Virucidal Activity of Different Mouthwashes Using a Novel Biochemical Assay
Abstract
:1. Introduction
2. Methods
2.1. Virus Samples and Products
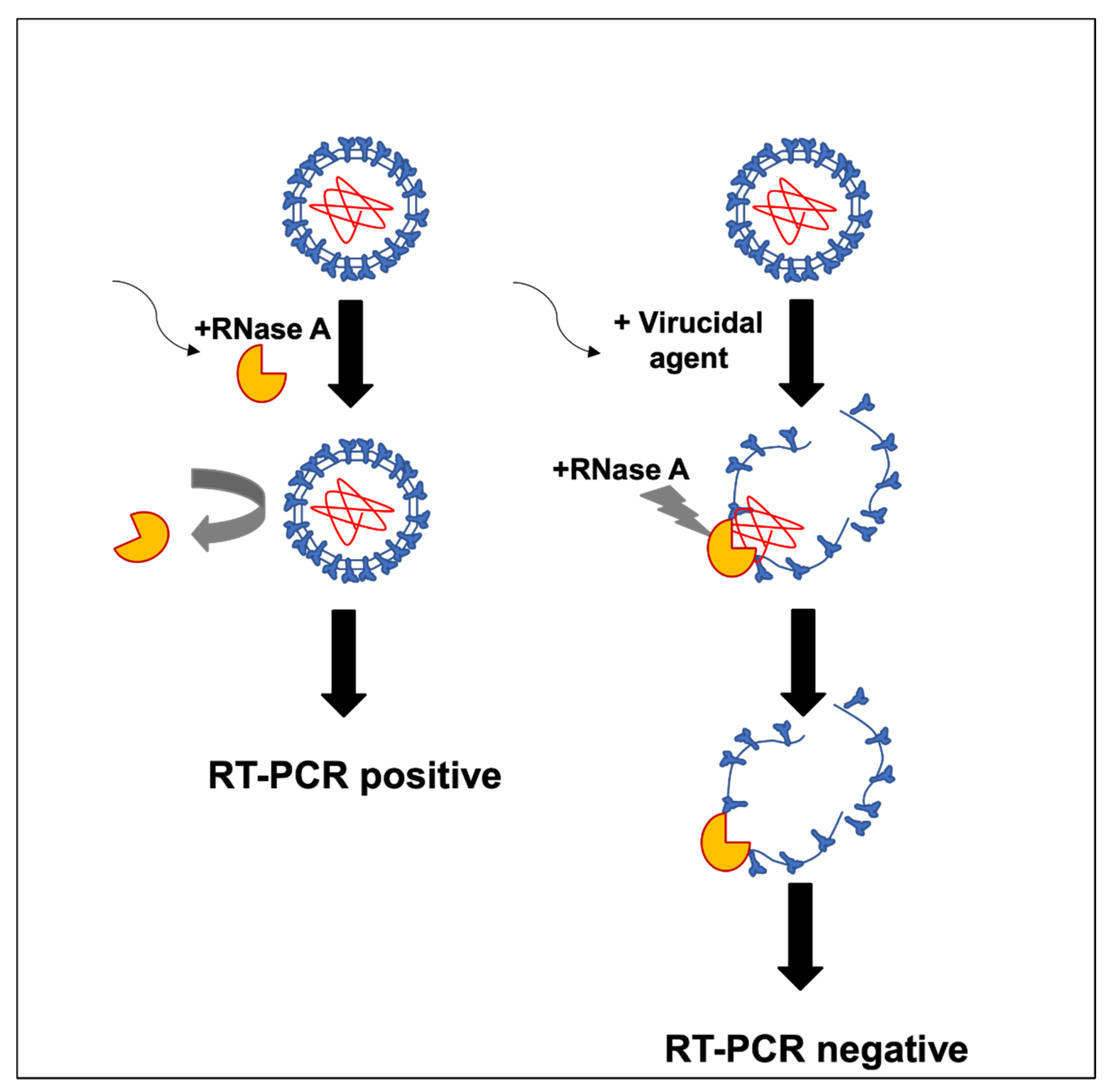
2.2. RNA Inactivating Assay
2.3. RT-qPCR
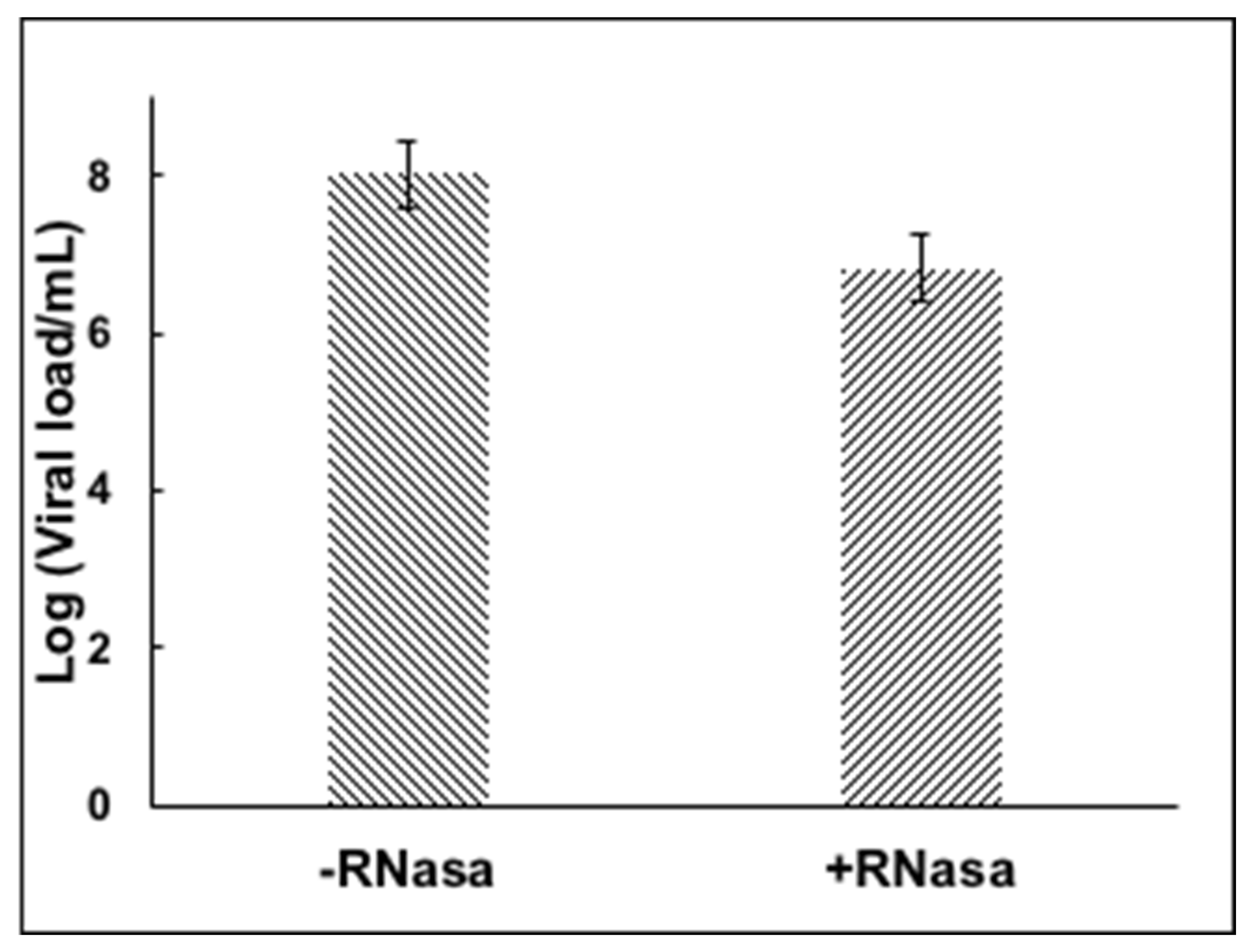
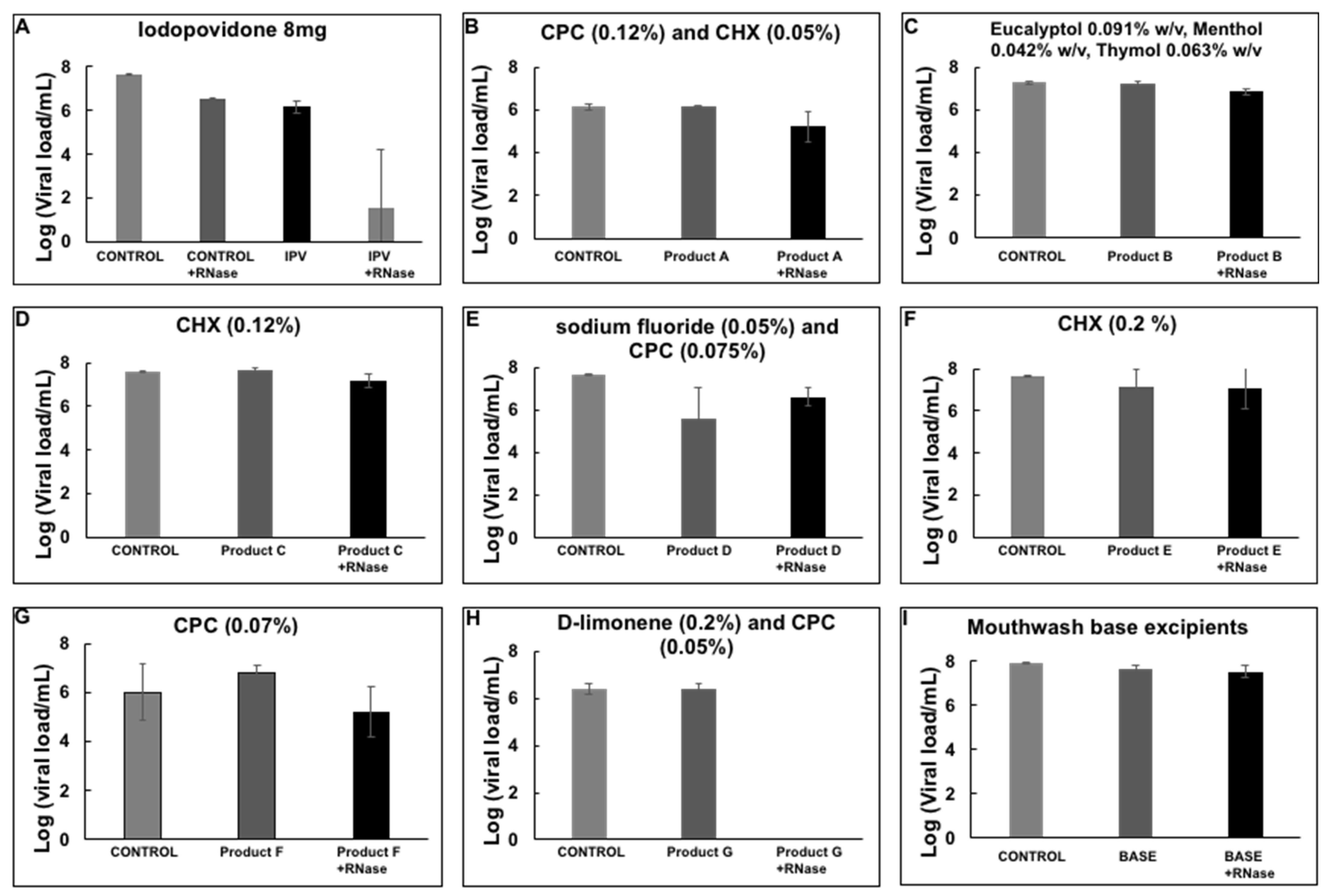
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| * | Isodine® Laboratorio Bussie. |
| † | Noncommercial mouthwash donated by Laboratorio Brix Medical Science. |
| ‡ | Noncommercial mouthwash donated by Laboratorio Brix Medical Science. |
| § | Noncommercial mouthwash donated by Laboratorio Brix Medical Science. |
| ** | Perio-Aid® Laboratorio Dentaid. |
| †† | Listerine® Zero Alcohol. Johnson & Johnson. |
| ‡‡ | Scope® Procter & Gamble. |
| §§ | Xyntrus® Laboratorio Brix Medical Science. |
| *** | Plax® Colgate. |
| ††† | Periogard® Colgate. |
| ‡‡‡ | Clorhexol® Laboratorios Farpag. |
References
- World Health Organization. WHO Coronavirus Disease (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 9 August 2021).
