Nutrition of Pregnant and Lactating Women in the First 1000 Days of Infant
Abstract
1. Introduction
2. Material and Methods
3. Results and Comments
3.1. Pregnant Women Nutrition
3.1.1. Energy Needs
3.1.2. Macronutrients
3.1.3. Proteins
3.1.4. Fats
3.1.5. Mineral Salts and Trace Elements
3.1.6. Vitamins
3.2. Lactating Mothers Nutrition
3.2.1. Energy
3.2.2. Proteins
3.2.3. Carbohydrates
3.2.4. Fat’s Composition
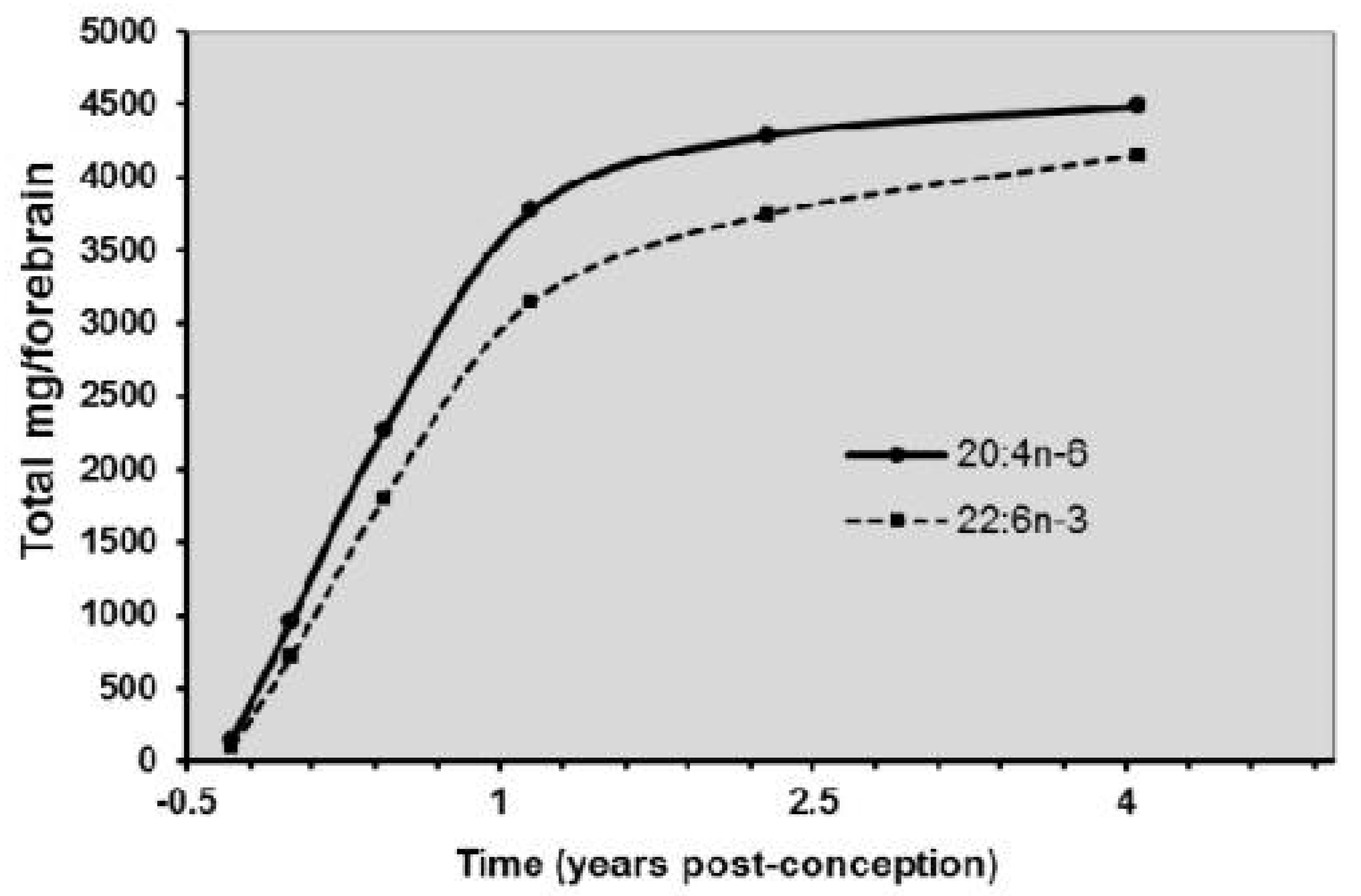
3.2.5. Essential Fatty Acids
3.2.6. Minerals, Vegetables, Oligo-Elements, Vitamins
Vegetables
Minerals–Trace-Elements
Vitamins
Proposal for a Typical Menu for Breastfeeding Woman
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mameli, C.; Mazzantini, S.; Zuccotti, G.V. Nutrition in the First 1000 Days: The Origin of Childhood Obesity. Int. J. Environ. Res. Public Health 2016, 13, 838. [Google Scholar] [CrossRef] [PubMed]
- WHO. Global Action Plan for the Prevention and Control of NCDs 2013–2020; WHO: Geneva, Switzerland, 2013. [Google Scholar]
- Molloy, A.M.; Kirke, P.N.; Brody, L.C.; Scott, J.M.; Mills, J.L. Effects of Folate and Vitamin B12 Deficiencies During Pregnancy on Fetal, Infant, and Child Development. Food Nutr. Bull. 2008, 29, S101–S111, discussion S112–S115. [Google Scholar] [CrossRef]
- Plecas, D.; Plesinac, S.; Kontic-Vucinic, O. Nutrition in pregnancy: Basic principles and recommendations. Srp. Arh. Celok. Lek. 2014, 142, 125–130. [Google Scholar] [CrossRef]
- Emmett, P.M.; Jones, L.R.; Golding, J. Pregnancy diet and associated outcomes in the Avon Longitudinal Study of Parents and Children. Nutr. Rev. 2015, 73 (Suppl. S3), 154–174. [Google Scholar] [CrossRef] [PubMed]
- Billeaud, C.; Roturier, M. Diététique et grossesse:aspects pratiques. Arch. De Pédiatrie 1997, 4 (Suppl. S2), 141s–145s. [Google Scholar] [CrossRef]
- Papoz, L.; Eschwege, E.; Cubeua, J.; Pequignot, G.; Barrat, J.; Le Lorier, G. Comportement alimentaire au cours de la grossesse [Dietary behavior during pregnancy (author’s transl)]. Rev. Epidemiol. Sante Publique 1980, 28, 155–167. [Google Scholar] [PubMed]
- Forsum, E.; Löf, M. Energy Metabolism During Human Pregnancy. Annu. Rev. Nutr. 2007, 27, 277–292. [Google Scholar] [CrossRef]
