Integration of Machine Learning Algorithms and Discrete-Event Simulation for the Cost of Healthcare Resources
Abstract
:1. Introduction
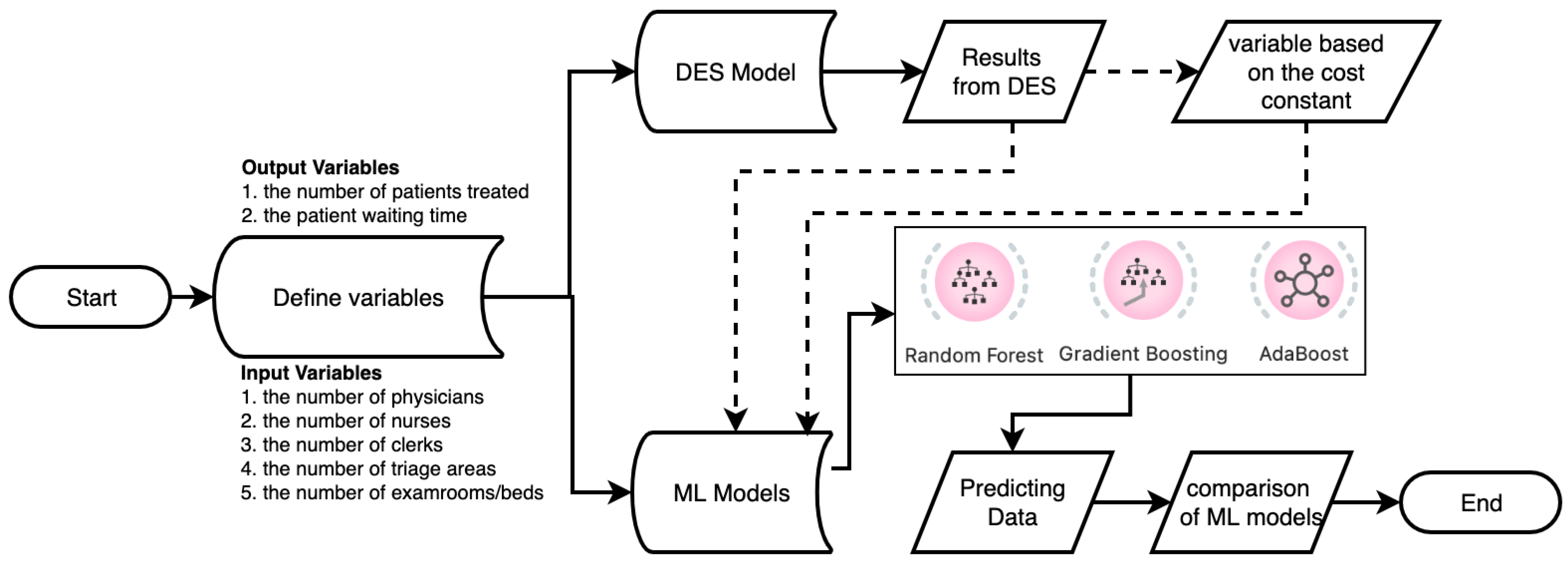
2. Methodology
2.1. Data Collection and Its Characteristics
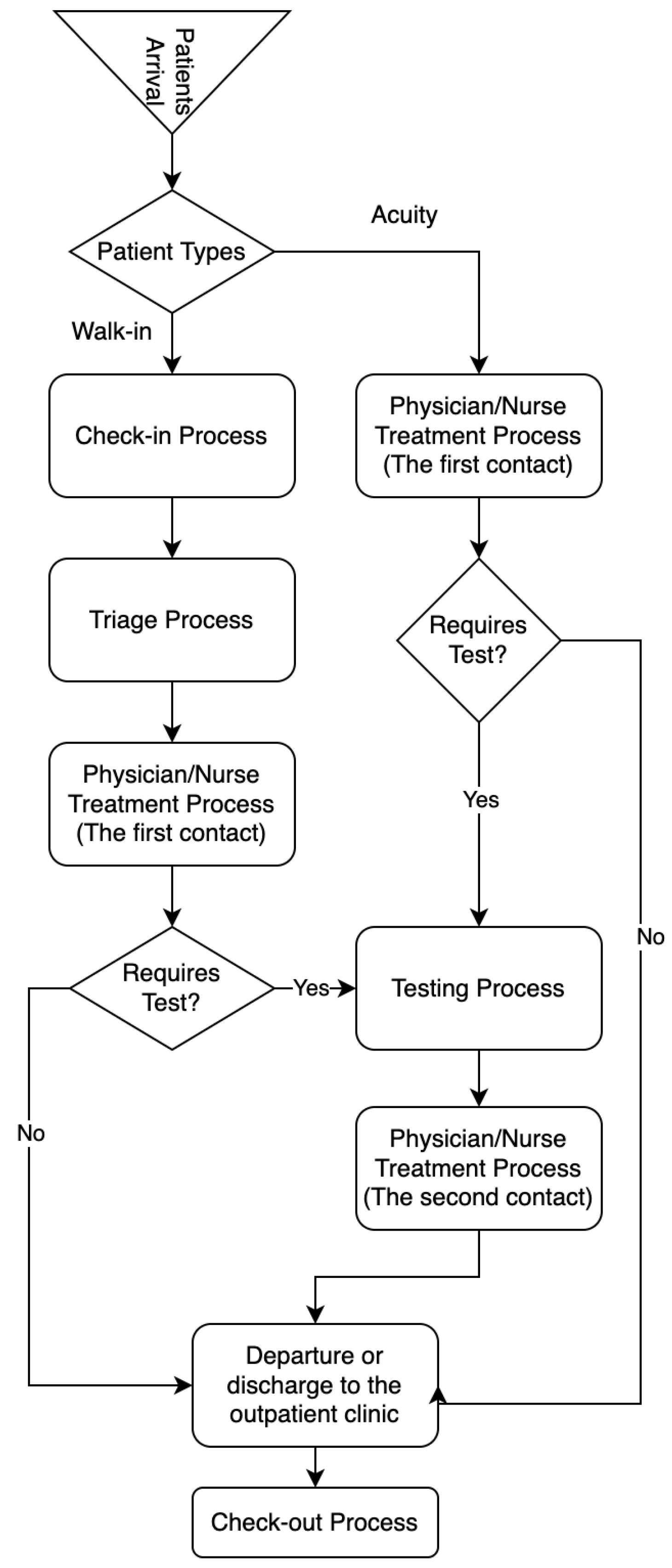
2.2. Discrete-Event Simulation
2.3. Machine Learning Models
2.3.1. Random Forest (RF)
2.3.2. Gradient Boosting (GB)
2.3.3. AdaBoost (AB)
| Algorithm 1. SAMME Algorithm |
| Step 1. Initialize the observation weights Step 2. For : Step 2.1. fit a classifier to the training data using weights . Step 2.2. compute: . Step 2.3. compute: . Step 2.4. set: . Step 2.5. re-normalize . Step 3. Output . SAMME.R algorithm: Step 1. Initialize the observation weights Step 2. For : Step 2.1. fit a classifier to the training data using weights . Step 2.2. obtain the weighted class probability estimates: Step 2.3. set: . Step 2.4. set: . Step 2.5. re-normalize . Step 3. Output . |
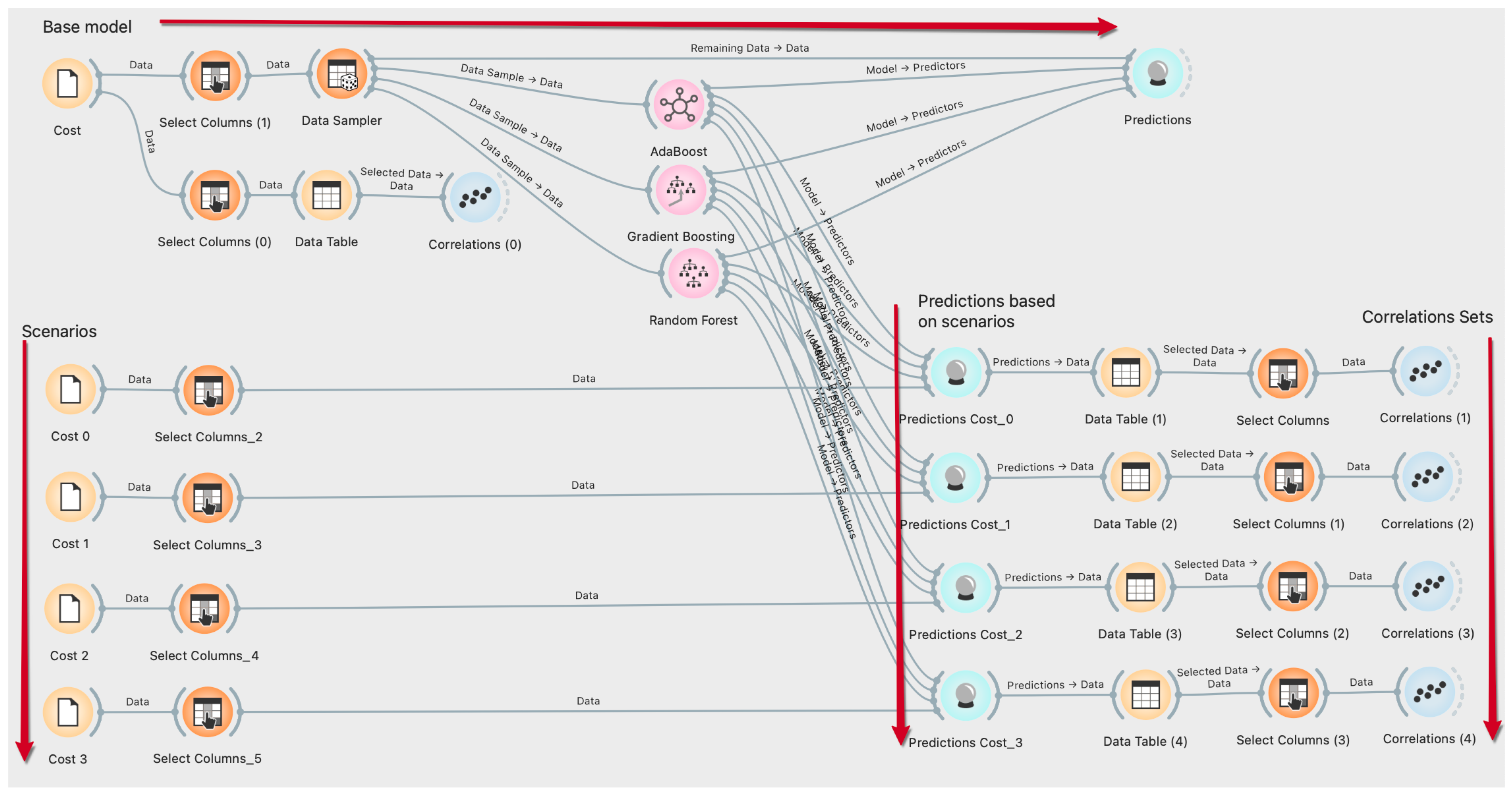
2.4. Healthcare Resources Cost
2.5. Model Metrics
3. Results and Discussion
3.1. Statistical Results
3.2. The Results of the Performance Evaluation
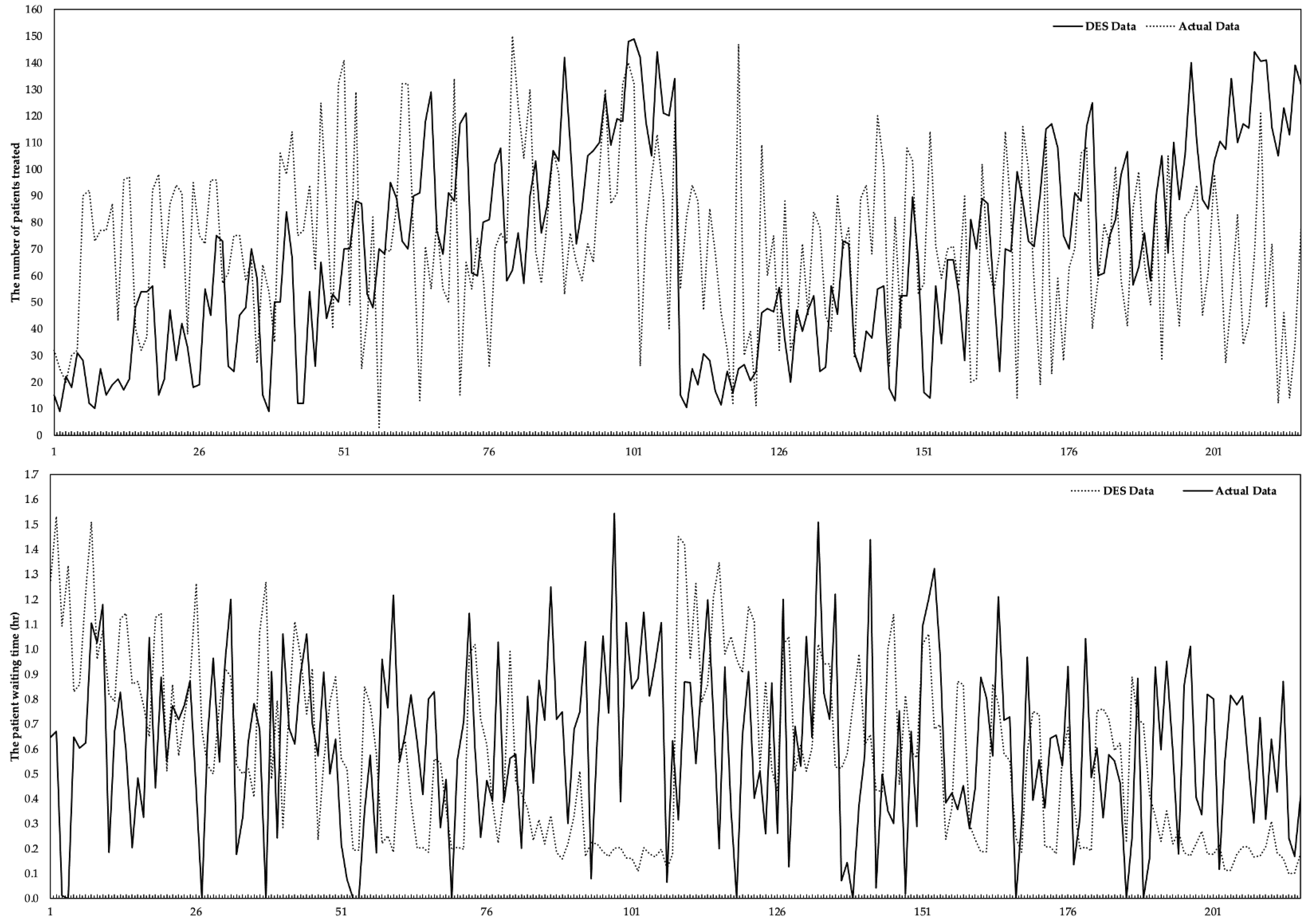
3.3. The Estimated Number of Patients Treated (
3.4. The Estimated Waiting Times (
4. Discussions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nahhas, A.; Awaldi, A.; Reggelin, T. Simulation and the Emergency Department Overcrowding Problem. Procedia Eng. 2017, 178, 368–376. [Google Scholar] [CrossRef]
- Guimarães, A.M.C.; Leal, J.E.; Mendes, P. Discrete-event simulation software selection for manufacturing based on the maturity model. Comput. Ind. 2018, 103, 14–27. [Google Scholar] [CrossRef]
- Atalan, A.; Dönmez, C.C. Optimizing experimental simulation design for the emergency departments. Braz. J. Oper. Prod. Manag. 2020, 17, 1–13. [Google Scholar] [CrossRef]
- Ahmed, M.A.; Alkhamis, T.M. Simulation optimization for an emergency department healthcare unit in Kuwait. Eur. J. Oper. Res. 2009, 198, 936–942. [Google Scholar] [CrossRef]
- Cimellaro, G.P.; Malavisi, M.; Mahin, S. Using Discrete Event Simulation Models to Evaluate Resilience of an Emergency Department. J. Earthq. Eng. 2017, 21, 203–226. [Google Scholar] [CrossRef]
- Bal, A.; Ceylan, C.; Taçoğlu, C. Using value stream mapping and discrete event simulation to improve efficiency of emergency departments. Int. J. Healthc. Manag. 2017, 10, 196–206. [Google Scholar] [CrossRef]
