Cost-Effectiveness of Upper Extremity Dry Needling in Chronic Stroke
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Intervention Conditions
2.4. Main Measures
2.4.1. EuroQol-5D
2.4.2. Modified Modified Ashworth Scale
2.5. Costs
2.6. Outcomes
2.6.1. Quality of Life (QOL)
2.6.2. Modified Modified Ashworth Scale (MMAS)
2.7. Statistical Analysis
3. Results
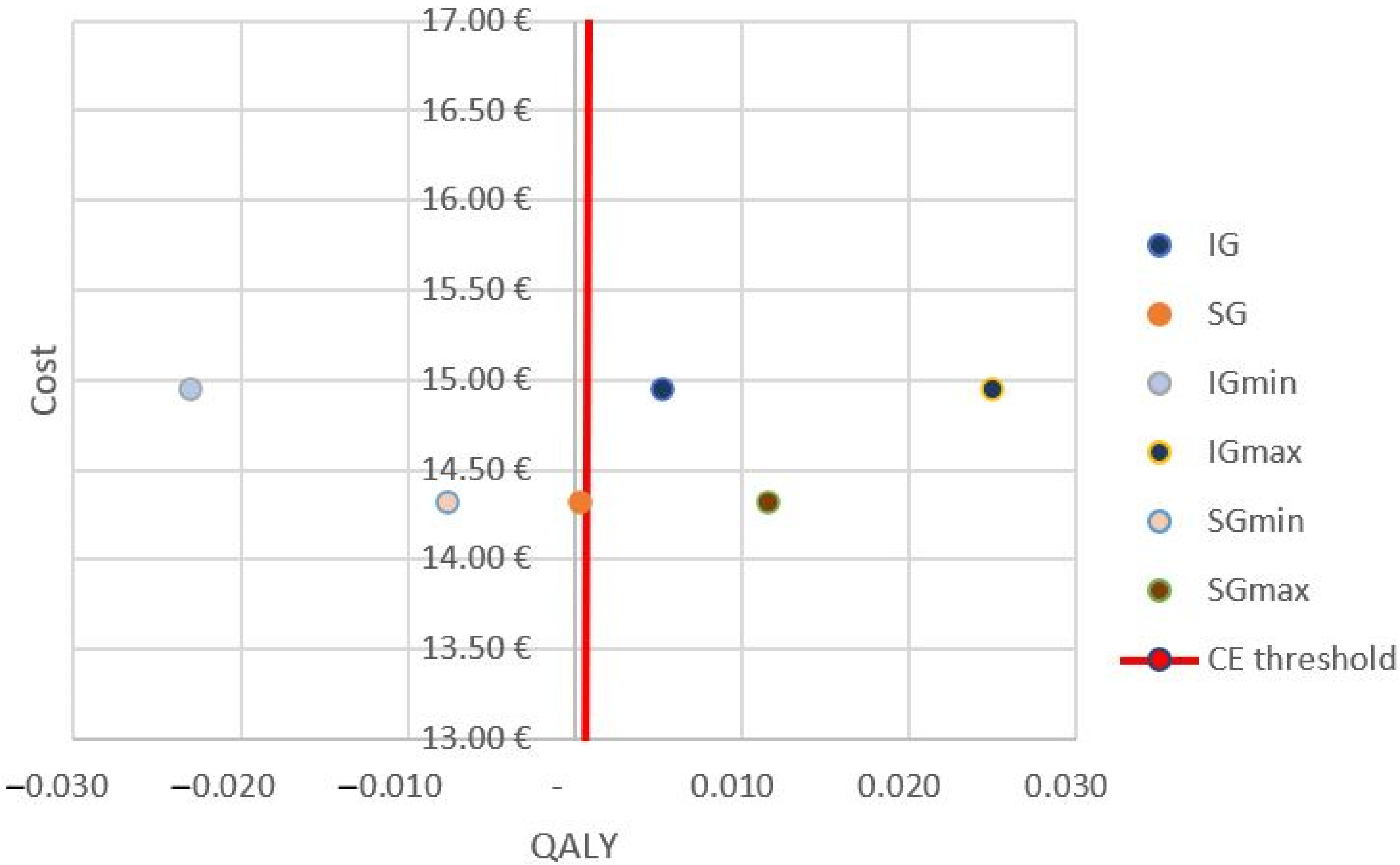
3.1. Costs
