1. Introduction
To assess the canal vestibular function in patients with acute vestibular syndrome (AVS) it is important to differentiate Vestibular Neuritis (VN) from a cerebellar or brainstem stroke [
1]. Indeed, combining the video Head Impulse Test (vHIT) with the HINTS protocol (Head-Impulse—Nystagmus—Test-of-Skew) could help clinicians in finding false positives for stroke [
2]. The evaluation of the Vestibulo Ocular Reflex (VOR) through the vHIT is nowadays a routine clinical test to measure the VOR gain and the correct eye movements during an unpredictable passive head turn [
3]. Specifically, two evaluation paradigms are available to study the canal vestibular function, the Head Impulse Paradigm (HIMP) and the Suppression Head Impulse Paradigm (SHIMP). In the first case, with 100% of sensitivity and specificity, the clinician can assess the functional condition of each semicircular canal, while the person maintains the fixation on an earth-fixed target during small, abrupt, passive, unpredictable impulsive turns following the plane of the tested canal [
4]. In the second paradigm, with the same reliability, it is possible to assess the function of the horizontal semicircular canal VOR slow phase velocity when the person maintains the fixation on a head-fixed target during small, abrupt, passive, unpredictable impulsive turns in the horizontal plane [
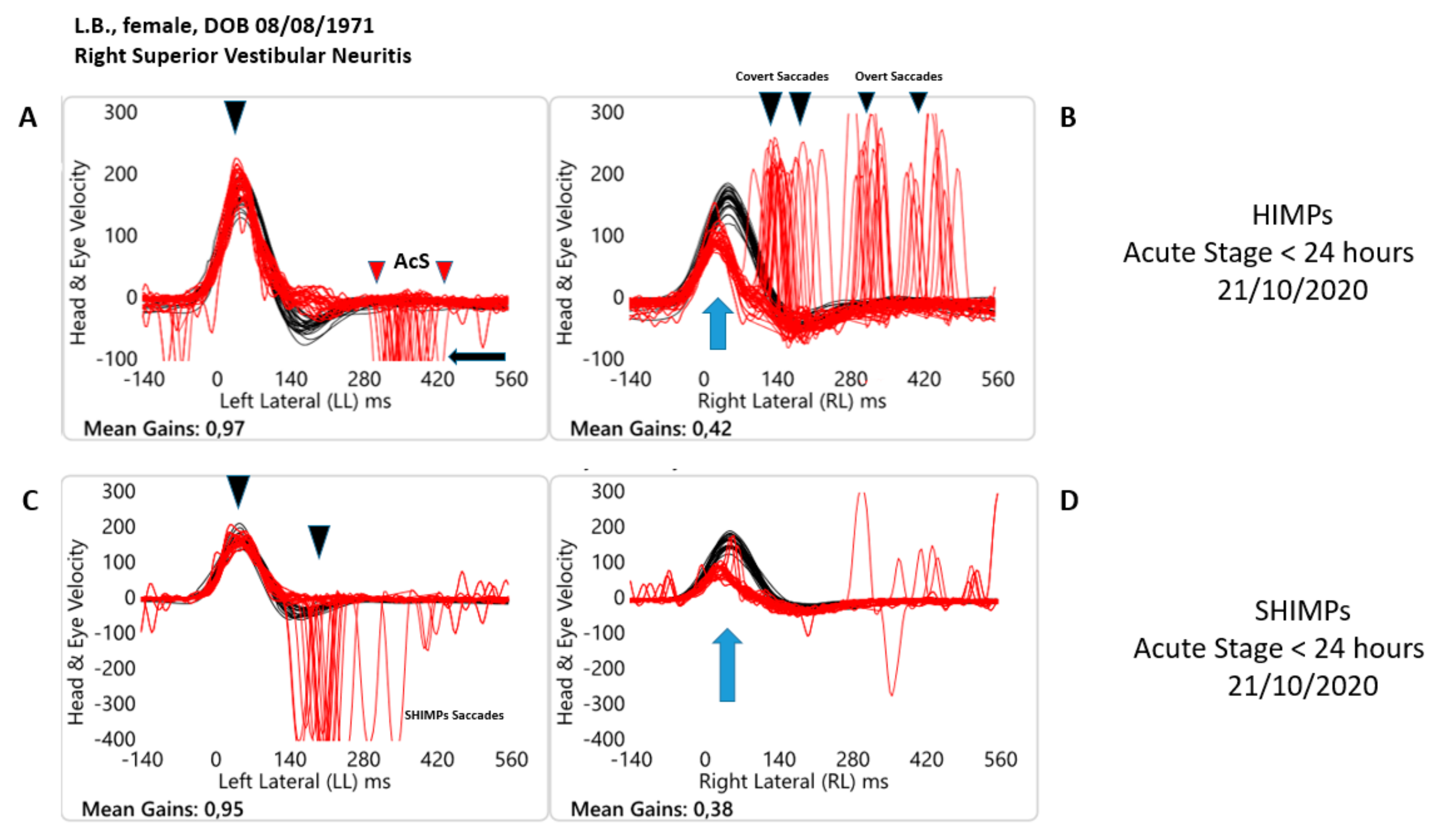
4]. In the case of vestibulopathy, during the HIMP a covert or overt saccade appears during the test, while during the SHIMP the patient does not make a corrective saccade (a “SHIMPs’’saccade) in both unilateral and bilateral vestibulopathy [
5,
6,
7]. Recently, a study [
8] reported the presence of anticompensatory saccades (AcS) also on the healthy side in patients with VN during the HIMP in the acute phase. Indeed, the presence of AcS could be a sign of VOR altered function, similar to what happens on the affected side. Conversely, the presence of the AcS during the SHIMP is a physiological response to regain the target at the end of the head rotation and their reappearance after an AVS is the clinical sign of a probable recovery of the VOR [
9]
Recently it has been argued that SHIMP gives more precise information on the VOR slow phase velocity value compared to HIMP because the evaluation of the gain is not affected by covert saccades [