- Qureshi, S.M.; Purdy, N.; Mohani, A.; Neumann, W.P. Predicting the effect of nurse–patient ratio on nurse workload and care quality using discrete event simulation. J. Nurs. Manag. 2019, 27, 971–980. [Google Scholar] [CrossRef]
- Hasan, I.; Bahalkeh, E.; Yih, Y. Evaluating intensive care unit admission and discharge policies using a discrete event simulation model. Simulation 2020, 96, 501–518. [Google Scholar] [CrossRef]
- Atalan, A. A cost analysis with the discrete-event simulation application in nurse and doctor employment management. J. Nurs. Manag. 2022, 30, 733–741. [Google Scholar] [CrossRef]
- Baril, C.; Gascon, V.; Vadeboncoeur, D. Discrete-event simulation and design of experiments to study ambulatory patient waiting time in an emergency department. J. Oper. Res. Soc. 2019, 70, 2019–2038. [Google Scholar] [CrossRef]
- Capocchi, L.; Santucci, J.-F.; Zeigler, B.P. Discrete Event Modeling and Simulation Aspects to Improve Machine Learning Systems. In Proceedings of the 2018 4th International Conference on Universal Village (UV), Boston, MA, USA, 21–24 October 2018; IEEE: Piscataway, NJ, USA, 2018; pp. 1–6. [Google Scholar]
- Mekov, E.; Miravitlles, M.; Petkov, R. Artificial intelligence and machine learning in respiratory medicine. Expert Rev. Respir. Med. 2020, 14, 559–564. [Google Scholar] [CrossRef]
- Zhou, Q.; Lu, S.; Wu, Y.; Wang, J. Property-Oriented Material Design Based on a Data-Driven Machine Learning Technique. J. Phys. Chem. Lett. 2020, 11, 3920–3927. [Google Scholar] [CrossRef]
- Sah, S. Machine Learning: A Review of Learning Types. Artif. Intell. Robot. 2020, preprints. [Google Scholar] [CrossRef]
- Shailaja, K.; Seetharamulu, B.; Jabbar, M.A. Machine Learning in Healthcare: A Review. In Proceedings of the 2018 Second International Conference on Electronics, Communication and Aerospace Technology (ICECA), Coimbatore, India, 29–31 March 2018; IEEE: Piscataway, NJ, USA, 2018; pp. 910–914. [Google Scholar]
- Raita, Y.; Goto, T.; Faridi, M.K.; Brown, D.F.M.; Camargo, C.A.; Hasegawa, K. Emergency department triage prediction of clinical outcomes using machine learning models. Crit. Care 2019, 23, 64. [Google Scholar] [CrossRef]
- Ceylan, Z.; Atalan, A. Estimation of healthcare expenditure per capita of Turkey using artificial intelligence techniques with genetic algorithm-based feature selection. J. Forecast. 2021, 40, 279–290. [Google Scholar] [CrossRef]
- Islam, M.M.; Shamsuddin, R. Machine learning to promote health management through lifestyle changes for hypertension patients. Array 2021, 12, 100090. [Google Scholar] [CrossRef]