- Koletzko, B.; Godfrey, K.M.; Poston, L.; Szajewska, H.; Van Goudoever, J.B.; De Waard, M.; Brands, B.; Grivell, R.M.; Deussen, A.R.; Dodd, J.M.; et al. Nutrition during pregnancy, lactation and early childhood and its implications for maternal and long-term child health: The early nutrition project recommendations. Ann. Nutr. Metab. 2019, 74, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Mazurier, E.; Rigourd, V.; Perez, P.; Buffin, R.; Couedelo, L.; Vaysse, C.; Belcadi, W.; Sitta, R.; Nacka, F.; Lamireau, D.; et al. Effects of Maternal Supplementation with Omega-3 Precursors on Human Milk Composition. J. Hum. Lact. 2017, 33, 319–328. [Google Scholar] [CrossRef]
- Lapillonne, A.; Groh-Wargo, S.; Gonzalez, C.H.L.; Uauy, R. Lipid Needs of Preterm Infants: Updated Recommendations. J. Pediatr. 2013, 162 (Suppl. S3), S37–S47. [Google Scholar] [CrossRef]
- Boué, C.; Combe, N.; Billeaud, C.; Mignerot, C.; Entressangles, B.; Thery, G.; Geoffrion, H.; Brun, J.L.; Dallay, D.; Leng, J.J. Trans fatty acids in adipose tissue of French women in relation to their dietary sources. Lipids 2000, 35, 561–566. [Google Scholar] [CrossRef]
- Carlson, S.E. Docosahexaenoic acid supplementation in pregnancy and lactation. Am. J. Clin. Nutr. 2008, 89, 678S–684S. [Google Scholar] [CrossRef]
- Billeaud, C.; Combe, N.; Couedelo, L.; Belcadi, W.; Dallay, D.; Leng, J.-J.; Vaysse, C. Transfatty Acids (TFAs) in Cord Blood and Cord Tissue, in France. J. Food Sci. Eng. 2017, 7, 413–422. [Google Scholar] [CrossRef][Green Version]
- Koletzko, B.; Müller, J. Cis- and Trans-Isomeric Fatty Acids in Plasma Lipids of Newborn Infants and Their Mothers. Neonatology 1990, 57, 172–178. [Google Scholar] [CrossRef]
- Boue, C.; Combe, N.; Billeaud, C.; Entressangles, B. Nutritional implications of trans fatty acids during perinatal period, in French pregnant women. OCL 2001, 8, 68–72. [Google Scholar] [CrossRef]
- Kominiarek, M.A.; Rajan, P. Nutrition Recommendations in Pregnancy and Lactation. Med. Clin. N. Am. 2016, 100, 1199–1215. [Google Scholar] [CrossRef]
- Arshad, M.; Jaberian, S.; Pazouki, A.; Riazi, S.; Rangraz, M.A.; Mokhber, S. Iron deficiency anemia and megaloblastic anemia in obese patients. Rom. J. Intern. Med. 2017, 55, 3–7. [Google Scholar] [CrossRef][Green Version]
- Zhao, W.; Li, X.; Xia, X.; Gao, Z.; Han, C. Iodine Nutrition During Pregnancy: Past, Present, and Future. Biol. Trace Elem. Res. 2018, 188, 196–207. [Google Scholar] [CrossRef]
- Pilz, S.; Zittermann, A.; Obeid, R.; Hahn, A.; Pludowski, P.; Trummer, C.; Lerchbaum, E.; Pérez-López, F.R.; Karras, S.N.; März, W. The Role of Vitamin D in Fertility and during Pregnancy and Lactation: A Review of Clinical Data. Int. J. Environ. Res. Public Health 2018, 15, 2241. [Google Scholar] [CrossRef]