9,
10]. Several studies [
11,
12] have already suggested the use of vHIT with HIMP to diagnose a VN in the acute phase and as a valid tool to diagnose peripheral vestibulopathy in the different stages of the disease [
3]. A recent systematic review [
13] highlighted the usefulness of the SHIMP in diagnosing a VOR alteration in patients with vestibulopathy suggesting to evaluate with new studies if this new paradigm could replace the HIMP in both the acute and chronic phases of vestibulopathy. For these reasons, we hypothesize that SHIMP could be compared, in terms of usefulness, to the HIMP in evaluating eye movement and visuo-vestibular interaction strategy alteration in patients with acute and chronic VN. Indeed, anti-compensatory saccades and their relationship with the vestibular input could be a useful mechanism for minimizing subjective symptoms after acute vestibular loss through efficient interaction between the visual and vestibular systems. Chen F. and colleagues [
14] performed an association analysis of HIMP and SHIMP quantitative parameters and underlined that the two vHIT-based paradigms potentially provide a comprehensive assessment of vestibular loss and function by measuring VOR gain in patients with VN evaluated within 7 days from the onset.
To date, no studies have compared the simultaneous application of SHIMPs and HIMPs paradigms in different stages of the disease of VN. Thus, this study aims to investigate the clinical usefulness of the SHIMP in comparison with the HIMP in the acute phase and during the follow-up in a large sample of patients with superior VN.
2. Materials and Methods
2.1. Study Design
This retrospective study compares SHIMP and HIMP to assess the VOR gain values of the horizontal canal across the various stages of the VN. The STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) principles were followed when conducting the study. All patients gave written consent to publish the results obtained from their clinical examinations and instrumental tests.
2.2. Setting
Medical records of patients with a diagnosis of VN in the early stages of the disease (from the first hours to six weeks since the AVS) and still symptomatic who were admitted to the ENT MSA Academy Center from January 2020 to June 2022 were reviewed.