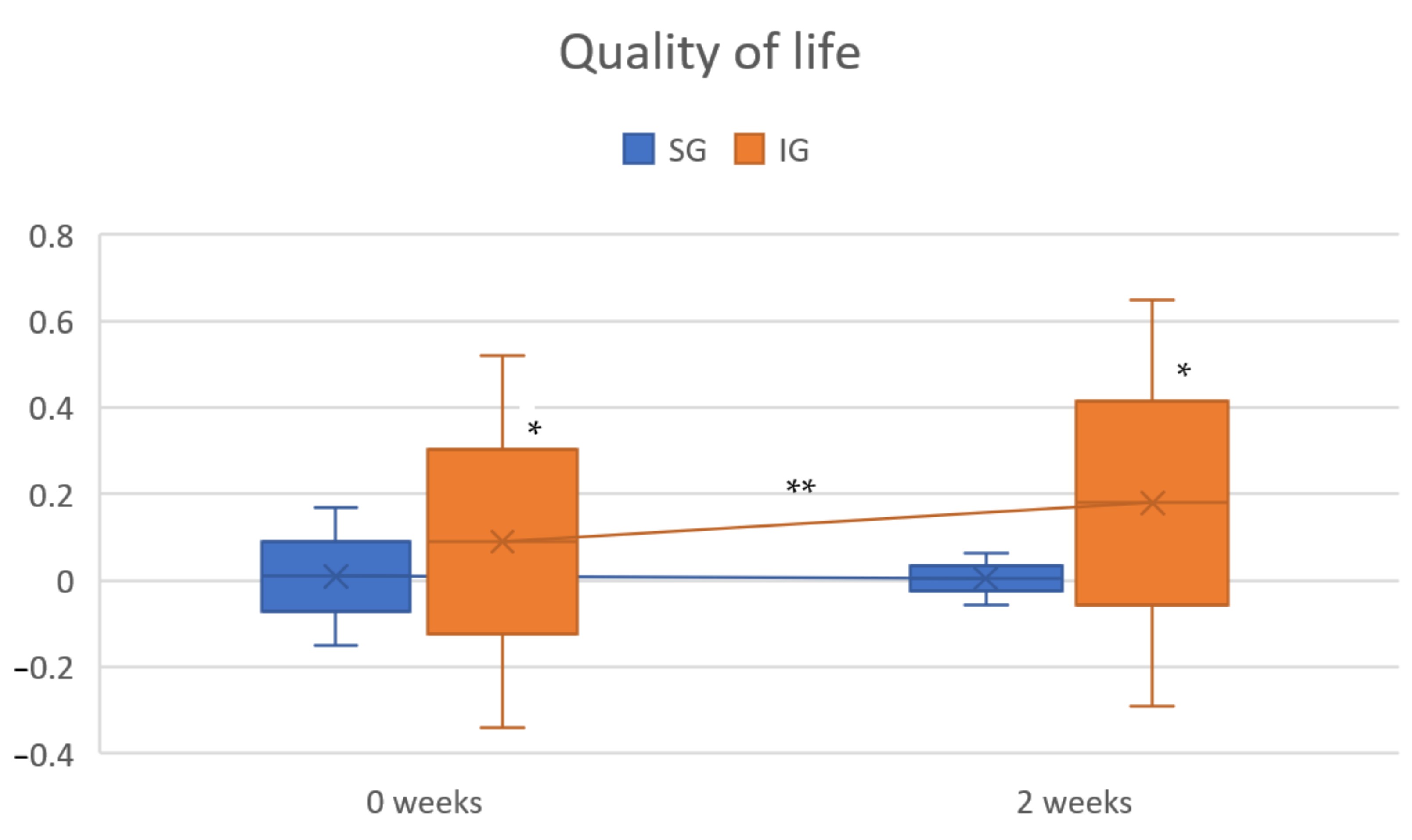
3.2. Quality of Life
3.3. Modified Modified Ashworth Scale
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- National Statistics Institute; Spanish Statistical Office. INEbase. Instituto Nacional de Estadística (INE). Available online: https://www.ine.es/dyngs/INEbase/es/operacion.htm?c=Estadistica_C&cid=1254736176780&menu=ultiDatos&idp=1254735573175 (accessed on 20 May 2021).
