4.1. Main Findings
The main aim of this article was to develop a multiscale electrophysiological model of rat heart ventricles with infarction injury which includes a detailed description of the processes at the cellular, tissue and whole organ levels. The rationale for this study was that many experimental studies of ischemia and infarction injury are performed in the rat heart and anatomical modeling of these processes can be a helpful addition to these studies. This was carried out after the detailed analysis of cellular models, their study in 1D and 2D systems, the generation of a mesh for the 3D anatomical model of the rat ventricles and the study of dynamics of various excitation sources in that model. Our computational model also includes the representation of the post-infarction scar.
To the best of our knowledge, this is the first model to simulate infarction injury in the rat heart (see
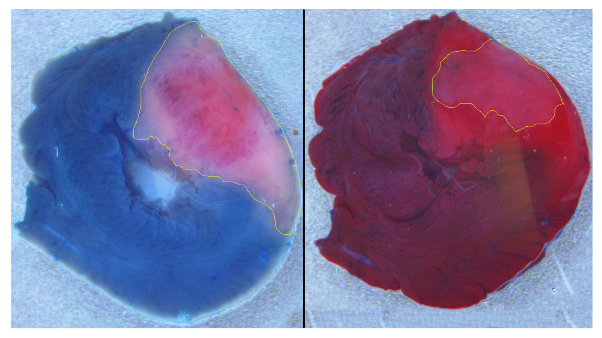