2.3. Participants
Patients who had a diagnosis of VN in the early stages (within 72 h of the AVS) met the inclusion criteria. Patients with another vestibular diagnosis (more than six weeks since the AVS, Ménière’s Disease, bilateral vestibular loss, vestibular migraine, benign paroxysmal positional vertigo, acoustic neuroma, superior semicircular canal dehiscence syndrome), somatic or psychiatric disorders or neurological diseases were not included in the study. All patients underwent a vestibular evaluation that included a bedside Head Impulse Test + video-Head Impulse Test, Air Conducted Sound, and Bone Conducted Vibration Cervical and Ocular VEMPs, an evaluation of the horizontal and vertical semicircular canals, and an audiological assessment.
The following criteria were used to diagnose VN: (a) a history of acute onset of severe, protracted, rotatory vertigo, nausea, and postural imbalance; (b) on clinical examination, the presence of horizontal spontaneous nystagmus with a rotational component toward the unaffected ear (fast phase); (c) abnormal bed-side HIT showing an ipsilateral deficit of the horizontal semicircular canal; [
4]; (d) alterations in the cervical and ocular VEMPs results compatible with the diagnosis of Vestibulopathy (e) an MRI was performed at least 48 h after the AVS onset, and data saved in the medical records of the patients [
15,
16]. After 30 days and during the acute phase (approximately 72 h), patients were examined.
2.4. Video Head Impulse Test
Using horizontal vHIT (OtosuiteV
®, GN Otometrics, Taastrup, Denmark), the semicircular canals’ function was assessed. Gain occurs during the VOR slow phase in the HIMP and SHIMP paradigms. The gain value of 0.76 has 100% specificity (48–100) and 100% sensitivity in identifying the affected side of Unilateral VN (74–100). The area under the curve (AUC) during HIMP is 1.0 (0.81–1.0,
p < 0.0001), and VOR gain is the ratio of that value [
4].
With 100% sensitivity (48–100) and 100% specificity (74–100) and an AUC of 1.0 (0.81–1.0,
p < 0.0001), SHIMP gains (0.66) detected the affected side of Unilateral VN [
9]. Comparatively, Anticompensatory Saccades (AcS) or fast eye movement on the contralesional side were defined as saccades (peak velocity exceeding 50°/s) in the direction of the head movement. Compensatory saccades on the affected side were defined as saccades in the direction of eye movement. AcS start was at 10°/s, latency was the difference between head impulse and AcS start. AcS occurrence rate was the percentage of impulses with AcS [
17].
2.5. Statistical Analysis
Statistical analyses were carried out using the IBM SPSS Statistic Software Version 23, IBM Corp., Armonk, NY, USA. Based on the skewness and kurtosis statistics, the variables were judged to be normally distributed when the skew and kurtosis levels were <|2.0| and <|9.0|, respectively [
18,
19]. Descriptive statistics were calculated for all variables of interest, and data are presented as mean and standard deviation. A paired samples
t-test was performed to analyze differences in the VOR gain during SHIMP and HIMP paradigms in the acute phase (T0) and during a follow-up (T1). Diagnostic accuracy for the two vHIT paradigms was performed using the Receiver Operating Characteristic (ROC) curve analysis with MedCalc software (Ostend, Belgium). The significance level was set at
p < 0.05.
3. Results
Forty-two patients (16 females, mean age 51.06 ± 12.96; 26 male, mean age 62.50 ±9.82) met the inclusion criteria and were enrolled in the study. Clinical and demographic characteristics are reported in
Table 1.
Based on skewness and kurtosis statistics, data were normally distributed for SHIMP in the lesional (−1.27 and 1.95) and contralesional side (−0.27 and −0.53), respectively; and for HIMP in the lesional (−1.03 and 2.33) and contralesional side (−0.85 and 2.43).