- Kuriakose, D.; Xiao, Z. Pathophysiology and Treatment of Stroke: Present Status and Future Perspectives. Int. J. Mol. Sci. 2020, 21, 7609. [Google Scholar] [CrossRef] [PubMed]
- Ma, V.Y.; Chan, L.; Carruthers, K.J. Incidence, Prevalence, Costs, and Impact on Disability of Common Conditions Requiring Rehabilitation in the United States: Stroke, Spinal Cord Injury, Traumatic Brain Injury, Multiple Sclerosis, Osteoarthritis, Rheumatoid Arthritis, Limb Loss, and Back Pain. Arch. Phys. Med. Rehabil. 2014, 95, 986–995.e1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, F.; Hernández, L.; Ward, A. Cost-effectiveness of stroke treatments and secondary preventions. Expert Opin. Pharmacother. 2012, 13, 1751–1760. [Google Scholar] [CrossRef] [PubMed]
- Thibaut, A.; Chatelle, C.; Ziegler, E.; Bruno, M.-A.; Laureys, S.; Gosseries, O. Spasticity after stroke: Physiology, assessment and treatment. Brain Inj. 2013, 27, 1093–1105. [Google Scholar] [CrossRef] [PubMed]
- Schinwelski, M.J.; Sitek, E.; Waz, P.; Slawek, J. Prevalence and predictors of post-stroke spasticity and its impact on daily living and quality of life. Neurol. i Neurochir. Polska 2019, 53, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Moggio, L.; De Sire, A.; Marotta, N.; Demeco, A.; Ammendolia, A. Exoskeleton versus end-effector robot-assisted therapy for finger-hand motor recovery in stroke survivors: Systematic review and meta-analysis. Top. Stroke Rehabil. 2021, 1–12. [Google Scholar] [CrossRef]
- Lee, H.S.; Park, Y.J.; Park, S.W. The Effects of Virtual Reality Training on Function in Chronic Stroke Patients: A Systematic Review and Meta-Analysis. BioMed Res. Int. 2019, 2019, 7595639. [Google Scholar] [CrossRef] [Green Version]
- Veerbeek, J.M.; Van Wegen, E.; Van Peppen, R.; Van Der Wees, P.J.; Hendriks, E.; Rietberg, M.; Kwakkel, G. What Is the Evidence for Physical Therapy Poststroke? A Systematic Review and Meta-Analysis. PLoS ONE 2014, 9, e87987. [Google Scholar] [CrossRef] [Green Version]
- Kinnear, B.Z.; Lannin, N.A.; Cusick, A.; A Harvey, L.; Rawicki, B. Rehabilitation Therapies After Botulinum Toxin-A Injection to Manage Limb Spasticity: A Systematic Review. Phys. Ther. 2014, 94, 1569–1581. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Mila, Z.; Salom-Moreno, J.; Fernández-De-Las-Peñas, C. Effects of Dry Needling on Post-Stroke Spasticity, Motor Function and Stability Limits: A Randomised Clinical Trial. Acupunct. Med. 2018, 36, 358–366. [Google Scholar] [CrossRef]
- Ghannadi, S.; Shariat, A.; Ansari, N.N.; Tavakol, Z.; Honarpishe, R.; Dommerholt, J.; Noormohammadpour, P.; Ingle, L. The Effect of Dry Needling on Lower Limb Dysfunction in Poststroke Survivors. J. Stroke Cerebrovasc. Dis. Off. J. Natl. Stroke Assoc. 2020, 29, 104814. [Google Scholar] [CrossRef]
- Zaldívar, J.N.C.; Calvo, S.; Bravo-Esteban, E.; Ruiz, P.O.; Santi-Cano, M.J.; Herrero, P. Effectiveness of dry needling for upper extremity spasticity, quality of life and function in subacute phase stroke patients. Acupunct. Med. 2021, 39, 299–308. [Google Scholar] [CrossRef]