Figure 2). We built a ventricular model with an inexcitable transmural scar and an associated border zone with modified activity reflecting cellular remodeling. The shape and dimensions of the scar and border zone were set to be similar to what we observed in our experiments on ischemia-reperfusion injury in the rat heart (see
Figure 1).
To develop the anatomical model, we first studied and updated the Gattoni2016 cellular model to be able to reproduce cellular activity at high pacing rates. We also made some modifications to increase the numerical stability of the model. This was an essential step in order to be able to reproduce excitation sources in the tissue with periods in a range of 10–20 Hz [
24,
35,
36,
37]. This is similar to what is measured in the rat heart during various arrhythmias, including those induced by ischemia-reperfusion interventions in rats [
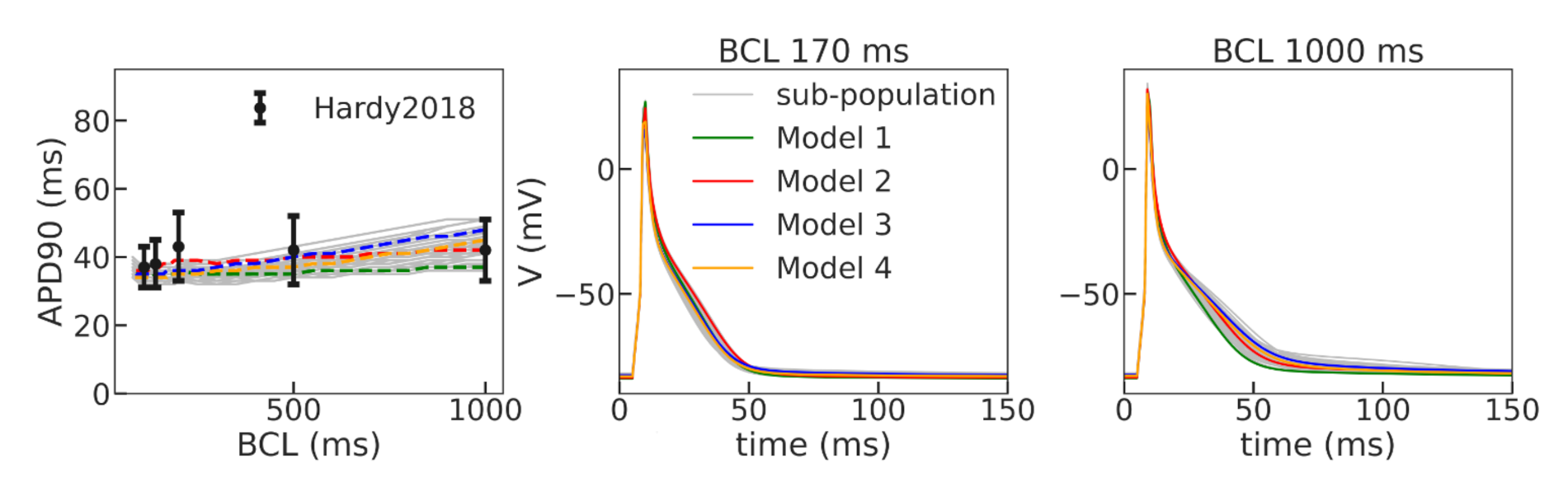
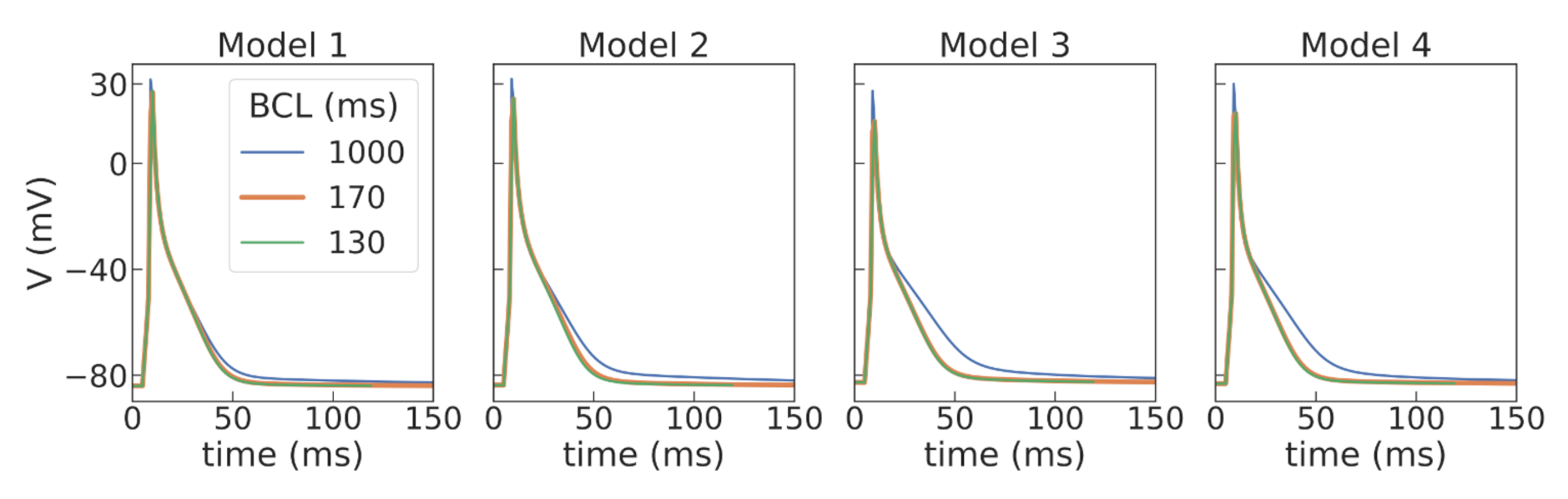
