Global Analysis of a Reaction-Diffusion Within-Host Malaria Infection Model with Adaptive Immune Response
Abstract
1. Introduction
2. A Reaction-Diffusion within-Host Malaria Model with both Cytotoxic T Lymphocyte and Antibody Immune Responses
- (i)
- The dynamics of uninfected red blood cells is similar to the one given in model (1);
- (ii)
- The infected red blood cell population is divided into ring, trophozoite, and schizont infected red blood cells;
- (iii)
- The ring infected red blood cells increase due to the invasion of healthy red blood cells, and they decrease due to either the development into trophozoite infected red blood cells or natural death;
- (iv)
- The trophozoite infected red blood cells decrease due to either the development into schizont infected red blood cells or natural death;
- (v)
- The schizont infected red blood cells are depleted due to either the CTLs or natural death;
- (vi)
- Free merozoites in the blood increase as a result of schizonts bursting, and they decrease due to either the removal by antibodies or natural death;
- (vii)
- CTLs are stimulated by schizont infected red blood cells, while the production of antibodies is stimulated by free merozoites;
- (viii)
- CTLs and antibodies decrease duo to natural decay rates.
3. Basic Properties of Model (3)
- (a)
- The disease-free equilibrium always exists.
- (b)
- The immune-free equilibrium exists if .
- (c)
- The antibody-activated equilibrium exists if .
- (d)
- The CTL-activated equilibrium exists if .
- (e)