- Siddique, S.; Chow, J.C.L. Machine Learning in Healthcare Communication. Encyclopedia 2021, 1, 220–239. [Google Scholar] [CrossRef]
- Rajula, H.S.R.; Verlato, G.; Manchia, M.; Antonucci, N.; Fanos, V. Comparison of conventional statistical methods with machine learning in medicine: Diagnosis, drug development, and treatment. Medicina 2020, 56, 455. [Google Scholar] [CrossRef]
- Battineni, G.; Chintalapudi, N.; Amenta, F. Machine learning in medicine: Performance calculation of dementia prediction by support vector machines (SVM). Inform. Med. Unlocked 2019, 16, 100200. [Google Scholar] [CrossRef]
- Dhiman, P.; Ma, J.; Navarro, C.A.; Speich, B.; Bullock, G.; Damen, J.A.; Kirtley, S.; Hooft, L.; Riley, R.D.; Van Calster, B.; et al. Reporting of prognostic clinical prediction models based on machine learning methods in oncology needs to be improved. J. Clin. Epidemiol. 2021, 138, 60–72. [Google Scholar] [CrossRef]
- Ali, M.; Aittokallio, T. Machine learning and feature selection for drug response prediction in precision oncology applications. Biophys. Rev. 2019, 11, 31–39. [Google Scholar] [CrossRef]
- Menden, M.P.; Iorio, F.; Garnett, M.; McDermott, U.; Benes, C.H.; Ballester, P.J.; Saez-Rodriguez, J. Machine Learning Prediction of Cancer Cell Sensitivity to Drugs Based on Genomic and Chemical Properties. PLoS ONE 2013, 8, e61318. [Google Scholar] [CrossRef]
- Aufegger, L.; Bicknell, C.; Soane, E.; Ashrafian, H.; Darzi, A. Understanding health management and safety decisions using signal processing and machine learning. BMC Med. Res. Methodol. 2019, 19, 121. [Google Scholar] [CrossRef]
- López-Martínez, F.; Núñez-Valdez, E.R.; García-Díaz, V.; Bursac, Z. A Case Study for a Big Data and Machine Learning Platform to Improve Medical Decision Support in Population Health Management. Algorithms 2020, 13, 102. [Google Scholar] [CrossRef]
- Anderson, D.; Bjarnadottir, M.V.; Nenova, Z. Machine Learning in Healthcare: Operational and Financial Impact. In Innovative Technology at the Interface of Finance and Operations; Springer: Berlin/Heidelberg, Germany, 2022; pp. 153–174. [Google Scholar]
- Gan, C.L. Prognostics and Health Management of Electronics: Fundamentals, Machine Learning, and the Internet of Things. Life Cycle Reliab. Saf. Eng. 2020, 9, 225–226. [Google Scholar] [CrossRef]
- Panicacci, S.; Donati, M.; Profili, F.; Francesconi, P.; Fanucci, L. Trading-Off Machine Learning Algorithms towards Data-Driven Administrative-Socio-Economic Population Health Management. Computers 2020, 10, 4. [Google Scholar] [CrossRef]