- Humphrey, J.H.; West, K.P.; Sommer, A. Vitamin A deficiency and attributable mortality among under-5-year-olds. Bull. World Health Organ. 1992, 70, 225–232. [Google Scholar]
- Castel, B.; Billeaud, C. Mother’s breastfeeding feeding. Cah. De Nutr. Et De Diet. 2017, 52, 89–93. [Google Scholar] [CrossRef]
- Martin, A. Apports Nutritionnels Conseillés Pour la Population Française; Tec & Doc; CNERNA-CNRS, AFSSA: Paris, France, 2001. [Google Scholar]
- Vaysse, C.; Simon, N.; Tressou, J.; Pasteau, S.; Buaud, B.; Guesnet, P.; Couedelo, L.; Billeaud, C. Polyunsaturated fatty acids consumption in lactating women in France: The INCA 2 study and evolution of essential fatty acids composition in breast milk from 1997 to 2014. In Cahiers de Nutrition et de Diététique; Elsevier Masson: Amsterdam, The Netherlands, 2018; Volume 54, pp. 35–43. [Google Scholar] [CrossRef]
- Cervera, P.; Ngo, J. Dietary guidelines for the breast-feeding woman. Public Health Nutr. 2001, 4, 1357–1362. [Google Scholar] [CrossRef] [PubMed][Green Version]
- American Academy of Pediatrics: A woman’s guide to breastfeeding. Pediatrics 1997, 100, 1035–1039.
- Trumbo, P.; Schlicker, S.; Yates, A.A.; Poos, M.; Food and Nutrition Board of the Institute of Medicine, The National Academies. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein and Amino Acids. J. Am. Diet. Assoc. 2002, 102, 1621–1630. [Google Scholar] [CrossRef]
- Wang, D.; Thielecke, F.; Fleith, M.; Afeiche, M.C.; De Castro, C.A.; Martínez-Costa, C.; Haaland, K.; Marchini, G.; Agosti, M.; Domellöf, M.; et al. Analysis of dietary patterns and nutritional adequacy in lactating women: A multicentre European cohort (ATLAS study). J. Nutr. Sci. 2021, 10. [Google Scholar] [CrossRef]
- Giuffrida, F.; Fleith, M.; Goyer, A.; Samuel, T.M.; Elmelegy-Masserey, I.; Fontannaz, P.; Cruz-Hernandez, C.; Thakkar, S.; Monnard, C.; De Castro, C.A.; et al. Human milk 1 fatty acid composition and its association with maternal blood and adipose tissue fatty acid content in a cohort of women from Europe: European. J. Nutr. 2021. accepted December 2021. [Google Scholar]
- Billeaud, C. Implication des Acides Gras Trans Chez la Femme Enceinte et Allaitante. (Bordeaux 1, 2000). Available online: https://www.theses.fr/121134032 (accessed on 1 October 2000).
