansfield M. Association between Body Weight and Body Mass Index and Patellar Tendinopathy in Elite Basketball and Volleyball Players, a Systematic Review and Meta-Analysis. Healthcare. 2022; 10(10):1928. https://doi.org/10.3390/healthcare10101928
Chicago/Turabian StyleDeng, Minghao, and Michael Mansfield. 2022. "Association between Body Weight and Body Mass Index and Patellar Tendinopathy in Elite Basketball and Volleyball Players, a Systematic Review and Meta-Analysis" Healthcare 10, no. 10: 1928. https://doi.org/10.3390/healthcare10101928
APA StyleDeng, M., & Mansfield, M. (2022). Association between Body Weight and Body Mass Index and Patellar Tendinopathy in Elite Basketball and Volleyball Players, a Systematic Review and Meta-Analysis. Healthcare, 10(10), 1928. https://doi.org/10.3390/healthcare10101928