- Calvo, S.; La Cruz, N.B.-D.; Jiménez-Sánchez, C.; Bravo-Esteban, E.; Herrero, P. Effects of dry needling on function, hypertonia and quality of life in chronic stroke: A randomized clinical trial. Acupunct. Med. 2021. [Google Scholar] [CrossRef]
- La Cruz, N.B.-D.; Calvo, S.; Rodríguez-Blanco, C.; Herrero, P.; Bravo-Esteban, E. Effects of dry needling on gait and muscle tone in Parkinson’s disease: A randomized clinical trial. Acupunct. Med. 2022, 40, 3–12. [Google Scholar] [CrossRef]
- Luque-Moreno, C.; Granja-Domínguez, A.; Moral-Munoz, J.; Izquierdo-Ayuso, G.; Lucena-Anton, D.; Heredia-Rizo, A. Effectiveness of Dry Needling versus Placebo on Gait Performance, Spasticity, Electromyographic Activity, Pain, Range-of-Movement and Quality of Life in Patients with Multiple Sclerosis: A Randomized Controlled Trial Protocol. Brain Sci. 2020, 10, 997. [Google Scholar] [CrossRef]
- Dong, Y.; Wu, T.; Hu, X.; Wang, T. Efficacy and safety of botulinum toxin type A for upper limb spasticity after stroke or traumatic brain injury: A systematic review with meta-analysis and trial sequential analysis. Eur. J. Phys. Rehabil. Med. 2017, 53, 256–267. [Google Scholar] [CrossRef]
- Kütük, S.G.; Özkan, Y.; Kütük, M.; Özdaş, T. Comparison of the Efficacies of Dry Needling and Botox Methods in the Treatment of Myofascial Pain Syndrome Affecting the Temporomandibular Joint. J. Craniofacial Surg. 2019, 30, 1556–1559. [Google Scholar] [CrossRef]
- Valencia-Chulián, R.; Heredia-Rizo, A.M.; Moral-Munoz, J.A.; Lucena-Anton, D.; Luque-Moreno, C. Dry needling for the management of spasticity, pain, and range of movement in adults after stroke: A systematic review. Complement. Ther. Med. 2020, 52, 102515. [Google Scholar] [CrossRef]
- Brady, S.; McEvoy, J.; Dommerholt, J.; Doody, C. Adverse events following trigger point dry needling: A prospective survey of chartered physiotherapists. J. Man. Manip. Ther. 2013, 22, 134–140. [Google Scholar] [CrossRef] [Green Version]
- Kaku, M.; Simpson, D. Spotlight on botulinum toxin and its potential in the treatment of stroke-related spasticity. Drug Des. Dev. Ther. 2016, 10, 1085–1099. [Google Scholar] [CrossRef] [Green Version]
- Sanchis, D.F.; Zaldívar, J.N.C.; Calvo, S.; Herrero, P.; Barrera, M.G. Cost-effectiveness of upper extremity dry needling in the rehabilitation of patients with stroke. Acupunct. Med. 2021. [Google Scholar] [CrossRef]
- Mitchell, U.H.; Stoneman, P.; Larson, R.E.; Page, G.L. The Construction of Sham Dry Needles and Their Validity. Evid. Based Complement. Altern. Med. 2018, 2018, 9567061. [Google Scholar] [CrossRef]
- Braithwaite, F.A.; Walters, J.L.; Moseley, G.L.; Williams, M.T.; McEvoy, M.P. Towards more homogenous and rigorous methods in sham-controlled dry needling trials: Two Delphi surveys. Physiotherapy 2020, 106, 12–23. [Google Scholar] [CrossRef]