- Tressou, J.; Buaud, B.; Simon, N.; Pasteau, S.; Guesnet, P. Very low inadequate dietary intakes of essential n-3 polyunsaturated fatty acids (PUFA) in pregnant and lactating French women: The INCA2 survey. Prostaglandins Leukot. Essent. Fat. Acids 2018, 140, 3–10. [Google Scholar] [CrossRef]
- National Academies of Sciences, Engineering, and Medicine. Nutrition During Pregnancy and Lactation: Exploring New Evidence: Proceedings of a Workshop; The National Academies Press: Washington, DC, USA, 2020. [Google Scholar] [CrossRef]
| Nutrients | W.I.C USA | LECLERC France | PAPOZ France | RDI | Needs |
|---|---|---|---|---|---|
| Energy Kcal/day | 1512–2400 | 2233 | 2136 | 2300 | +100–450 |
| Protein g/day | 68–110 | 85 g 15% | 78 g 15% | 60 g 13% | Excess Quality |
| Fats g/day | 104 g 42% | 103 g 43% | 90 g 35% | Excess Quality | |
| Carbohydrates g/day | 235 g 42% | 211 g 40% | 340 g 55% | +100 Quality |
| Oils | Peanuts | Rapeseed | Hazelnut | Olive | Grapeseed | Soja | Sunflower | Sunflower Oleic |
|---|---|---|---|---|---|---|---|---|
| Saturated FA SFA | 48–66 | 55–62 | 24–32 | 61–80 | 14–22 | 17–26 | 15–25 | 75–83 |
| Monounsaturated MUFA | 49–68 | 56–65 | 25–33 | 32–81 | 15–23 | 18–27 | 16–26 | 75–84 |
| Linoleic Ac C18:2 n-6 | 14–28 | 18–22 | 55–62 | 3–14 | 65–73 | 50–62 | 62–70 | 10–21 |
| Linolenic Ac C18:3 n-3 | <0.3 | 8–10 | <2 | <1 | <0.5 | 4–10 | ≤0.2 | ≤0.3 |
| Polyunsaturated PUFA | 14–28 | 26–32 | 57–64 | 4–15 | 65–73 | 54–72 | 62–70 | 10–22 |
| Oily Fish | Arachidonic Acid (AA) (mg/100 g) | Eicosapentaenoic Acid EPA (mg/100 g) | DHA (mg/100 g) |
|---|---|---|---|
| Mackerel | 70 | 1020 | 1940 |
| Sardine | - | 1250 | 1790 |
| Salmon | 41 | 527 | 842 |
| Albacore Tuna | 42 | 562 | 313 |
| Lean Fish | Arachidonic Acid (AA) (mg/100 g) | EPA (mg/100 g) | DHA (mg/100 g) |
| Wild Sea Bar | - | 220 | 293 |
| Cod | <10 | 77 | 194 |
| Dab | <14 | 102 | 189 |
| Sole | <11 | 19 | 81 |
| Nutrients | W.I.C USA | LECLERC France | PAPOZ France | RDI | Needs |
|---|---|---|---|---|---|
| Calcium mg/d | 668–1670 | 975 | 869 | 1000 | 200 |
| Magnesium mg/d | 187–269 | 339 | 260 | 480 | 300 |
| Iron mg/d | 11.4–17 | 13.7 | 12.4 | 30 | 30 |
| Zinc mg/d | 6–12 | 19 | 15 |
| Nutrients | W.I.C USA | LECLERC France | PAPOZ France | RDI | Needs |
|---|---|---|---|---|---|
| Vit D mg | 3–5 | 3.4 | 10 | +10 | |
| Vit B1 mg/day | 1.2–1.7 | 1.3 | 1.3 | 1.5 | +2.5 |
| Vit B6 mg/day | 0.6–2.1 | 1.6 | 1.7 | 2.5 | +2 |
| Folates (B9) mg/day | 144–243 | 255 | 53 | 500 | +300 |
| Fatty Acids % | 1997 PhD n = 18 | 2007 JFRN n = 142 | 2012 Barcelona n = 22 | 2014 [10] (1) PHRC ω3 n = 80 | 2014 [10] (2) PHRC ω3 n = 80 | 2020 [29] ATLAS n = 85 |
|---|---|---|---|---|---|---|