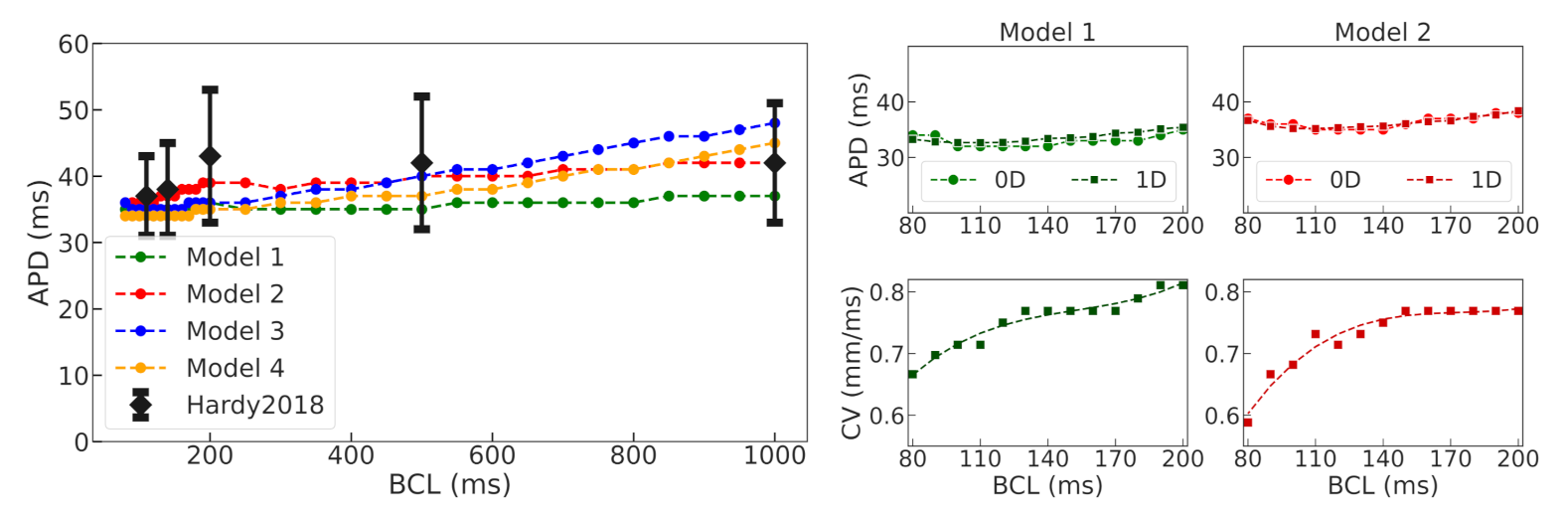
37]. Using a population-of-models approach, we tested 10,000 Gattoni2016 cellular models with varied parameters and selected four examples of cellular models which had APD restitution curves consistent with the experimental data (see
Figure 5 and
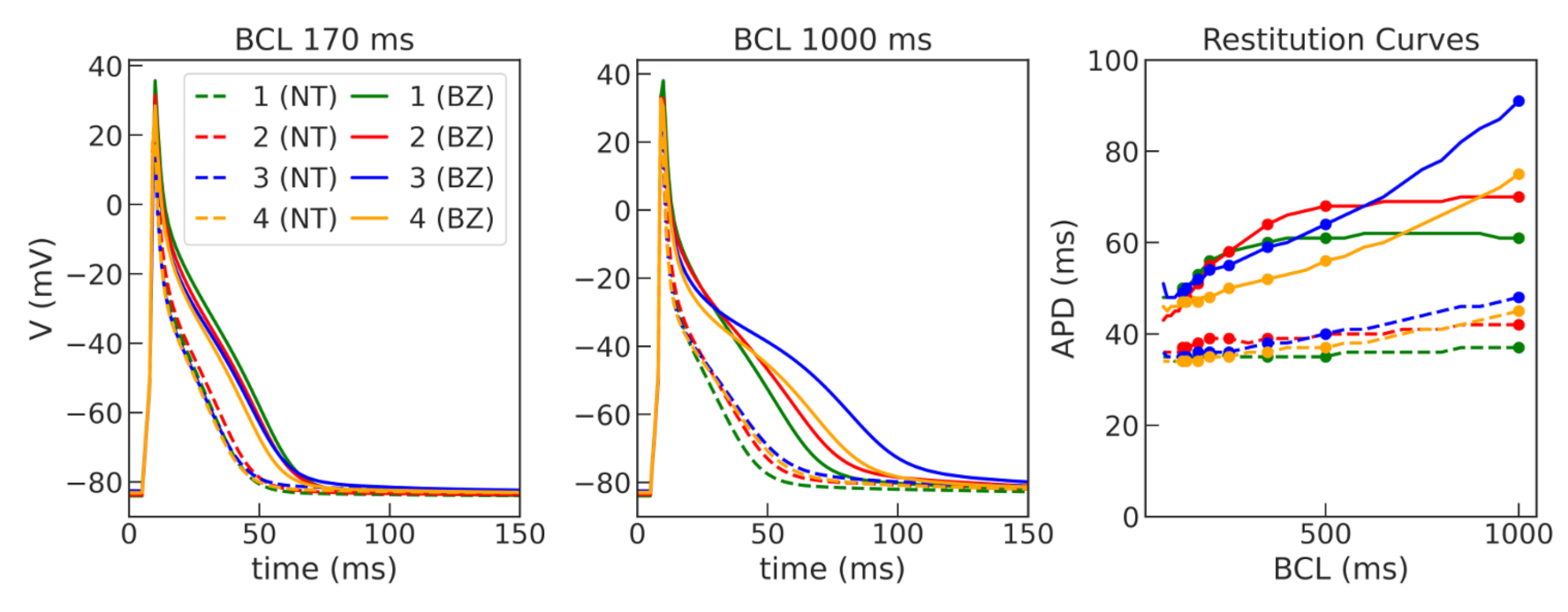
Figure A2) and were suitable for stable long-running simulations at high pacing rates for thousands of cycles. The baseline models for normal tissue were then modified to simulate APD elongation in the border zone of the post-infarction scar (see
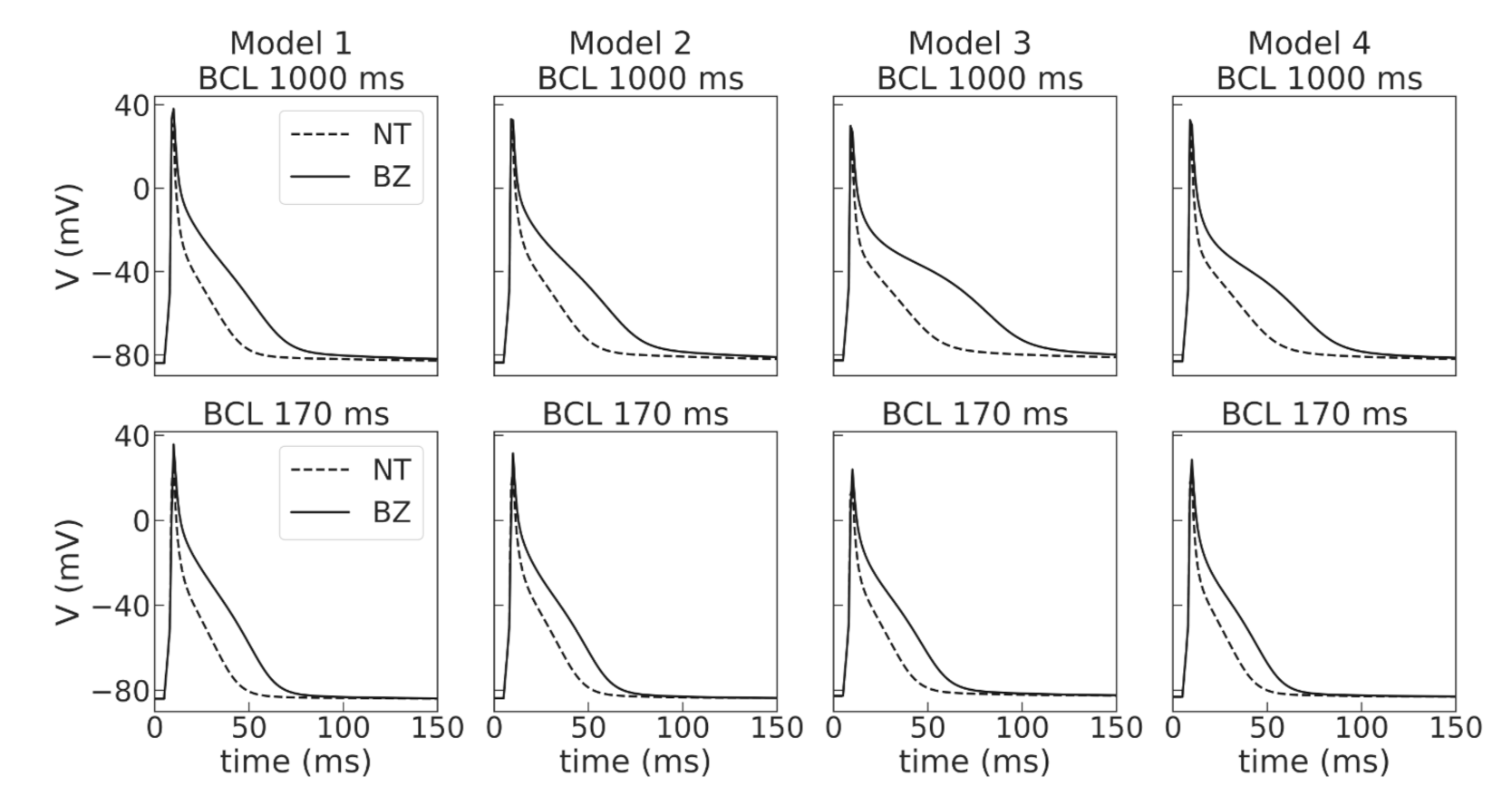
Figure 4), as reported in experimental studies [
39]. Then, we performed simulations in 1D tissue strands composed of normal tissue cells to check if the APD restitution curves for propagating waves are close to those of single cells, and conduction velocity in the tissue models is in the physiological range.