- Herrero, P.; Calvo, S.; Ortiz, M. Dry Needling for Hypertonia an Spasticity (DNHS). In Advanced Techniques in Musculoskeletal Medicine & Physiotherapy Using Minimally Invasive Therapies; Minaya, I.F.V.F., Ed.; Chruchill-Livingston; Elsevier: London, UK, 2015. [Google Scholar]
- Del Moral, O.M.; Lacomba, M.T.; Russell, I.J.; Méndez, Ó.S.; Sánchez-Sánchez, B. Validity and Reliability of Clinical Examination in the Diagnosis of Myofascial Pain Syndrome and Myofascial Trigger Points in Upper Quarter Muscles. Pain Med. 2018, 19, 2039–2050. [Google Scholar] [CrossRef]
- Mahesh, P.K.B.; Gunathunga, M.W.; Jayasinghe, S.; Arnold, S.M.; Senanayake, S.; Senanayake, C.H.; De Silva, L.S.D.; Kularatna, S. Construct validity and reliability of EQ-5D-3L for stroke survivors in a lower middle income setting. Ceylon. Med. J. 2019, 64, 52–58. [Google Scholar] [CrossRef]
- Pinto, E.; Maso, I.; Vilela, R.N.R.; Santos, L.C.; Oliveira-Filho, J. Validation of the EuroQol quality of life questionnaire on stroke victims. Arq. Neuro-Psiquiatr. 2011, 69, 320–323. [Google Scholar] [CrossRef] [Green Version]
- Bohannon, R.W.; Smith, M.B. Interrater Reliability of a Modified Ashworth Scale of Muscle Spasticity. Phys. Ther. 1987, 67, 206–207. [Google Scholar] [CrossRef]
- Meseguer-Henarejos, A.-B.; Sánchez-Meca, J.; López-Pina, J.-A.; Carles-Hernández, R. Inter- and intra-rater reliability of the Modified Ashworth Scale: A systematic review and meta-analysis. Eur. J. Phys. Rehabil. Med. 2018, 54, 576–590. [Google Scholar] [CrossRef]
- Ansari, N.N.; Naghdi, S.; Mashayekhi, M.; Hasson, S.; Fakhari, Z.; Jalaie, S. Intra-rater reliability of the Modified Modified Ashworth Scale (MMAS) in the assessment of upper-limb muscle spasticity. Neurorehabilitation 2012, 31, 215–222. [Google Scholar] [CrossRef]
- Rychlik, R.; Kreimendahl, F.; Schnur, N.; Lambert-Baumann, J.; Dressler, D. Quality of life and costs of spasticity treatment in German stroke patients. Health Econ. Rev. 2016, 6, 27. [Google Scholar] [CrossRef] [Green Version]
- BOA ORDEN SAN/1221/2017, de 21 de Julio, Por la Que se Establecen los Precios y Tarifas Máximas Aplicables en la Prestación de Servicios Sanitarios con Medios Ajenos al Sistema de Salud de Aragón. Available online: http://www.boa.aragon.es/cgi-bin/EBOA/BRSCGI?CMD=VEROBJ&MLKOB=977342223030 (accessed on 22 October 2021).
- Junta de Castilla y León. JRESOLUCIÓN de 21 de Noviembre de 2011, del PRESIDENTE de la Gerencia Regional de Salud, por la Que se Fijan las Tarifas Máximas y los Porcentajes de Revisión de las Condiciones Económicas Aplicables en el año 2011, a la Prestación de Servicios de Asistencia Sanitaria Concertada en el Ámbito de la Gerencia Regional de Salud. Available online: https://www.saludcastillayleon.es/institucion/es/resumen-bocyl-legislacion-sanitaria/resolucion-21-noviembre-2011-presidente-gerencia-regional-1 (accessed on 22 October 2021).