| ALA | 0.52 (0.2) b | 0.83 (0.14) b | 0.86 (001) | 0.96 (0.50) a | 2.15 (0.74) a | 0.93 (0.26) |
| LA | 13.33 (19.62) b | 11.14 (10.24) | 9.27 (0.34) b | 10.03 (3.0) | 10.77 (2.11) | 10.51 (1.46) |
| LA/ALA | 27.63 b | 13.42 b | 10.77 | 10.73 a | 5.54 (2.11) a | 11.30 |
| EPA | 0.08 (0.003) | 0.07 (0.002) | 0.06 (0.0001) | 0.09 (0.05) a | 0.17 (0.01) a | 0.09 (0.03) |
| DHA | 0.26 (0.01) | 0.24 (0.01) | 0.24 (0.003) | 0.29 (0.16) a | 0.56 (0.40) a | 0.33 (0.11) |
| AA | 0.38 (0.05) | 0.40 (0.01) | 0.39 (0.001) | 0.36 (0.07) a | 0.33 (0.22) a | 0.43 (0.07) a |
| AA/DHA | 1.46 | 1.67 | 1.63 | 1.24 a | 0.82 (0.15) a | 1.52 |
| SFA | 48.05 (27.67) | 47.50 (27.98) | 48.84 (4.28) | 46.71 (4.38) | 43.76 (3.88) | 43.96 (0.59) |
| MUFA | 32.80 (13.39) | 37.76 (15.84) | 38.60 (2.62) | 39.72 (3.12) | 40.60 (7,63) | 43.1 (3.79) |
| PUFA | 15.35 (21.72) | 13.45 (11.83) | 11.64 (0.45) | 12.54 (3.3) a | 14.70 (2.7) a | 12.95 (2.6) |
| TFAs | 2.10 (0.62) b | 1.30 (0.36) b | 0.92 (0.09) b | 1.03 (0.29) | 0.94 (0.27) | - |
| Nutrients | Pregnancy | Lactancy | ||
|---|---|---|---|---|
| Intake | RDI | Intake | RDI | |
| Proteins % TEI | 18 | 13 | 17 | 20 |
| Fats % TEI | 44 | 35 | 43 | 40 |
| SFA % TEI | 13 | ≤ | 15 | 12 |
| MUFA% TEI | 13 | 15 | 8 | 20 |
| ω6 PUFA % TEI | 4.1 | = | 3.9 | 4 |
| ω3 PUFA % TEI | 0.4 | = | 0.3 | 1 |
| ω6/ω3 | 10 | ≤ | 13 | 5 |
| Carbohydrates % TEI | 38 | 40 | 40 | 55 |
| Simple carbohydrates % TEI | 14 | ≤ | 18 | 10 |
| Fibers (g) | 12 | 25 | 20 | 30 |
| Alcohol (g) | 3 | = | 5 | 0 |
| Calcium (mg) | 900 | 1000 | 800 | 1000 |
| Magnesium (mg) | 200 | 400 | 250 | 390 |
| Iron (mg) | 14 | 30 | 9.5 | 10 |
| Zinc (mg) | 5 | 14 | 9 | 19 |
| Vitamin D (µg) | 2.4 | 1.9 | 10 | |
| Vitamin B6 (mg) | 1.5 | = | 1 | 2 |
| Vitamin B9 (µg) | 154 | = | 242 | 400 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Billeaud, C.; Brines, J.; Belcadi, W.; Castel, B.; Rigourd, V. Nutrition of Pregnant and Lactating Women in the First 1000 Days of Infant. Healthcare 2022, 10, 65. https://doi.org/10.3390/healthcare10010065
Billeaud C, Brines J, Belcadi W, Castel B, Rigourd V. Nutrition of Pregnant and Lactating Women in the First 1000 Days of Infant. Healthcare. 2022; 10(1):65. https://doi.org/10.3390/healthcare10010065
Chicago/Turabian StyleBilleaud, Claude, Juan Brines, Wafae Belcadi, Bérénice Castel, and Virginie Rigourd. 2022. "Nutrition of Pregnant and Lactating Women in the First 1000 Days of Infant" Healthcare 10, no. 1: 65. https://doi.org/10.3390/healthcare10010065
APA StyleBilleaud, C., Brines, J., Belcadi, W., Castel, B., & Rigourd, V. (2022). Nutrition of Pregnant and Lactating Women in the First 1000 Days of Infant. Healthcare, 10(1), 65. https://doi.org/10.3390/healthcare10010065