The means of the VOR gain for both paradigms were respectively 0.38 ± 0.12 (SHIMP) and 0.46 ± 0.13 (HIMP) at T0, and 0.55 ± 0.20 (SHIMP) and 0.64 ± 0.19 (HIMP) at T1 for the lesional side. For the contralesional side, 0.85 ± 0.12 (SHIMP) and 0.91 ± 0.14 (HIMP) at T0, and 0.91 ± 0.12 (SHIMP) and 0.99 ± 0.13 (HIMP) at T1.
Participants showed a significant larger VOR gain values with HIMP when compared with SHIMP (∆ 0.08 ± 0.08) at T0 for the lesional,
t(41) = −6.54,
p < 0.001,
d = 0.60; and contralesional sides,
t(41) = −3.34,
p = 0.002,
d = 0.47 (see
Figure 1).
Similarly, significant larger VOR gain values for HIMP were found at T1 for the lesional,
t(41) = −7.87,
p < 0.001,
d = 0.45; and contralesional sides
t(41) = −5.52,
p < 0.001,
d = 0.63 when compared to SHIMP (∆ 0.09 ± 0.01) (see
Figure 2 and
Figure 3). VOR gain descriptive statistics are reported in
Table 2.
For the HIMP, the gain value <0.76 identified the affected side of VN with 100% sensitivity (92–100) and 100% specificity (91–100). For the SHIMP, the gain value <0.66 identified the affected side of VN with 100% sensitivity (92–100) and 100% specificity (91–100) and an AUC of 1.0 (0.96–1.0, p < 0.0001). During both paradigms, the VOR gain represented the ratio of the area under the curve (AUC) of 1.0 (0.95–1.0, p < 0.0001).
4. Discussion
This study aimed to investigate the clinical usefulness of the SHIMP paradigm in comparison with HIMP in the acute phase and during the follow-up in patients with VN.
HIMP and SHIMP are two different paradigms for the clinical evaluation of the state of health of the vestibulo-oculomotor reflex (HIMP) and in addition to this also for the evaluation of the visual and vestibular interaction (SHIMP).
Our results confirm that both paradigms had a 100% sensitivity and specificity in the evaluation of the VOR function in patients with VN during the acute phase and after at least 30 days from the onset. Indeed, the mean of the VOR gain values was <0.76 for the HIMP and <0.66 for the SHIMP paradigms for the affected side. In the same way during acute and subacute or chronic stages, the paradigms present among themselves have different values of the VOR gain.
The angular velocity of the slow phase of the VOR in the HIMP paradigm presents values greater than those highlighted with the SHIMP and this seems to confirm the hypothesis that the contribution of the covert saccades is decisive in recording that type of value in the case of the classic paradigm proposed by Halmagyi and Curthoys [
4,
5]. For this reason, the execution of the SHIMP paradigm appears to be able to better evaluate the effective value of the VOR gain. In both acute and chronic stages, the paradigms present different values of the VOR gain. The difference between the two paradigms of the VOR gain mean at T0 is 0.08 ± 0.01 and 0.09 ± 0.01 at T1. From a clinical point of view, this result is a relevant and interesting fact because the evaluation of the angular velocity of the slow phase in both paradigms is lower than the physiological range and at the same time both paradigms can highlight this deficit during a VN.
Interestingly, this difference was also found for the contralesional side at T0 (0.06 ± 0.02) and T1 (0.08 ± 0.00). Indeed, since its first description, the SHIMP paradigm has highlighted some peculiarities due to the difference in execution compared to the HIMP paradigm. The two peculiarities were essentially characterized in healthy people by the presence of anticompensatory saccades at the end of the head turns and these saccades were considered indicators of vestibular function.
MacDougall and colleagues [
4] have demonstrated that in SHIMPs, the gain of the VOR was slightly lower than HIMPs gains also on the contralesional side as confirmed by our results. From our point of view, we can hypothesize that this difference can be due to two kinds of factors.
Firstly, healthy people can, after a delay, suppress their slow phase eye velocity response elicited by semicircular canal stimulation in the SHIMP paradigm. This phenomenon has been well described by Crane and Demer [
20]. For this reason, it is necessary that the person executes a refixation saccade in the direction of the movement of the head at the end of the head turns to bring the aim back to the fovea.