The next important step of our study was to simulate spiral waves in 2D tissue. We compared wave dynamics, using four selected cellular ionic models, with baseline parameters for normal tissue and modified parameters for the border zone (see
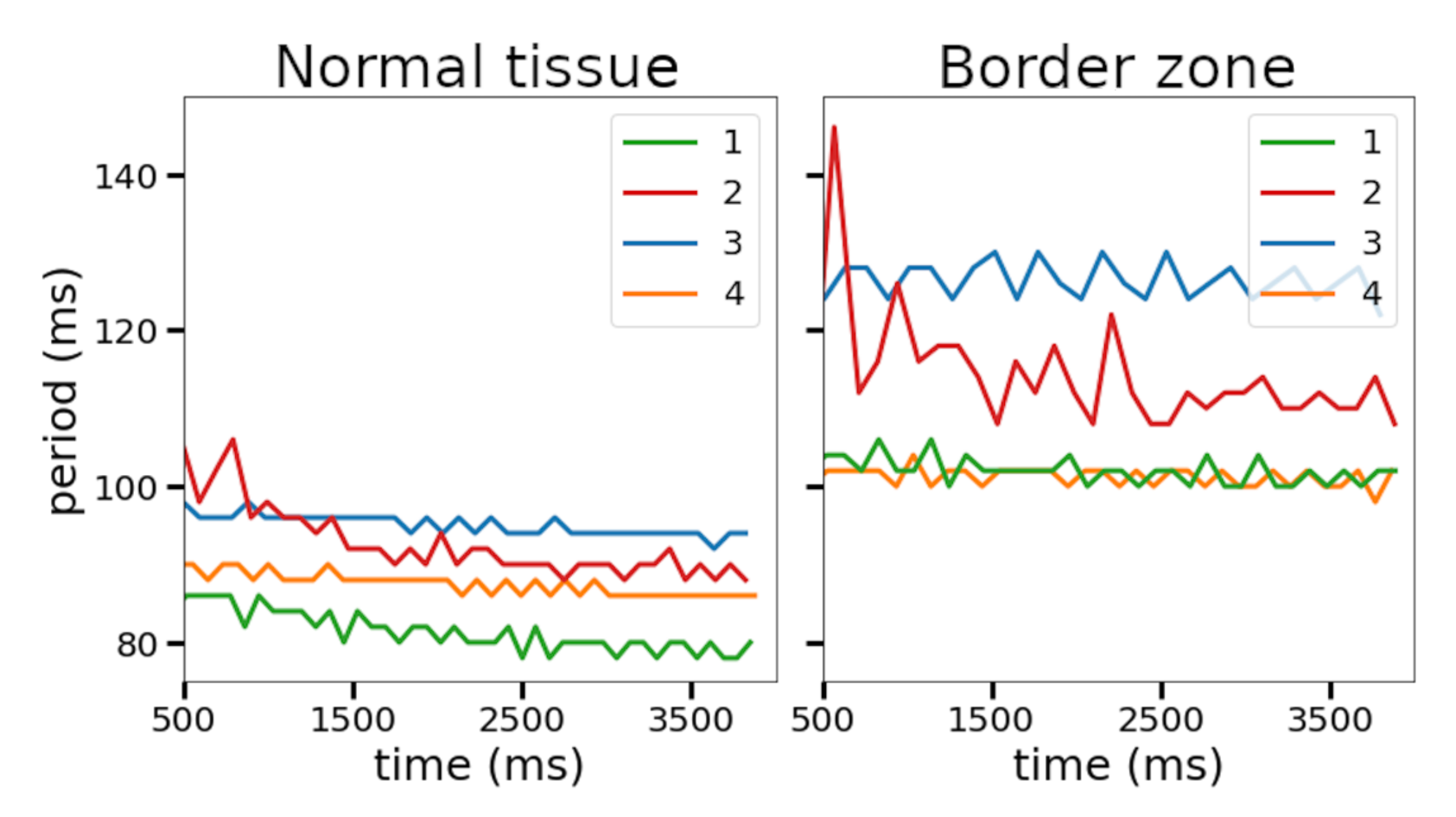
Figure 6). In this setting, we found periods of spiral waves varying from 82 to 126 ms (see
Table 4), which is consistent with experimental studies [
24,
37]. The longer periods of waves were produced by modified models with longer action potentials. Additionally, all models showed meandering dynamics of the spiral waves with a different degree of meandering. One of the models (Model 2) in the border zone tissue showed unstable behavior, leading to the formation of additional wave breaks (row 2R in
Figure 6). The stable Model 1 and unstable Model 2 were then used to simulate scroll waves in the ventricular rat models.
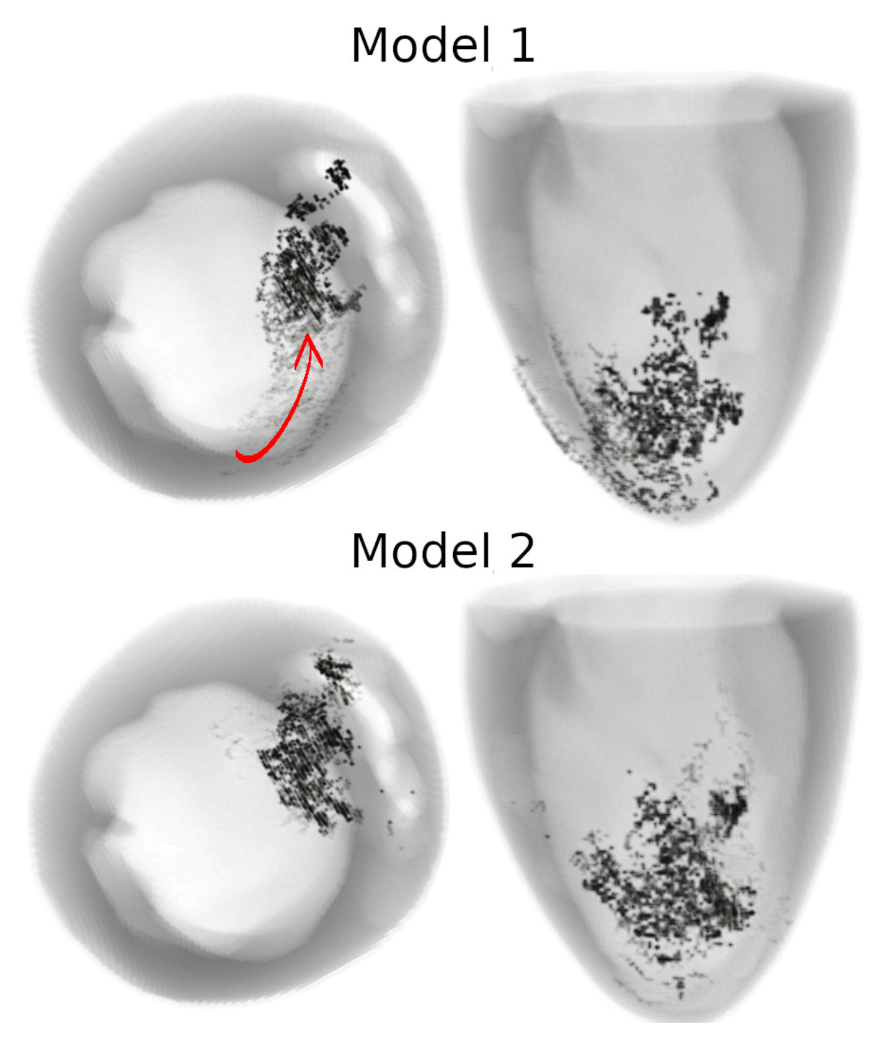
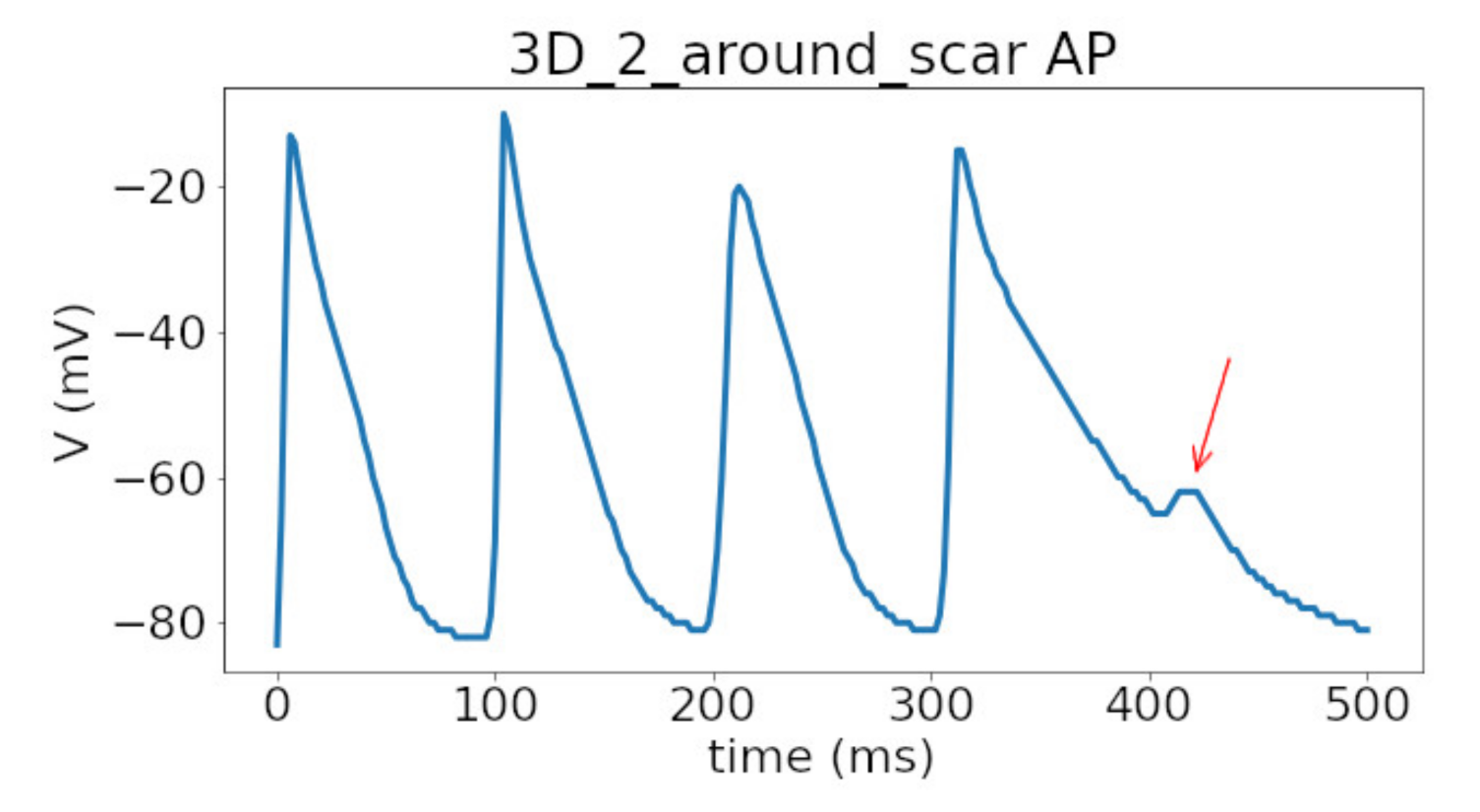
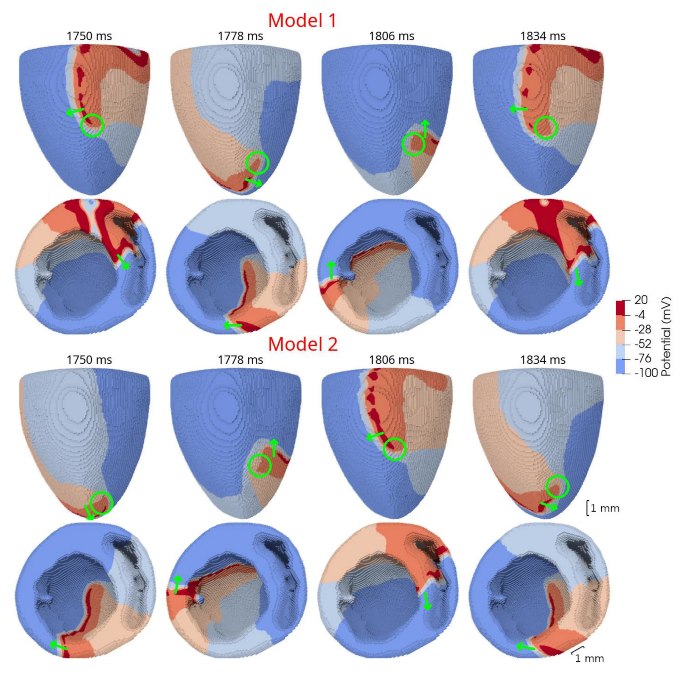
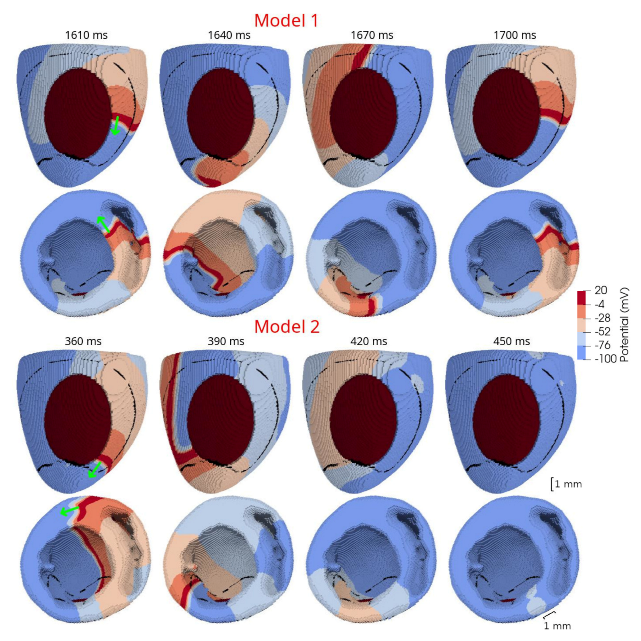
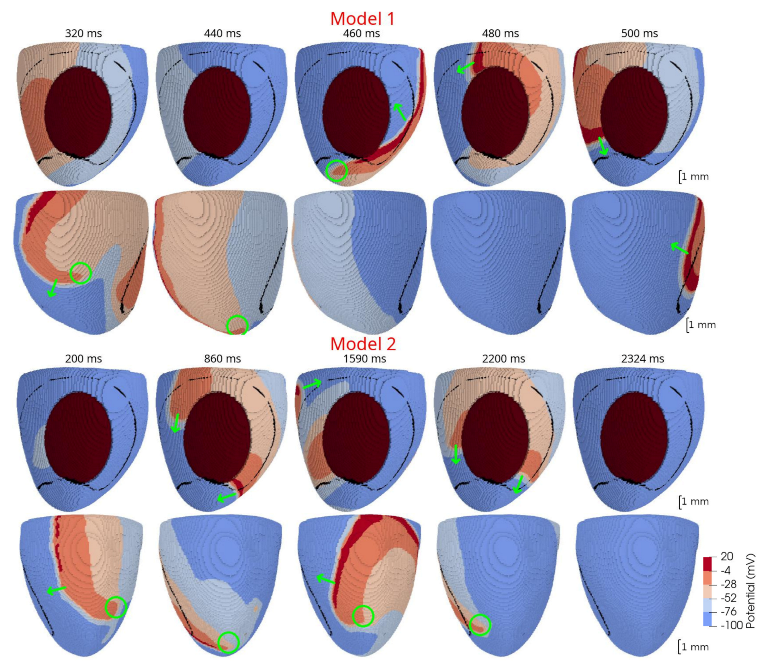
In 3D models of rat ventricles, we introduced an inexcitable post-infarction scar, with a border zone containing cells with modified ionic activity. In the models without the scar, we found no qualitative difference between the ventricular models utilizing the Cellular Models 1 and 2. Both models produce stable scroll waves with rotation periods of 83-90 ms, which is consistent with