- Gartner, D.; Padman, R. Machine learning for healthcare behavioural OR: Addressing waiting time perceptions in emergency care. J. Oper. Res. Soc. 2020, 71, 1087–1101. [Google Scholar] [CrossRef]
- Mahyoub, M.A. Improving Health Referral Processing Using Machine-Learning-Guided Simulation: A Care Management Setting Case Study. Master’s Thesis, State University of New York, Binghamton, NY, USA, 2020. [Google Scholar]
- Hosseini_Shokouh, S.; Mohammadi, K.; Yaghoubi, M. Optimization of Service Process in Emergency Department Using Discrete Event Simulation and Machine Learning Algorithm. Arch. Acad. Emerg. Med. 2022, 10, e44. [Google Scholar]
- Kim, J.; Lim, H.; Ahn, J.-H.; Lee, K.H.; Lee, K.S.; Koo, K.C. Optimal Triage for COVID-19 Patients Under Limited Health Care Resources with a Parsimonious Machine Learning Prediction Model and Threshold Optimization Using Discrete-Event Simulation: Development Study. JMIR Med. Inform. 2021, 9, e32726. [Google Scholar] [CrossRef]
- Olave-Rojas, D.; Nickel, S. Modeling a pre-hospital emergency medical service using hybrid simulation and a machine learning approach. Simul. Model. Pract. Theory 2021, 109, 102302. [Google Scholar] [CrossRef]
- Elbattah, M.; Molloy, O.; Zeigler, B.P. Designing care pathways using simulation modeling and machine learning. In Proceedings of the 2018 Winter Simulation Conference (WSC), Gothenburg, Sweden, 9–12 December 2018; IEEE: Piscataway, NJ, USA, 2018; pp. 1452–1463. [Google Scholar]
- Košinár, M.; Štrba, R. Simulations of Agile Software Processes for Healthcare Information Systems Development Based on Machine Learning Methods. IFAC Proc. Vol. 2013, 46, 175–180. [Google Scholar] [CrossRef]
- Rigatti, S.J. Random Forest. J. Insur. Med. 2017, 47, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, Z.-P.; Zhang, X.-S.; Chen, L. Prediction of hot spots in protein interfaces using a random forest model with hybrid features. Protein Eng. Des. Sel. 2012, 25, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Zhang, J.; Zhang, Q.; Wei, X. Risk prediction of type II diabetes based on random forest model. In Proceedings of the 2017 Third International Conference on Advances in Electrical, Electronics, Information, Communication and Bio-Informatics (AEEICB), Chennai, India, 27–28 February 2017; IEEE: Piscataway, NJ, USA, 2017; pp. 382–386. [Google Scholar]