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Tan, W. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Zeidler, A.; Karpinski, T.M. SARS-CoV, MERS-CoV, SARS-CoV-2 comparison of three emerging Coronaviruses. Jundishapur J. Microbiol. 2020, 13, e103744. [Google Scholar] [CrossRef]
- Liu, J.; Liao, X.; Qian, S.; Yuan, J.; Wang, F.; Liu, Y.; Wang, Z.; Wang, F.S.; Liu, L.; Zhang, Z. Community Transmission of Severe Acute Respiratory Syndrome Coronavirus 2, Shenzhen, China, 2020. Emerg. Infect. Dis. 2020, 26, 1320–1323. [Google Scholar] [CrossRef] [PubMed]
- Morawska, L.; Cao, J. Airborne transmission of SARS-CoV-2: The world should face the reality. Environ. Int. 2020, 139, 105730. [Google Scholar] [CrossRef]
- van Doremalen, N.; Bushmaker, T.; Morris, D.H.; Holbrook, M.G.; Gamble, A.; Williamson, B.N.; Tamin, A.; Harcourt, J.L.; Thornburg, N.J.; Gerber, S.I.; et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N. Engl. J. Med. 2020, 382, 1564–1567. [Google Scholar] [CrossRef]
- Wang, W.; Xu, Y.; Gao, R.; Lu, R.; Han, K.; Wu, G.; Tan, W. Detection of SARS-CoV-2 in Different Types of Clinical Specimens. JAMA 2020, 323, 1843–1844. [Google Scholar] [CrossRef] [Green Version]
- Chang, L.; Zhao, L.; Gong, H.; Wang, L.; Wang, L. Severe Acute Respiratory Syndrome Coronavirus 2 RNA Detected in Blood Donations. Emerg. Infect. Dis. 2020, 26, 1631–1633. [Google Scholar] [CrossRef]
- Breastfeeding and COVID-19. World Health Organization: Geneva, Switzerland. 2020. Available online: https://www.who.int/news-room/commentaries/detail/breastfeeding-and-covid-19 (accessed on 9 August 2021).
- Karia, R.; Gupta, I.; Khandait, H.; Yadav, A.; Yadav, A. COVID-19 and its Modes of Transmission. SN Compr. Clin. Med. 2020, 2, 1798–1801. [Google Scholar] [CrossRef]
- Andersen, K.G.; Rambaut, A.; Lipkin, W.I.; Holmes, E.C.; Garry, R.F. The proximal origin of SARS-CoV-2. Nat. Med. 2020, 26, 450–452. [Google Scholar] [CrossRef] [Green Version]
- Zhou, P.; Yang, X.L.; Wang, X.G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.R.; Zhu, Y.; Li, B.; Huang, C.L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef] [Green Version]
- WHO. Transmission of SARS-CoV-2: Implication for Infection Prevention Precautions. World Health Organization. 2020. Available online: https://www.who.int/news-room/commentaries/detail/transmission-of-sars-cov-2-implications-for-infection-prevention-precautions (accessed on 21 April 2020).