experimental data. The presence of the scar obstacle and cellular heterogeneity in the myocardium lead to the divergent behavior of the scroll waves in the two models. For Cellular Model 1 a scroll wave initiated near the scar remained stable VT-like (single scroll wave filament) for 2 s (20 rotations), while for Cellular Model 2, the scroll wave self-terminated after four rotations in the ventricles. When initiated outside of the scar region, the scroll wave drifted to the scar area and anchored around the scar in the case of Cellular Model 1, while it rotated with the initial core location before terminating in the case of Model 2.
The results of our study confirm the applicability of a multi-scale species-specific computational model of a rat heart, which reproduces a ventricular geometry and fiber orientation in combination with a detailed ionic model of cellular electrophysiology to study VT and VF-like arrhythmia in the rat heart under myocardial injury. The model provides the scientific community with a tool to study the effects of specific interventions aimed to reduce myocardial injury both in terms of dimensions of the scar and in terms of the change in the functional properties of cardiac tissue.
4.2. On Species-Specific Models of Rat Heart to Study Cardiac Arrhythmia
A curious fact is that despite the rat being the most widely used laboratory animal for studying cardiac physiology and pathology, only a few rat-specific cardiac models have been developed and used to analyze experimental data. Actually, to the best of our knowledge, there are only two groups who reported on both generic models of myocardial tissue and anatomical models of rat ventricles which can be used to study arrhythmia in a species-specific environment.
In Whittaker and co-authors’ first study [
16], a detailed realistic model of the gross anatomy of rat heart ventricles and myocyte orientations in tissue was developed based on the data from DT MRI. The authors focused on the effects of variations in myocyte orientations on arrhythmia induction and on the dynamics of re-entry. In contrast to our study, they used a phenomenological cellular model, created by updating a Fenton–Karma three-variable model, to simulate cellular action potentials in rat cardiomyocytes. They showed that differences in myocyte orientations could critically affect the inducibility and persistence of arrhythmias. This aspect should be further addressed by using cellular ionic models.
In the second recent article of Bi and co-authors [
17], a multi-scale 3D rat ventricle model was implemented using one of the bi-ventricular geometry models from [
16] and a Pandit ionic model [
19] of rat cardiomyocytes. The authors were mostly focused on the numerical aspects of model calculation and a parallelization solution based on the GPU platform. While they demonstrated an example of scroll waves initiated by an S1–S2 protocol, no specific detail on the wave characteristics was provided to compare with our findings.