- Natekin, A.; Knoll, A. Gradient boosting machines, a tutorial. Front. Neurorobot. 2013, 7, 21. [Google Scholar] [CrossRef] [PubMed]
- Touzani, S.; Granderson, J.; Fernandes, S. Gradient boosting machine for modeling the energy consumption of commercial buildings. Energy Build. 2018, 158, 1533–1543. [Google Scholar] [CrossRef]
- Schapire, R.E. Explaining AdaBoost. In Empirical Inference; Springer: Berlin/Heidelberg, Germany, 2013; pp. 37–52. [Google Scholar]
- Vezhnevets, A.; Vezhnevets, V. Modest AdaBoost-teaching AdaBoost to generalize better. In Proceedings of the Graphicon, Novosibirsk, Russia, 20–24 June 2005; Volume 12, pp. 987–997. [Google Scholar]
- Pandey, P.; Prabhakar, R. An analysis of machine learning techniques (J48 & AdaBoost)-for classification. In Proceedings of the 2016 1st India International Conference on Information Processing (IICIP), Delhi, India, 12–14 August 2016; IEEE: Piscataway, NJ, USA, 2016; pp. 1–6. [Google Scholar]
- Hastie, T.; Rosset, S.; Zhu, J.; Zou, H. Multi-class adaboost. Stat. Interface 2009, 2, 349–360. [Google Scholar] [CrossRef]
- Breiman, L.; Friedman, J.H.; Olshen, R.A.; Stone, C.J. Classification and Regression Trees; Wadsworth Publishing: Belmont, CA, USA, 1984; ISBN 9781315139470. [Google Scholar]
- Ahmad, S.; Kalra, A.; Stephen, H. Estimating soil moisture using remote sensing data: A machine learning approach. Adv. Water Resour. 2010, 33, 69–80. [Google Scholar] [CrossRef]
- Lin, W.-C.; Goldstein, I.H.; Hribar, M.R.; Sanders, D.S.; Chiang, M.F. Predicting Wait Times in Pediatric Ophthalmology Outpatient Clinic Using Machine Learning. In Proceedings of the AMIA Annual Symposium Proceedings, Washington, DC, USA, 16–20 November 2019. [Google Scholar]
- Pak, A.; Gannon, B.; Staib, A. Predicting waiting time to treatment for emergency department patients. Int. J. Med. Inform. 2021, 145, 104303. [Google Scholar] [CrossRef]
- Kim, E.; Han, K.S.; Cheong, T.; Lee, S.W.; Eun, J.; Kim, S.J. Analysis on Benefits and Costs of Machine Learning-Based Early Hospitalization Prediction. IEEE Access 2022, 10, 32479–32493. [Google Scholar] [CrossRef]
- Berg, B.; Denton, B.; Nelson, H.; Balasubramanian, H.; Rahman, A.; Bailey, A.; Lindor, K. A Discrete Event Simulation Model to Evaluate Operational Performance of a Colonoscopy Suite. Med. Decis. Mak. 2010, 30, 380–387. [Google Scholar] [CrossRef]





| Reference | Unit | ML Algorithms | The Purpose(s) of the Problem |
|---|---|---|---|
| [30] | ED | Naïve Bayes, Bayesian Networks, classification Trees | Examining patient satisfaction, waiting time estimation, |