- BOCM-20170821-1 ORDEN 727/2017, de 7 de agosto, del Consejero de Sanidad, por la que Se fijan los Precios Públicos por la Prestación de los Servicios y Actividades de Naturaleza Sanitaria de la Red de Centros de la Comunidad de Madrid. Available online: http://www.madrid.org/wleg_pub/secure/normativas/contenidoNormativa.jsf?opcion=VerHtml&nmnorma=9930#no-back-button (accessed on 22 October 2021).
- TARIFAS PARA FACTURACIÓN DE SERVICIOS SANITARIOS Y DOCENTES DE OSAKIDETZA PARA EL AÑO 2019. Available online: https://www.osakidetza.euskadi.eus/contenidos/informacion/osk_servic_para_empresas/es_def/adjuntos/LIBRO-DE-TARIFAS_2020_osakidetza.pdf (accessed on 22 October 2021).
- ORDRE SLT/42/2012, de 24 de Febrer, per la qual es Regulen els Supòsits i Conceptes Facturables i S’aproven els Preus Públics Corresponents als Serveis que Presta L’Institut Català de la Salut. Mar 2, 2012. Available online: http://ics.gencat.cat/web/.content/documents/transparencia/economia/ICF_2n2014.pdf (accessed on 22 October 2021).
- York, Consortium YHE. Quality-Adjusted Life Year (QALY) 2016. Available online: https://yhec.co.uk/glossary/quality-adjusted-life-year-qaly/ (accessed on 22 October 2021).
- YHE Consortium. Incremental Cost-Effectiveness Ratio (ICER). Available online: https://yhec.co.uk/glossary/incremental-cost-effectiveness-ratio-icer/ (accessed on 22 October 2021).
- Vallejo-Torres, L.; García-Lorenzo, B.; Serrano-Aguilar, P. Estimating a cost-effectiveness threshold for the Spanish NHS. Health Econ. 2018, 27, 746–761. [Google Scholar] [CrossRef] [PubMed]
- Arias-Buría, J.L.; Martín-Saborido, C.; Cleland, J.; Koppenhaver, S.L.; Plaza-Manzano, G.; Fernández-De-Las-Peñas, C. Cost-effectiveness Evaluation of the Inclusion of Dry Needling into an Exercise Program for Subacromial Pain Syndrome: Evidence from a Randomized Clinical Trial. Pain Med. 2018, 19, 2336–2347. [Google Scholar] [CrossRef] [PubMed]
- Shaw, L.; Rodgers, H.; Price, C.; Van Wijck, F.; Shackley, P.; Steen, N.; Barnes, M.; Ford, G.; Graham, L. BoTULS: A multicentre randomised controlled trial to evaluate the clinical effectiveness and cost-effectiveness of treating upper limb spasticity due to stroke with botulinum toxin type A. Health Technol. Assess. 2010, 14, 1–113. [Google Scholar] [CrossRef] [PubMed]
- Herrero, P.; Del Moral, O.M. A Case Study Looking at the Effectiveness of Deep Dry Needling for the Management of Hypertonia. J. Musculoskelet. Pain 2007, 15, 55–60. [Google Scholar] [CrossRef]
- Andrés-Nogales, F.d.; Morell, A.; Aracil, J.; Torres, C.; Oyagüez, I.; Casado, M.A. Análisis de costes del uso de toxina botulínica A en España. J. Farm. Hosp. 2014, 38, 193–201. [Google Scholar]
- Wissel, J. [Multidisciplinary therapy for focal spasticity treatment]. Nervenarzt 2008, 79 (Suppl. 1), 24–28. [Google Scholar]
- Dörfler, E.; Kulnik, S.T. Despite communication and cognitive impairment—person-centred goal-setting after stroke: A qualitative study. Disabil. Rehabil. 2020, 42, 3628–3637. [Google Scholar] [CrossRef] [Green Version]