Compared with previous studies, our research has novelty in several aspects. The first one is the choice of the cellular ionic model. There is a family of cellular ionic models first developed by Pandit and co-authors in 2001 [
19] and then updated by several groups [
18,
40]. However, none of the models has been systematically validated for studying cardiac arrhythmia, where the frequency of cardiac cycling essentially increases over a physiological range. In the experimental rat models of VT and VF, the frequency of spontaneous activity measured in the heart is in a range of 10–20 Hz [
36]. In this case, cellular models which are appropriate to study VT and VF should reproduce activity at high pacing rates and allow stable long-term runs in these conditions. However, the most frequent reference pacing rate used to develop cellular models is 1 Hz (e.g., [
19,
40]), which is out of the physiological and especially pathological ranges in rats. Unfortunately, some existing models were not specifically curated to ensure a robust performance at high frequencies. In particular, we have not been able to run the Pandit model at pacing rates higher than 6 Hz using Myokit software with an adaptive time step provided by the CVODE solver. Moreover, for those BCLs where we were able to calculate the models, we found a negative restitution curve which is not consistent with most of the experimental data on rats. The recent Gattoni2016 cellular model [
18] inherited the Pandit description of ionic currents but was upgraded for the description of Ca
dynamics in cells. It was specifically developed to simulate activity at high pacing frequencies in rat cardiomyocytes with a focus on the frequency-dependence of Ca
dynamics in the cells. Indeed, the reference model reproduces experimental data on APD shortening and an increase in the duration of the intracellular Ca
, which occurs with an increase in the pacing rate. This was achieved through manually setting the frequency-dependence of the SERCA pump rate via increasing the
parameter with increasing pacing rate. The model was tested by the authors at frequencies of up to 6 Hz, so additional testing had to be carried out to ensure its applicability for even faster pacing. Moreover, the suggested phenomenological dependence of the SERCA rate on pacing frequency is reasonable for the steady state, reflecting the cumulative effects of several regulatory mechanisms adjusting cellular activity to the pacing rate. Actually, in the intact heart, this adaptation needs several cycles to approach the new level of cellular parameters. Such a model seems not quite appropriate in a dynamic setting where the cycle length may change from cycle to cycle and there is no time to adjust the model parameters for new cycle length conditions. However, the Gattoni2016 cellular model failed to reproduce a positive restitution curve with any constant
parameter assigned to BCLs of either 1000 ms or 600 ms. Moreover, the model also showed unstable behavior at frequencies higher than 6 Hz. Therefore, the first step of our study was to search for an appropriate cellular model to be used in the dynamic VT-like simulations in the tissue.
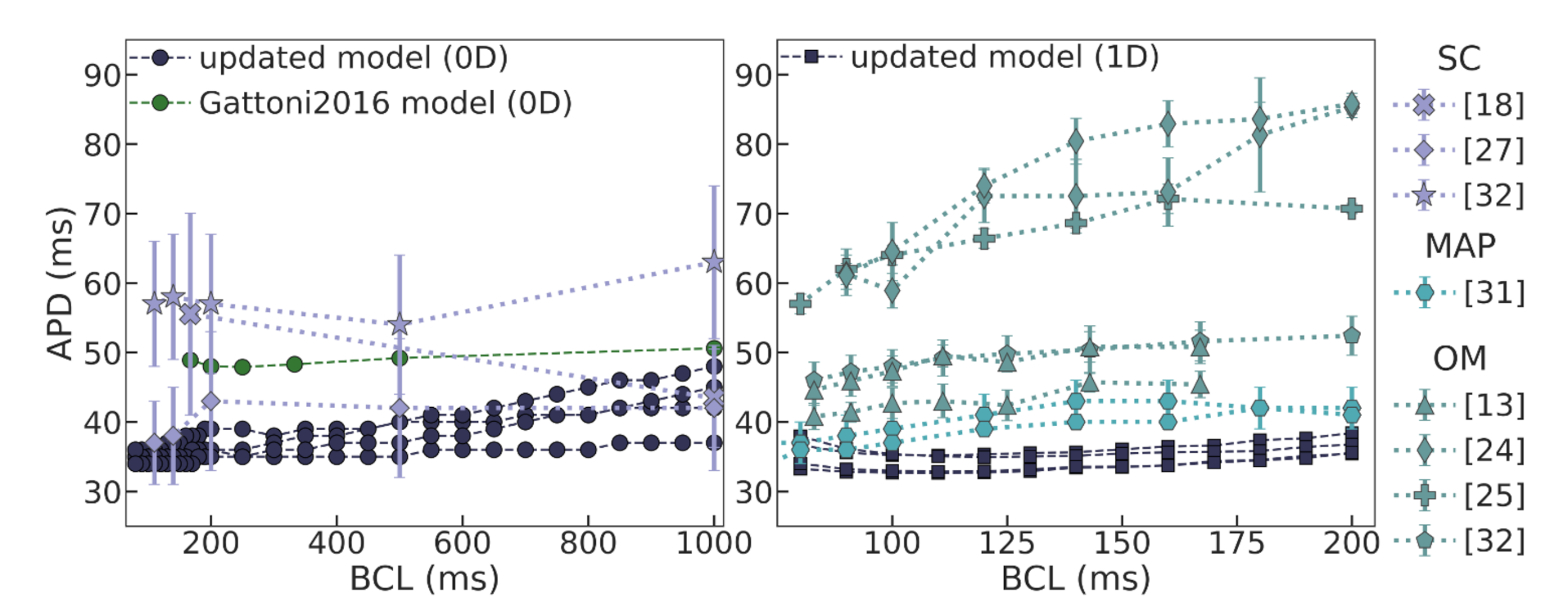
We utilized a baseline Gattoni2016 cellular model and built a population of models by varying a number of model parameters in a range of 0–200% of the original reference values. Models demonstrating instabilities during the series of calculations and/or not meeting calibration criteria for action potential and Ca
transient characteristics based on experimental data were rejected. The next step of model validation was to ensure consistency with experimental data on APD restitution in cells and myocardial tissue. In this study, we did not intend to reproduce particular restitution curves measured in experiments. For the purpose of our study, we were satisfied with any model that produces a positive restitution curve with a flat area at high pacing rates within an experimental range. Therefore, we have selected four such models with varying combinations of parameters as non-implausible samples from a population of applicable cellular models (see
Table 1). Despite the essential variety in model parameters, especially those for the L-type calcium current (
), the SERCA pump (
) and sodium-calcium and sodium-potassium exchangers (
and
) governing intracellular Ca
dynamics, our selected models produce action potential and restitution curves with similar characteristics (see
Figure 3 and
Figure 5,
Table 2). This indicates rather wide uncertainty in the model parameters, which could affect the simulation results as we showed in tissue settings (see discussion below). In case research aims to provide a more precise simulation of experimental restitution curves, one may use an approach suggested recently by Coveney and co-authors [
41], which is aimed to decrease uncertainty in the model parameter choice.