| [31] | Hospitals, RCU | KNN, neural network, Decision Tree, Random Forest, Support Vector Machine | Improving health referrals processing |
| [32] | ED | Artificial Neural Network algorithm, Genetic Algorithm | Minimizing patients’ waiting time, the percentage of units’ engagement to enhance the ED efficiency |
| [33] | General * | XGBoost, Logistic Regression | Discovering optimal thresholds to effectively triage COVID-19 patients, minimizing death rates while preserving health system capacity. |
| [34] | ECC | Generalized Linear Model, Multivariate Adaptive Regression Splines, Random Forest, Support Vector Machine, Decision Tree | Analyzing critical crew capacity |
| [35] | HCU | K-Means Algorithm | To design health pathways and evaluate the return on investment of implementation |
| [36] | General * | Neural Network, | Development of healthcare information systems |
| This research | ED | Random Forest, Gradient Boosting, AdaBoost | , analysis of healthcare resources cost |
| Features | |||||||
|---|---|---|---|---|---|---|---|
| Status | Input | Input | Input | Input | Input | Output | Output |
| Data Type | Numeric * | Numeric * | Binary ** | Numeric * | Binary ** | Numeric * | Numeric |
| Mean | 2.000 | 2.000 | 1.500 | 2.000 | 1.500 | 68.570 | 0.579 |
| SE Mean | 0.056 | 0.056 | 0.034 | 0.056 | 0.034 | 2.5500 | 0.024 |
| StDev | 0.818 | 0.818 | 0.501 | 0.818 | 0.501 | 37.550 | 0.358 |
| Variance | 0.670 | 0.670 | 0.251 | 0.670 | 0.251 | 1409.9 | 0.128 |
| CoefVar | 40.92 | 40.92 | 33.41 | 40.92 | 33.41 | 54.760 | 61.78 |
| Minimum | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 9.0000 | 0.098 |
| Median | 2.000 | 2.000 | 1.500 | 2.000 | 1.500 | 68.000 | 0.539 |
| Maximum | 3.000 | 3.000 | 2.000 | 3.000 | 2.000 | 149.00 | 1.530 |
| Skewness | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.2100 | 0.510 |
| Kurtosis | −1.510 | −1.510 | −2.020 | −1.510 | −2.020 | −0.950 | −0.720 |
| Staff | Gender | Quantity | Type | Responsible Process | Process Distribution |
|---|---|---|---|---|---|
| Physicians | Female/Male | 3 | Human-based | diagnosis/examination/treatment | Uniform (10.0, 30.0, 0.0) |
| Nurses | Female/Male | 3 | Human-based | assisting the doctor, to follow the patient, provide control, triage process, perform additional operations * | Triangular (3.0, 15.0, 5.0) |
| Clerks | Female/Male | 2 | Human-based | check-in/check-out | Uniform (3.0, 5.0, 0.0) |