- Tonelli, M.R.; Sullivan, M.D. Person-centred shared decision making. J. Eval. Clin. Pract. 2019, 25, 1057–1062. [Google Scholar] [CrossRef]
| Unitary Cost | Sham Group | Intervention Group | |
|---|---|---|---|
| Dry needling (material per session) | EUR 0.64 | - | EUR 0.64 |
| Mean physiotherapy cost per session | EUR 14.32 ± 4.39 | EUR 14.32 | EUR 14.96 |
| Sham Group | Intervention Group | ||
|---|---|---|---|
| QOL | Pre-test | 0.01 ± 0.16 | 0.09 ± 0.43 * |
| 2 weeks | 0.005 ± 0.06 | 0.18 ± 0.47 * | |
| QALY | 0.0003 (min. −0.0077; max. 0.0115) | 0.0052 (min. −0.0230, max. 0.02493) | |
| ICER (EUR/QALY) | 130.14 (min. −41.57, max. 47.51) | ||
| Post-Intervention | 2 Weeks | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | Intervention (DNHS®) | Control | Intervention (DNHS®) | |||||||||||||
| n | % Responder | EUR/Responder | n | % Responder | EUR/Responder | McNemar Test | ICER | n | % Responder | EUR/Responder | n | % Responder | EUR/Responder | McNemar Test | ICER | |
| Elbow flexors | 12 | 33% | EUR 42.96 | 11 | 27% | EUR 54.85 | 1.000 | EUR −10.52 | 12 | 33% | EUR 42.96 | 11 | 27% | EUR 54.85 | 1.000 | EUR −10.52 |
| Elbow extensors | 12 | 8% | EUR 171.84 | 11 | 73% | EUR 20.57 | 0.039 * | EUR 0.99 | 12 | 25% | EUR 57.28 | 11 | 73% | EUR 20.57 | 0.125 | EUR 1.34 |
| Wrist–dorsal flexors | 12 | 25% | EUR 57.28 | 11 | 36% | EUR 41.13 | 1.000 | EUR 5.61 | 12 | 17% | EUR 85.92 | 11 | 45% | EUR 32.91 | 0.375 | EUR 2.22 |
| Wrist–palmar flexors | 12 | 17% | EUR 85.92 | 11 | 18% | EUR 82.27 | 1.000 | EUR 42.09 | 12 | 17% | EUR 85.92 | 11 | 27% | EUR 54.85 | 1.000 | EUR 6.01 |
| Thumb adductor | 12 | 17% | EUR 85.92 | 10 | 40% | EUR 37.39 | 0.375 | EUR 2.73 | 12 | 50% | EUR 28.64 | 10 | 30% | EUR 49.86 | 0.625 | EUR −3.19 |
| Means | 20% | EUR 88.78 | 39% | EUR 47.24 | EUR 8.18 | 28% | EUR 60.14 | 41% | EUR 42.60 | EUR −0.83 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Sanchis, D.; Brandín-de la Cruz, N.; Jiménez-Sánchez, C.; Gil-Calvo, M.; Herrero, P.; Calvo, S. Cost-Effectiveness of Upper Extremity Dry Needling in Chronic Stroke. Healthcare 2022, 10, 160. https://doi.org/10.3390/healthcare10010160
Fernández-Sanchis D, Brandín-de la Cruz N, Jiménez-Sánchez C, Gil-Calvo M, Herrero P, Calvo S. Cost-Effectiveness of Upper Extremity Dry Needling in Chronic Stroke. Healthcare. 2022; 10(1):160. https://doi.org/10.3390/healthcare10010160
Chicago/Turabian StyleFernández-Sanchis, Daniel, Natalia Brandín-de la Cruz, Carolina Jiménez-Sánchez, Marina Gil-Calvo, Pablo Herrero, and Sandra Calvo. 2022. "Cost-Effectiveness of Upper Extremity Dry Needling in Chronic Stroke" Healthcare 10, no. 1: 160. https://doi.org/10.3390/healthcare10010160
APA StyleFernández-Sanchis, D., Brandín-de la Cruz, N., Jiménez-Sánchez, C., Gil-Calvo, M., Herrero, P., & Calvo, S. (2022). Cost-Effectiveness of Upper Extremity Dry Needling in Chronic Stroke. Healthcare, 10(1), 160. https://doi.org/10.3390/healthcare10010160