- Chia, P.Y.; Coleman, K.K.; Tan, Y.K.; Ong, S.W.X.; Gum, M.; Lau, S.K.; Marimuthu, K. Detection of air and surface contamination by SARS-CoV-2 in hospital rooms of infected patients. Nat. Commun. 2020, 11, 2800. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, H.; Hao, S.; Chen, Y.; He, J.; Liu, Y.; Chen, L.; Yu, Y.; Hua, S. Digital PCR is a sensitive new technique for SARS-CoV-2 detection in clinical applications. Clin. Chim. Acta 2020, 511, 346–351. [Google Scholar] [CrossRef]
- Liu, Y.; Ning, Z.; Chen, Y.; Guo, M.; Liu, Y.; Gali, N.K.; Sun, L.; Duan, Y.; Cai, J.; Westerdahl, D.; et al. Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals. Nature 2020, 582, 557–560. [Google Scholar] [CrossRef]
- Santarpia, J.L.; Rivera, D.N.; Herrera, V.L.; Morwitzer, M.J.; Creager, H.M.; Santarpia, G.W.; Crown, K.K.; Brett-Major, D.M.; Schnaubelt, E.R.; Broadhurst, M.J.; et al. Aerosol and surface contamination of SARS-CoV-2 observed in quarantine and isolation care. Sci. Rep. 2020, 10, 12732. [Google Scholar] [CrossRef]
- Xu, H.; Zhong, L.; Deng, J.; Peng, J.; Dan, H.; Zeng, X.; Chen, Q. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int. J. Oral Sci. 2020, 12, 8. [Google Scholar] [CrossRef]
- Ge, Z.Y.; Yang, L.M.; Xia, J.J.; Fu, X.H.; Zhang, Y.Z. Possible aerosol transmission of COVID-19 and special precautions in dentistry. J. Zhejiang Univ. Sci. B 2020, 21, 361–368. [Google Scholar] [CrossRef] [Green Version]
- Meethil, A.P.; Saraswat, S.; Chaudhary, P.P.; Dabdoub, S.M.; Kumar, P.S. Sources of SARS-CoV-2 and Other Microorganisms in Dental Aerosols. J. Dent. Res. 2021, 100, 817–823. [Google Scholar] [CrossRef]
- Liu, L.; Wei, Q.; Alvarez, X.; Wang, H.; Du, Y.; Zhu, H.; Chen, Z. Epithelial cells lining salivary gland ducts are early target cells of severe acute respiratory syndrome coronavirus infection in the upper respiratory tracts of rhesus macaques. J. Virol. 2011, 85, 4025–4030. [Google Scholar] [CrossRef] [Green Version]
- To, K.K.; Tsang, O.T.; Yip, C.C.; Chan, K.H.; Wu, T.C.; Chan, J.M.; Leung, W.S.; Chik, T.S.; Choi, C.Y.; Kandamby, D.H.; et al. Consistent detection of 2019 Novel Coronavirus in Saliva. Clin. Infect. Dis. 2020, 71, 841–843. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Li, Y.; Gan, F.; Du, Y.; Yao, Y. Salivary glands: Potential reservoirs for COVID-19 asymptomatic infection. J. Dent. Res. 2020, 99, 989. [Google Scholar] [CrossRef] [Green Version]
- Herrera, D.; Serrano, J.; Roldán, S.; Sanz, M. Is the oral cavity relevant in SARS-CoV-2 pandemic? Clin. Oral Investig. 2020, 24, 2925–2930. [Google Scholar] [CrossRef]
- O’Donnell, V.B.; Thomas, D.; Stanton, R.; Maillard, J.Y.; Murphy, R.C.; Jones, S.A.; Humphreys, I.; Wakelam, M.J.O.; Fegan, C.; Wise, M.P.; et al. Potential role of oral rinses targeting the viral lipid envelope in SARS-CoV-2 infection. Function 2020, 1, zqaa002. [Google Scholar] [CrossRef]
- Seneviratne, C.J.; Balan, P.; Ko, K.; Udawatte, N.S.; Lai, D.; Ng, D.; Venkatachalam, I.; Lim, K.S.; Ling, M.L.; Oon, L.; et al. Efficacy of commercial mouth-rinses on SARS-CoV-2 viral load in saliva: Randomized control trial in Singapore. Infection 2021, 49, 305–311. [Google Scholar] [CrossRef]
- Mateos Moreno, M.V.; Obrador, A.M.; Márquez, V.A.; Ferrer García, M.D. Oral antiseptics against Coronavirus: In vitro and clinical evidence. J. Hosp. Infect. 2021, 113, 30–43. [Google Scholar] [CrossRef]
- Corman, V.M.; Haage, V.C.; Bleicker, T.; Schmidt, M.L.; Mühlemann, B.; Zuchowski, M.; Jo, W.K.; Tscheak, P.; Möncke-Buchner, E.; Müller, M.A.; et al. Comparison of seven commercial SARS-CoV-2 rapid point-of-care antigen tests: A single-centre laboratory evaluation study. Lancet Microbe 2021, 2, e311–e319. [Google Scholar] [CrossRef]
- Anderson, D.E.; Sivalingam, V.; Kang, A.E.Z.; Ananthanarayanan, A.; Arumugam, H.; Jenkins, T.M.; Hadjiat, Y.; Eggers, M. Povidone-iodine demonstrates rapid in vitro virucidal activity against SARS-CoV-2, the virus causing COVID-19 disease. Infect. Dis. Ther. 2020, 9, 669–675. [Google Scholar] [CrossRef]
- Transmission of SARS-CoV-2: Implications for Infection Prevention Precautions. Scientific Brief. 9 July 2020. Available online: https://www.who.int/publications/i/item/modes-of-transmission-of-virus-causing-covid-19-implications-for-ipc-precaution-recommendations (accessed on 9 August 2021).