| Beds or Exam Rooms | - | 5 | Location-based | the area reserved for the patient during the treatment or examination process | Triangular (3.0, 15.0, 5.0) ** Uniform (10.0, 30.0, 0.0) |
| Triage areas and equipment | - | 2 | Location-based | the area where the patient’s first health check is provided, and the values are measured | Triangular (3.0, 15.0, 5.0) |
| Waiting Room | - | 1 (Limit: 50 seats) | Location-based | the area where patients wait until health resources become available | Triangular (0.0, 0.18, 1.68) *** |
| Parameters | % | Cumulative % | |||||
|---|---|---|---|---|---|---|---|
| 1.00 | 0.80 | 0.60 | 0.50 | 0.40 | 0.00 | 1.00 | |
| 1.20 | 0.96 | 0.72 | 0.60 | 0.48 | 0.20 | 1.20 | |
| 1.56 | 1.25 | 0.94 | 0.78 | 0.62 | 0.30 | 1.30 | |
| 2.34 | 1.87 | 1.40 | 1.17 | 0.94 | 0.50 | 1.50 | |
| Sum | 6.10 | 4.88 | 3.66 | 3.05 | 2.44 | 1.00 | 5.00 |
| Values | Feature 1 | Feature 2 | Feature 3 | ||
|---|---|---|---|---|---|
| 0.844 | −0.726 | ||||
| 0.372 | −0.540 | ||||
| 0.328 | −0.293 | ||||
| −0.015 | −0.246 | ||||
| −0.211 | 0.232 | ||||
| 0.777 | −0.682 | ||||
| 0.456 | −0.538 | ||||
| 0.381 | −0.397 | ||||
| −0.218 | −0.247 | ||||
| −0.210 | 0.227 | ||||
| 0.722 | −0.622 | ||||
| 0.559 | −0.584 | ||||
| 0.299 | −0.381 | ||||
| −0.261 | −0.225 | ||||
| −0.201 | 0.201 | ||||
| 0.774 | −0.627 | ||||
| 0.424 | −0.351 | ||||
| 0.367 | −0.506 | ||||
| −0.275 | −0.281 | ||||
| −0.201 | 0.218 |
| Outputs | Algorithm | Training Dataset | Testing Dataset | ||||||
|---|---|---|---|---|---|---|---|---|---|
| MSE | RMSE | MAE | R2 | MSE | RMSE | MAE | R2 | ||
| RF | 0.4022 * | 0.0634 * | 0.0463 * | 0.9703 | 0.7830 * | 0.0885 * | 0.0699 * | 0.9539 | |
| GB | 0.4433 * | 0.0666 * | 0.0511 * | 0.9672 | 0.8457 * | 0.0920 * | 0.0721 * | 0.9502 | |
| AB | 0.2185 * | 0.0467 * | 0.0307 * | 0.9838 | 0.3107 * | 0.0557 * | 0.0385 * | 0.9817 | |
| RF | 0.0089 | 0.0943 | 0.0704 | 0.9275 | 0.0096 | 0.0981 | 0.0732 | 0.9216 | |
| GB | 0.0098 | 0.0988 | 0.0746 | 0.9205 | 0.0098 | 0.0988 | 0.0746 | 0.9205 | |
| AB | 0.0062 | 0.0789 | 0.0566 | 0.9492 | 0.0062 | 0.0789 | 0.0566 | 0.9492 | |
| Outputs | Algorithm | Training Dataset | Testing Dataset | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MSE | RMSE | MAE | R2 | MSE | RMSE | MAE | R2 | |||
| RF | 0.4194 | 0.0648 | 0.0466 | 0.9690 | 3.3397 | 0.1827 | 0.1395 | 0.7620 | ||
| GB | 0.4433 | 0.0666 | 0.0511 | 0.9672 | 2.8690 | 0.1694 | 0.1315 | 0.7956 | ||
| AB | 0.2185 | 0.0467 | 0.0307 | 0.9838 | 3.7999 | 0.1949 | 0.1491 | 0.7292 | ||