Then, the four models were used to simulate cellular remodeling in the myocardium of the post-infarction scar border zone. Consistent with experimental data, we modified the model parameters of potassium currents
and
, as suggested in [
29], in the selected models to simulate APD elongation in the border zone of an infarct scar. Of note, the response of our models was different to the same relative parameter change. Model 2 showed the highest effect of the ionic remodeling on APD, so we had to change the relative decrease in g
from 45 to 35% and in
from 39 to 29% compared to other models to avoid the EAD-like prolongation of action potentials during the repolarization phase in the single-cell simulations, as in the presence of EADs it is impossible to fit experimentally observed APD restitution curves. However, there is experimental data of either no significant change in APD [
35,
42] or even the opposite, namely APD shortening in the border zone [
43]. The direction of APD change depends on the stage of ischemia development, where APD shortening may reflect the activation of ATP-dependent potassium currents in the acute phase of ischemia development. APD elongation, on the other hand, may be associated with long-term opposite processes of cellular adaptation with a partial inhibition of potassium currents after reperfusion [
43]. Different scenarios of cellular remodeling could be analyzed further within the ventricular models.
The four selected ionic models were used to compare the dynamics of spiral waves initiated in 2D isotropic tissue using S1–S2 protocols. We computed generic tissue sheet models composed of either baseline cardiomyocyte models for normal tissue or modified border zone models with elongated action potentials. The tissue model with modified Cellular Model 2 demonstrated a specific behavior compared to the other models. The variation of the wave rotation period and meandering area was the highest in this model (see
Figure 6 and
Figure 7). Moreover, a spiral wave initiated via an S1–S2 protocol in this model was broken up at 490 ms, and then the two waves collapsed into one wave again which continued rotation. Note that the conduction velocity restitution curves for Models 1 and 2 show similar dependencies (see
Figure 5). Thus, we think that the instabilities which we observe are related to cellular (EAD) dynamics and not to conduction velocity restitution. The dependence of spiral waves on cellular properties has been demonstrated by Pathmanathan and co-authors in canine models of myocardial tissue [
44]. They studied the effects of parameter uncertainties in a cellular model on spiral wave dynamics in 2D tissue. Among all the parameters varied in the study,
uncertainty had the greatest impact on the tissue results, strongly affecting the type of re-entrant behavior. Consistent with these results, our Model 2 has the greatest value of maximal conductance
of the
current among the models we tested, suggesting its potential contribution to instability in the tissue.
In the third part of our study, we used a rat ventricular geometry and myocyte orientation model from [
16] to study scroll waves in 3D tissue. For the first time, we included a generic model of post-infarction scar and border zone into the LV model and used two different cellular ionic models to compare scroll wave behavior. The dimensions and location of the scar were similar to what we observed in experimental models of ischemia reperfusion in rats. Note that we did not intend to reproduce the exact shape and structure of the infarction injury area as observed in the experiment. In contrast, we used a simplified description of the scar and border zone, simulating the former as a non-excitable area and the latter as a uniform area containing cardiomyocytes with modified properties compared to normal tissue. It has been well documented that the activity in the infarct border zone within the intact heart depends not only on the properties of surviving myocardial cells, but also on structural changes in the area around the scar. It was shown that this region is extremely heterogeneous with fibrous inclusions and discontinuities in myocyte orientation, which can significantly influence electrical activity in the tissue along with cellular remodeling [
7,
35,
43,
45]. Comprehensive imaging and analysis of the structure and function in the infarct border zone and simulations of their consequences for excitation abnormalities in a rat ventricular strand were performed by Rutherford and co-authors [
46]. They demonstrated that structural heterogeneity in the per-infarct region provides a substrate for unidirectional propagation, rate-dependent regional slowing, and a conduction block. In our study, we were focused on the more general research question related to the possibility of simulating the instabilities of scroll wave rotation with a realistic anatomical ventricular geometry with the presence of a heterogeneity associated with myocardial damage accompanied by ischemia-reperfusion remodeling. Using different baseline cellular models, we were able to observe different behaviors of the scroll waves. We found either stable VT-like behavior of a scroll wave anchoring on the border of the scar independently of the two starting locations that we considered (see
Figure 9 and
Figure 10, Model 1), a self-terminating scroll wave initiated at the scar border, and a stabilized wave initiated outside of the scar area in the case of Cellular Model 2 (see
Figure 9 and
Figure 10, Model 2). Our results are in line with simulations by Whittaker and co-authors [
16], who have also observed different scenarios of scroll wave evolution in rat ventricular models without myocardial damage depending on the fiber orientation and the location of the wave induction area.
A comparison of scroll waves produced by different cellular models in a subject-specific anatomical ventricular model of swine heart with ischemia damage was recently performed by Ramirez and co-authors [
47]. The authors implemented a hybrid model of swine ventricular geometry with two cellular models of action potential in human cardiomyocytes using a phenomenological FK model [
30,
48] and an ionic TP06 model [
49]. They concluded that model choice is essential in terms of variations in local APD restitution within ventricles with heterogeneous cellular properties along and across the wall within the LV and between the two ventricles. At the same time, the propensity and characteristics of VF are mainly controlled by anatomy and structural parameters, rather than by regional restitution properties. In contrast, the results of our simulations suggest that even little variations in the regional properties in small rat hearts may essentially affect macroscopic scroll wave dynamics. This result points out possible difficulties in transferring the results of experiments and simulations from a small rat heart to a large human heart.