Secondly, the de-saccading algorithm [
21], which vHIT technology uses to remove the catch-up saccades during the time window for VOR gain measurements, may be responsible for this systematic difference between the two paradigms. Our results confirm MacDougall and Colleagues [
21] hypothesis that “...this, in turn, would be an additional argument in favor of the new SHIMP paradigm, as it usually delays any saccades until after the end of the head impulse….” These two characteristics that differentiate the new paradigm from the older are particularly intriguing for researchers and clinicians. Therefore, it appears reductive to consider only the compensatory saccades data in the clinical evaluation process of a patient affected by AVS.
Strengths and Limitations
To the best of our knowledge, this is the first study with a large sample aiming to compare the two paradigms in patients with VN, but further studies are needed and routine application will be necessary to acquire more experience about the clinical utility of SHIMP at the bedside. We are aware that this study presents some limitations that should be mentioned. First, this is a retrospective study, with intrinsic potential bias. Second, we did not recruit children. Third, vHIT was performed using only the OtosuiteV® tool but more instruments should be compared.
5. Conclusions
Our results confirm that the SHIMP paradigm has a diagnostic accuracy equal to the classic HIMP paradigm in patients with VN. This confirmation comes from the data of the angular velocity of the slow phase of the VOR and therefore the SHIMP paradigm can replace HIMP with the same clinical efficacy. The clinician can formulate a diagnosis and follow the trend of the recovery of the VOR gain even just using the SHIMP paradigm.
Author Contributions
Conceptualization, L.M. and M.T.; methodology, L.M.; data curation, A.S.O.B.; writing—original draft preparation, M.T.; writing—review and editing, A.A.P. and A.S.O.B.; supervision, L.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of ENT MSA Academy Center with protocol number IRB/MSAENT20220429.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patients to publish this paper.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kattah, J.C.; Talkad, A.V.; Wang, D.Z.; Hsieh, Y.H.; Newman-Toker, D.E. HINTS to diagnose stroke in the acute vestibular syndrome: Three-step bedside oculomotor examination more sensitive than early MRI diffusion-weighted imaging. Stroke 2009, 40, 3504–3510. [Google Scholar] [CrossRef] [PubMed]
- Mantokoudis, G.; Tehrani, A.S.; Wozniak, A.; Eibenberger, K.; Kattah, J.C.; Guede, C.I.; Zee, D.S.; Newman-Toker, D.E. VOR gain by head impulse video-oculography differentiates acute vestibular neuritis from stroke. Otol. Neurotol. 2015, 36, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Manzari, L.; Princi, A.A.; De Angelis, S.; Tramontano, M. Clinical value of the video head impulse test in patients with vestibular neuritis: A systematic review. Eur. Arch. Oto-Rhino-Laryngol. 2021, 278, 4155–4167. [Google Scholar] [CrossRef] [PubMed]
- MacDougall, H.G.; McGarvie, L.A.; Halmagyi, G.M.; Rogers, S.J.; Manzari, L.; Burgess, A.M.; Curthoys, I.S.; Weber, K.P. A new saccadic indicator of peripheral vestibular function based on the video head impulse test. Neurology 2016, 87, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Halmagyi, G.M.; Curthoys, I.S. A clinical sign of canal paresis. Arch. Neurol. 1988, 45, 737–739. [Google Scholar] [CrossRef] [PubMed]
- MacDougall, H.G.; Weber, K.P.; McGarvie, L.A.; Halmagyi, G.M.; Curthoys, I.S. The video head impulse test: Diagnostic accuracy in peripheral vestibulopathy. Neurology 2009, 73, 1134–1141. [Google Scholar] [CrossRef] [PubMed]