- Li, B.; Deng, A.; Li, K.; Hu, Y.; Li, Z.; Xiong, Q.; Liu, Z.; Guo, Q.; Zou, L.; Zhang, H.; et al. Viral infection and transmission in a large, well-traced outbreak caused by the SARS-CoV-2 Delta variant. Medrxiv 2021, 7, 21260122. [Google Scholar] [CrossRef]
- Jan, C.; Craig, R.; Magaly, A.-M.; Miriam, B.; Thibault, C.; Manas, D.; Anne-Marie, G.; Beatriz, G.; Thomas, L.; Derek, R.; et al. 16 May 2020 Recommendations for the Re-Opening of Dental Services: A Rapid Review of International Sources (Substantial Update 16 May 2020) Cochrane Database of Systematic Reviews. Available online: https://oralhealth.cochrane.org/news/recommendations-re-opening-dental-services-rapid-review-international-sources (accessed on 9 August 2021).
- Vergara-Buenaventura, A.; Castro-Ruiz, C. Use of mouthwashes against COVID-19 in dentistry. Br. J. Oral Maxillofac. Surg. 2020, 58, 924–927. [Google Scholar] [CrossRef]
- Muñoz-Basagoiti, J.; Perez-Zsolt, D.; León, R.; Blanc, V.; Raïch-Regué, D.; Cano-Sarabia, M.; Izquierdo-Useros, N. Mouthwashes with CPC reduce the infectivity of SARS-CoV-2 variants in vitro. J. Dent. Res. 2021, 100, 1265–1272. [Google Scholar] [CrossRef]
- Komine, A.; Yamaguchi, E.; Okamoto, N.; Yamamoto, K. Virucidal activity of oral care products against SARS-CoV-2 in vitro. J. Oral Maxillofac. Surg. Med. Pathol. 2021, 33, 475–477. [Google Scholar] [CrossRef]
- Meister, T.L.; Brüggemann, Y.; Todt, D.; Conzelmann, C.; Müller, J.A.; Groß, R.; Steinmann, E. Virucidal efficacy of different oral rinses against severe acute respiratory syndrome Coronavirus 2. J. Infect. Dis. 2020, 222, 1289–1292. [Google Scholar] [CrossRef]
- Baker, N.; Williams, A.J.; Tropsha, A.; Ekins, S. Repurposing quaternary ammonium compounds as potential treatments for COVID-19. Pharm. Res. 2020, 37, 104. [Google Scholar] [CrossRef]
- Kampf, G.; Todt, D.; Pfaender, S.; Steinmann, E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J. Hosp. Infect. 2020, 104, 246–251. [Google Scholar] [CrossRef] [Green Version]
- Khokhar, M.; Roy, D.; Purohit, P.; Goyal, M.; Setia, P. Viricidal treatments for prevention of coronavirus infection. Pathog. Glob. Health 2020, 114, 349–359. [Google Scholar] [CrossRef]
- Carrouel, F.; Valette, M.; Gadea, E.; Esparcieux, A.; Illes, G.; Langlois, M.E.; Perrier, H.; Dussart, C.; Tramini, P.; Ribaud, M.; et al. Use of an antiviral mouthwash as a barrier measure in the SARS-CoV-2 transmission in adults with asymptomatic to mild COVID-19: A multicentre, randomized, double-blind controlled trial. Clin. Microbiol. Infect. 2021, 27, 1494–1501. [Google Scholar] [CrossRef]