| RF | 0.4086 | 0.0639 | 0.0446 | 0.9709 | 1.4427 | 0.1201 | 0.0891 | 0.8972 | ||
| GB | 0.4390 | 0.0663 | 0.0498 | 0.9687 | 0.9857 | 0.0993 | 0.0759 | 0.9298 | ||
| AB | 0.2207 | 0.0470 | 0.0306 | 0.9843 | 1.6923 | 0.1301 | 0.0915 | 0.8794 | ||
| RF | 0.4273 | 0.0654 | 0.0466 | 0.9684 | 2.9333 | 0.1713 | 0.1387 | 0.7910 | ||
| GB | 0.4433 | 0.0666 | 0.0511 | 0.9672 | 2.4027 | 0.1550 | 0.1285 | 0.8288 | ||
| AB | 0.2185 | 0.0467 | 0.0307 | 0.9838 | 1.8117 | 0.1346 | 0.0945 | 0.8709 | ||
| RF | 0.4127 | 0.0642 | 0.0460 | 0.9706 | 6.2546 | 0.2501 | 0.2116 | 0.5543 | ||
| GB | 0.4390 | 0.0663 | 0.0498 | 0.9687 | 5.7007 | 0.2388 | 0.2068 | 0.5938 | ||
| AB | 0.2160 | 0.0465 | 0.0307 | 0.9846 | 3.6574 | 0.1912 | 0.1506 | 0.7394 | ||
| RF | 0.0089 | 0.0944 | 0.0690 | 0.9300 | 0.0150 | 0.1226 | 0.0917 | 0.8818 | ||
| GB | 0.0094 | 0.0972 | 0.0738 | 0.9258 | 0.0150 | 0.1225 | 0.0929 | 0.8820 | ||
| AB | 0.0062 | 0.0787 | 0.0571 | 0.9514 | 0.0193 | 0.1390 | 0.0996 | 0.8482 | ||
| RF | 0.0086 | 0.0930 | 0.0692 | 0.9320 | 0.0163 | 0.1276 | 0.0948 | 0.8720 | ||
| GB | 0.0094 | 0.0972 | 0.0738 | 0.9258 | 0.0150 | 0.1225 | 0.0929 | 0.8821 | ||
| AB | 0.0061 | 0.0784 | 0.0578 | 0.9517 | 0.0197 | 0.1403 | 0.1004 | 0.8454 | ||
| RF | 0.0085 | 0.0923 | 0.0666 | 0.9331 | 0.0162 | 0.1272 | 0.0939 | 0.8728 | ||
| GB | 0.0094 | 0.0972 | 0.0738 | 0.9258 | 0.0150 | 0.1225 | 0.0929 | 0.8819 | ||
| AB | 0.0062 | 0.0787 | 0.0571 | 0.9514 | 0.0193 | 0.1390 | 0.0996 | 0.8482 | ||
| RF | 0.0083 | 0.0914 | 0.0677 | 0.9344 | 0.0151 | 0.1231 | 0.0928 | 0.8810 | ||
| GB | 0.0094 | 0.0972 | 0.0738 | 0.9258 | 0.0150 | 0.1225 | 0.0929 | 0.8818 | ||
| AB | 0.0062 | 0.0787 | 0.0571 | 0.9514 | 0.0193 | 0.1390 | 0.0996 | 0.8482 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atalan, A.; Şahin, H.; Atalan, Y.A. Integration of Machine Learning Algorithms and Discrete-Event Simulation for the Cost of Healthcare Resources. Healthcare 2022, 10, 1920. https://doi.org/10.3390/healthcare10101920
Atalan A, Şahin H, Atalan YA. Integration of Machine Learning Algorithms and Discrete-Event Simulation for the Cost of Healthcare Resources. Healthcare. 2022; 10(10):1920. https://doi.org/10.3390/healthcare10101920
Chicago/Turabian StyleAtalan, Abdulkadir, Hasan Şahin, and Yasemin Ayaz Atalan. 2022. "Integration of Machine Learning Algorithms and Discrete-Event Simulation for the Cost of Healthcare Resources" Healthcare 10, no. 10: 1920. https://doi.org/10.3390/healthcare10101920