- De Waele, C.; Shen, Q.; Magnani, C.; Curthoys, I.S. A Novel Saccadic Strategy Revealed by Suppression Head Impulse Testing of Patients with Bilateral Vestibular Loss. Front. Neurol. 2017, 8, 419. [Google Scholar] [CrossRef] [PubMed]
- Manzari, L.; Tramontano, M. Identifying the Clinical Signs on the Healthy Side Using Video Head Impulse Test During Different Stages of Vestibular Neuritis. J. Int. Adv. Otol. 2021, 17, 433–437. [Google Scholar] [CrossRef] [PubMed]
- Manzari, L.; Tramontano, M. Suppression Head Impulse Paradigm (SHIMP) in evaluating the vestibulo-saccadic interaction in patients with vestibular neuritis. Eur. Arch. Oto-Rhino-Laryngol. 2020, 277, 3205–3212. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.; Magnani, C.; Sterkers, O.; Lamas, G.; Vidal, P.P.; Sadoun, J.; Curthoys, I.S.; de Waele, C. Saccadic Velocity in the New Suppression Head Impulse Test: A New Indicator of Horizontal Vestibular Canal Paresis and of Vestibular Compensation. Front. Neurol. 2016, 7, 160. [Google Scholar] [CrossRef] [PubMed]
- Guan, Q.; Zhang, L.; Hong, W.; Yang, Y.; Chen, Z.; Lu, P.; Zhang, D.; Hu, X. Video Head Impulse Test for Early Diagnosis of Vestibular Neuritis Among Acute Vertigo. Can. J. Neurol. Sci. 2017, 44, 556–561. [Google Scholar] [CrossRef] [PubMed]
- Roberts, H.N.; McGuigan, S.; Infeld, B.; Sultana, R.V.; Gerraty, R.P. A video-oculographic study of acute vestibular syndromes. Acta Neurol. Scand. 2016, 134, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Manzari, L.; De Angelis, S.; Princi, A.A.; Galeoto, G.; Tramontano, M. The Clinical Use of the Suppression Head Impulse Paradigm in Patients with Vestibulopathy: A Systematic Review. Healthcare 2022, 10, 1182. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Chen, Z.; Zhang, Y.; Wei, X.; Zhao, H.; Hu, J.; Cheng, Y.; Ren, X.; Zhang, Q. Association Analysis of HIMP and SHIMP Quantitative Parameters in Patients With Vestibular Neuritis and Healthy Participants. Front. Neurol. 2021, 12, 748990. [Google Scholar] [CrossRef] [PubMed]
- Manzari, L.; Graziano, D.; Tramontano, M. The Different Stages of Vestibular Neuritis from the Point of View of the Video Head Impulse Test. Audiol. Res. 2020, 10, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Manzari, L.; Koch, G.; Tramontano, M. Selective Asymmetry of Ocular Vestibular-Evoked Myogenic Potential in Patients with Acute Utricular Macula Loss. J. Int. Adv. Otol. 2021, 17, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Luis, L.; Lehnen, N.; Muñoz, E.; de Carvalho, M.; Schneider, E.; Valls-Solé, J.; Costa, J. Anticompensatory quick eye movements after head impulses: A peripheral vestibular sign in spontaneous nystagmus. J. Vestib. Res. 2016, 25, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Posten, H.O. Robustness of the Two-Sample T-Test. In Robustness of Statistical Methods and Nonparametric Statistics. Theory and Decision Library; Rasch, D., Tiku, M.L., Eds.; Springer: Dordrecht, The Netherlands, 1984; Volume 1. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis. Curr. Dir. Psychol. Sci. 1992, 1, 98–101. [Google Scholar] [CrossRef]
- Crane, B.T.; Demer, J.L. Latency of voluntary cancellation of the human vestibulo-ocular reflex during transient yaw rotation. Exp. Brain Res. 1999, 127, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Macdougall, H.G.; McGarvie, L.A.; Halmagyi, G.M.; Curthoys, I.S.; Weber, K.P. The video Head Impulse Test (vHIT) detects vertical semicircular canal dysfunction. PLoS ONE 2013, 8, e61488. [Google Scholar] [CrossRef] [PubMed]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).