- Eduardo, F.P.; Corrêa, L.; Heller, D.; Daep, C.A.; Benitez, C.; Malheiros, Z.; Stewart, B.; Ryan, M.; Machado, C.M.; Hamerschlak, N.; et al. Salivary SARS-CoV-2 load reduction with mouthwash use: A randomized pilot clinical trial. Heliyon 2021, 7, e07346. [Google Scholar] [CrossRef]
- da Silva Santos, P.S.; da Fonseca Orcina, B.; Machado, R.; Vilhena, F.V.; da Costa Alves, L.M.; Zangrando, M.; de Oliveira, R.C.; Soares, M.; Simão, A.; Pietro, E.; et al. Beneficial effects of a mouthwash containing an antiviral phthalocyanine derivative on the length of hospital stay for COVID-19: Randomised trial. Sci. Rep. 2021, 11, 19937. [Google Scholar] [CrossRef]

| Product | Name (Laboratory) | Active Ingredient | |
|---|---|---|---|
| * | IPV | Isodine® (Bussie) | Iodopovidone (8 mg) |
| † | D-limonene | D-Limonene | D-limonene (0.3%) |
| ‡ | CPC | Cetylpyridinium chloride | Cetylpyridinium chloride (0.1%) |
| § | CHX | Chlorhexidine gluconate | Chlorhexidine gluconate 10% |
| ** | Product A | PERIO-AID® (DENTAID) | CPC (0.12%) and CHX (0.05%) |
| †† | Product B | Listerine® Zero Alcohol (Johnson & Johnson) | Eucalyptol 0.091% w/v, Menthol 0.042% w/v, Thymol 0.063% w/v |
| ††† | Product C | Periogard® (Colgate) | CHX (0.12%) |
| *** | Product D | Plax® (Colgate) | Sodium fluoride (0.05%) and CPC (0.075%) |
| ‡‡‡ | Product E | Clorhexol® (Farpag) | CHX (0.2%) |
| ‡‡ | Product F | Scope® (P&G) | CPC (0.07%) |
| §§ | Product G | Xyntrus® (Brix Medical Science) | D-limonene (0.2%) and CPC (0.05%) |
| §§§ | Mouthwash base excipients | Control reaction | Water, glycerin, citric acid, colorant, sodium citrate |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Casanovas, H.J.; la Rosa, M.D.; Bello-Lemus, Y.; Rasperini, G.; Acosta-Hoyos, A.J. Virucidal Activity of Different Mouthwashes Using a Novel Biochemical Assay. Healthcare 2022, 10, 63. https://doi.org/10.3390/healthcare10010063
Rodríguez-Casanovas HJ, la Rosa MD, Bello-Lemus Y, Rasperini G, Acosta-Hoyos AJ. Virucidal Activity of Different Mouthwashes Using a Novel Biochemical Assay. Healthcare. 2022; 10(1):63. https://doi.org/10.3390/healthcare10010063
Chicago/Turabian StyleRodríguez-Casanovas, Héctor J., Manuel De la Rosa, Yesit Bello-Lemus, Giulio Rasperini, and Antonio J. Acosta-Hoyos. 2022. "Virucidal Activity of Different Mouthwashes Using a Novel Biochemical Assay" Healthcare 10, no. 1: 63. https://doi.org/10.3390/healthcare10010063
APA StyleRodríguez-Casanovas, H. J., la Rosa, M. D., Bello-Lemus, Y., Rasperini, G., & Acosta-Hoyos, A. J. (2022). Virucidal Activity of Different Mouthwashes Using a Novel Biochemical Assay. Healthcare, 10(1), 63. https://doi.org/10.3390/healthcare10010063