- The CTL/antibody coexistence equilibrium exists if and .
- (i)
- If and , then the equilibrium conditions are shortened toFrom Equations (13)–(17) we get two equilibrium points. The first one is the disease-free equilibrium , where . As , the equilibrium always exists. The second one is the immune-free equilibrium , whereandHence, the equilibrium exists if . Here, is the within-host reproductive number and it represents the average number of new infected cells generated by one infected cell in a healthy cell population [18]. It follows that the condition is needed for successful establishment of malaria infection in the absence of CTL and antibody immune responses.
- (ii)
- If and , then we get the antibody-activated equilibrium . The other components of are given bywhereConsequently, the equilibrium exists if . The threshold condition is required for activating the antibody immune response during malaria infection.
- (iii)
- If and , then we obtain the CTL-activated equilibrium . The other components of are given bywhereThus, the equilibrium exists if . The threshold condition is needed for activating the CTL immune response during malaria infection.
- (iv)
- If and , then we get the CTL/antibody coexistence equilibrium . The other components of are given bywhereand
4. Global Properties of Model (3)
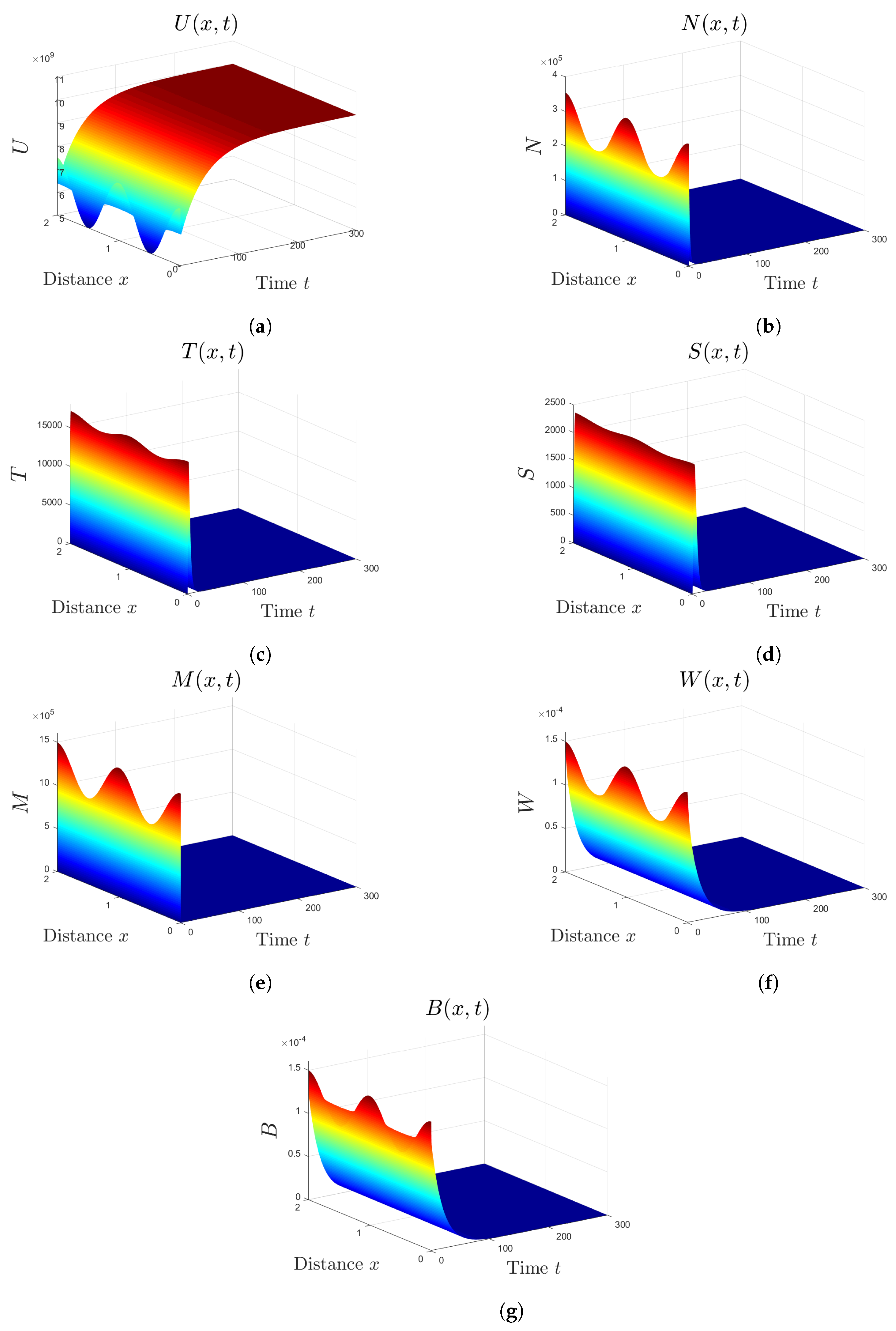
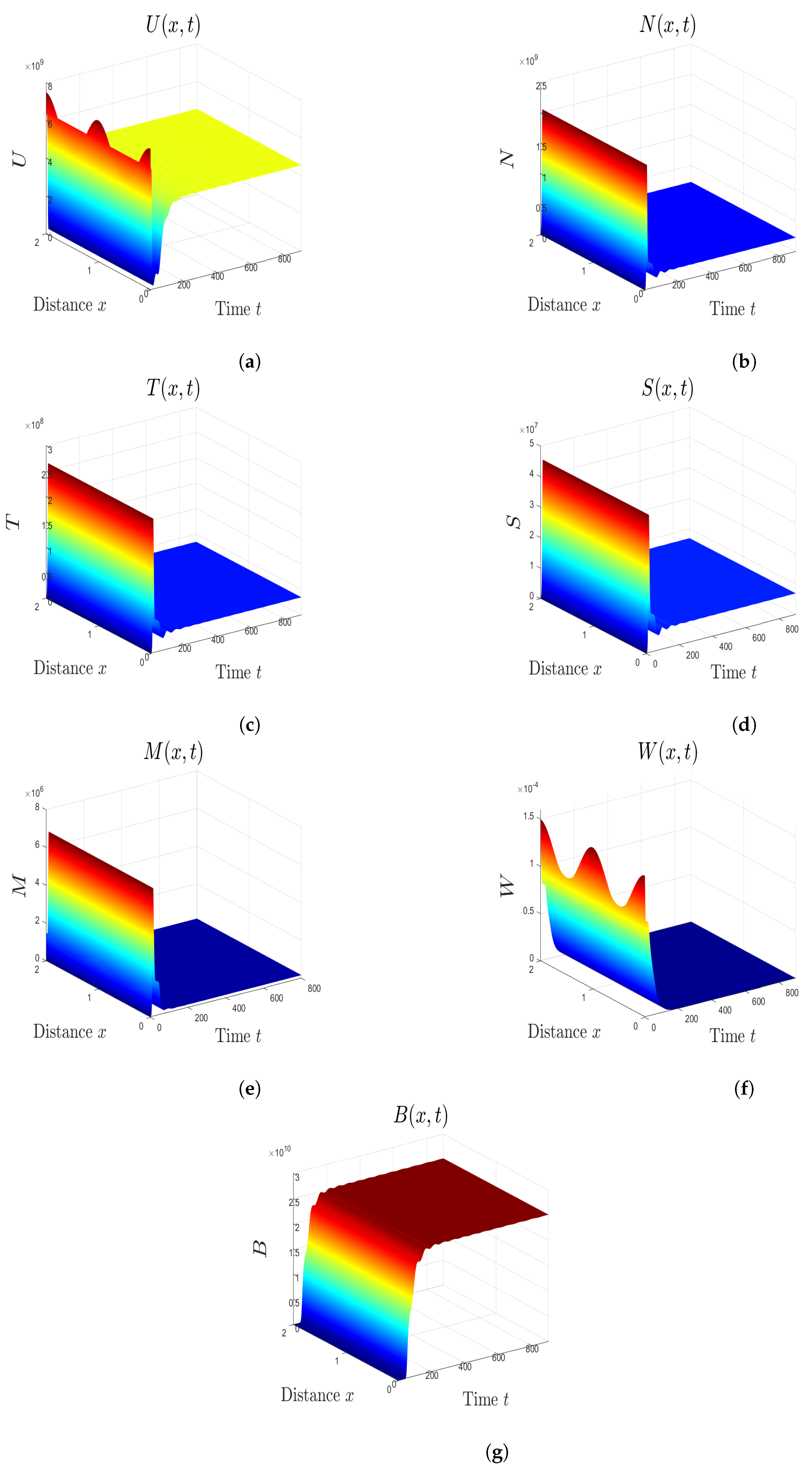
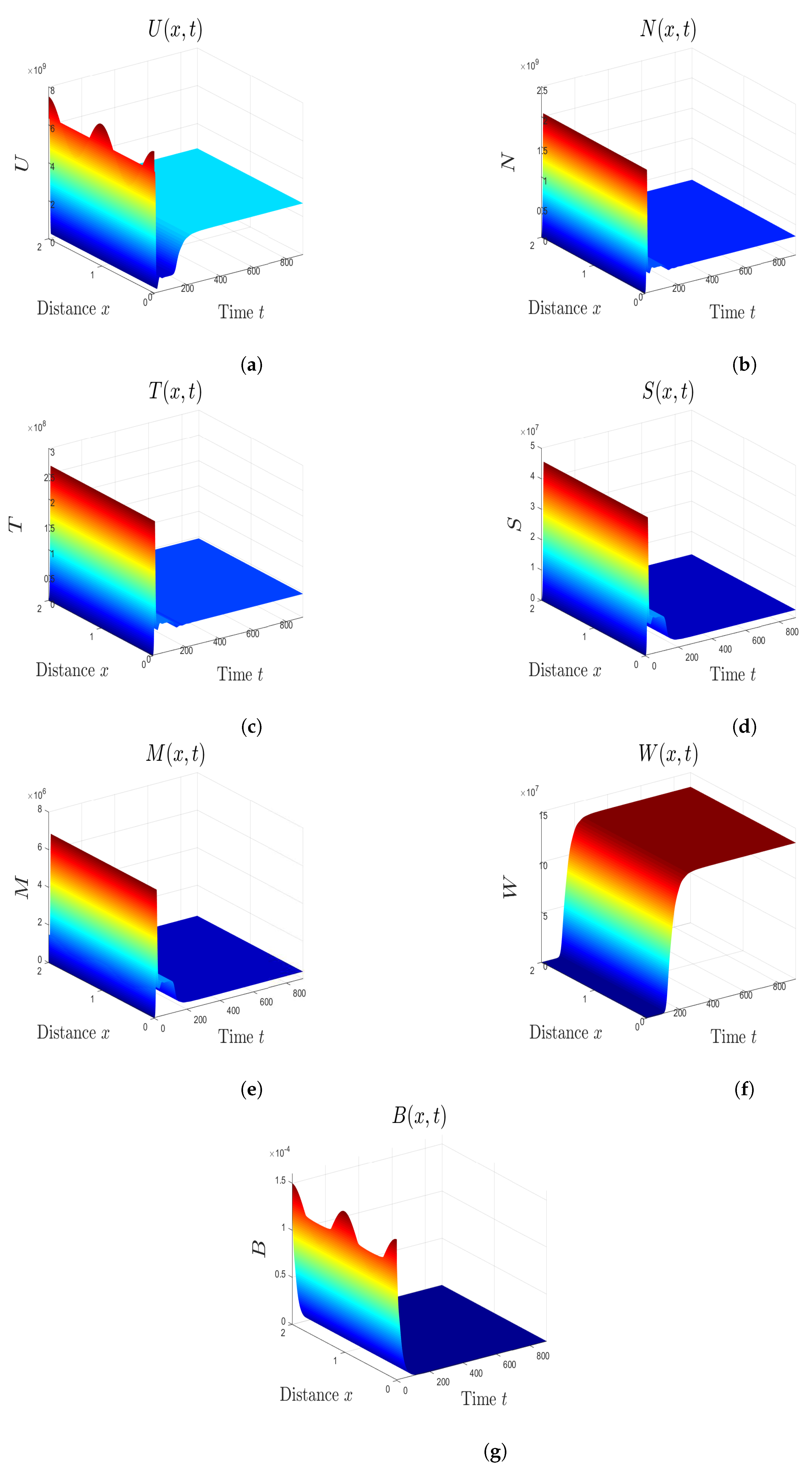
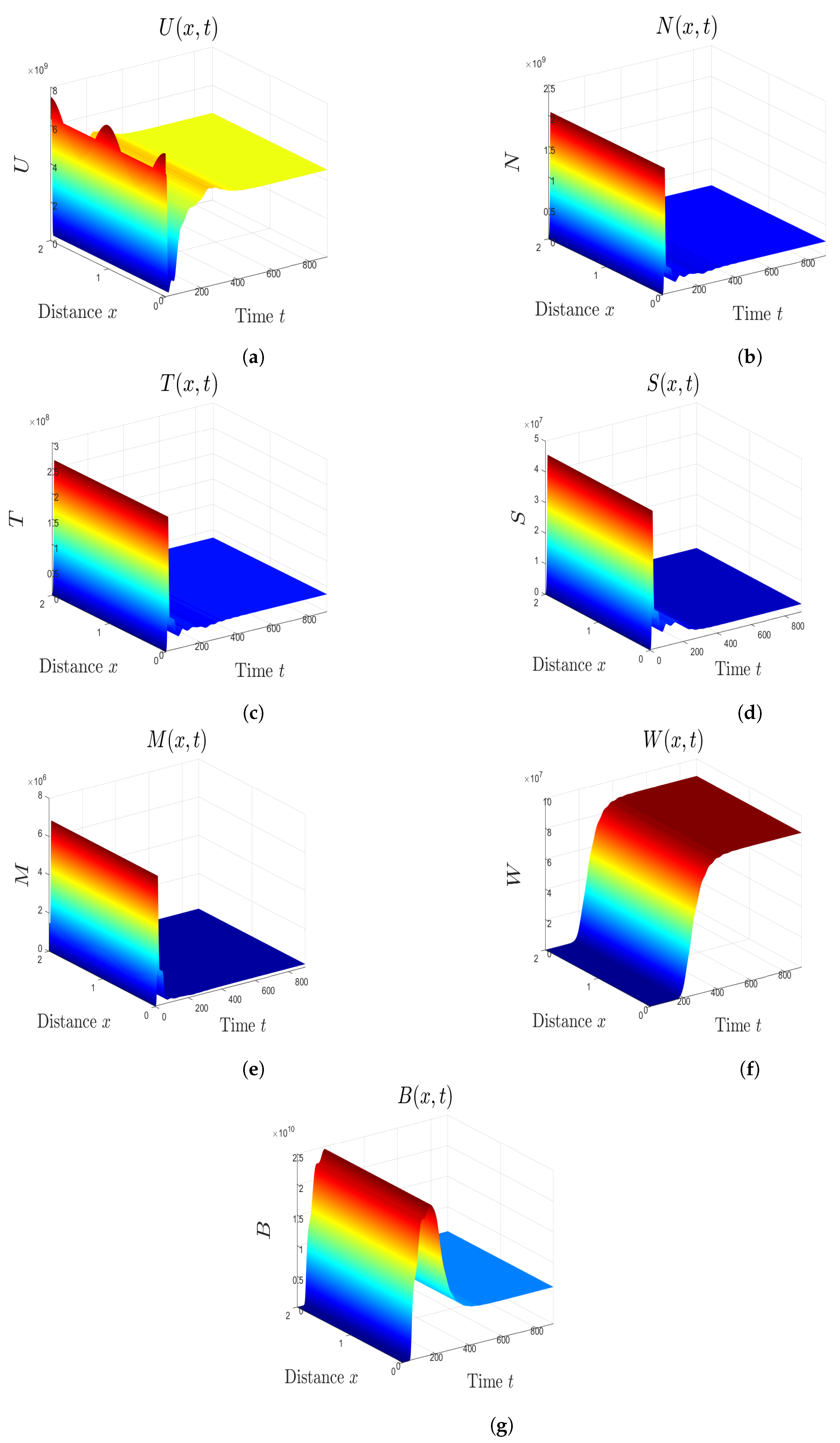
5. Numerical Simulations
5.1. Stability of Equilibria
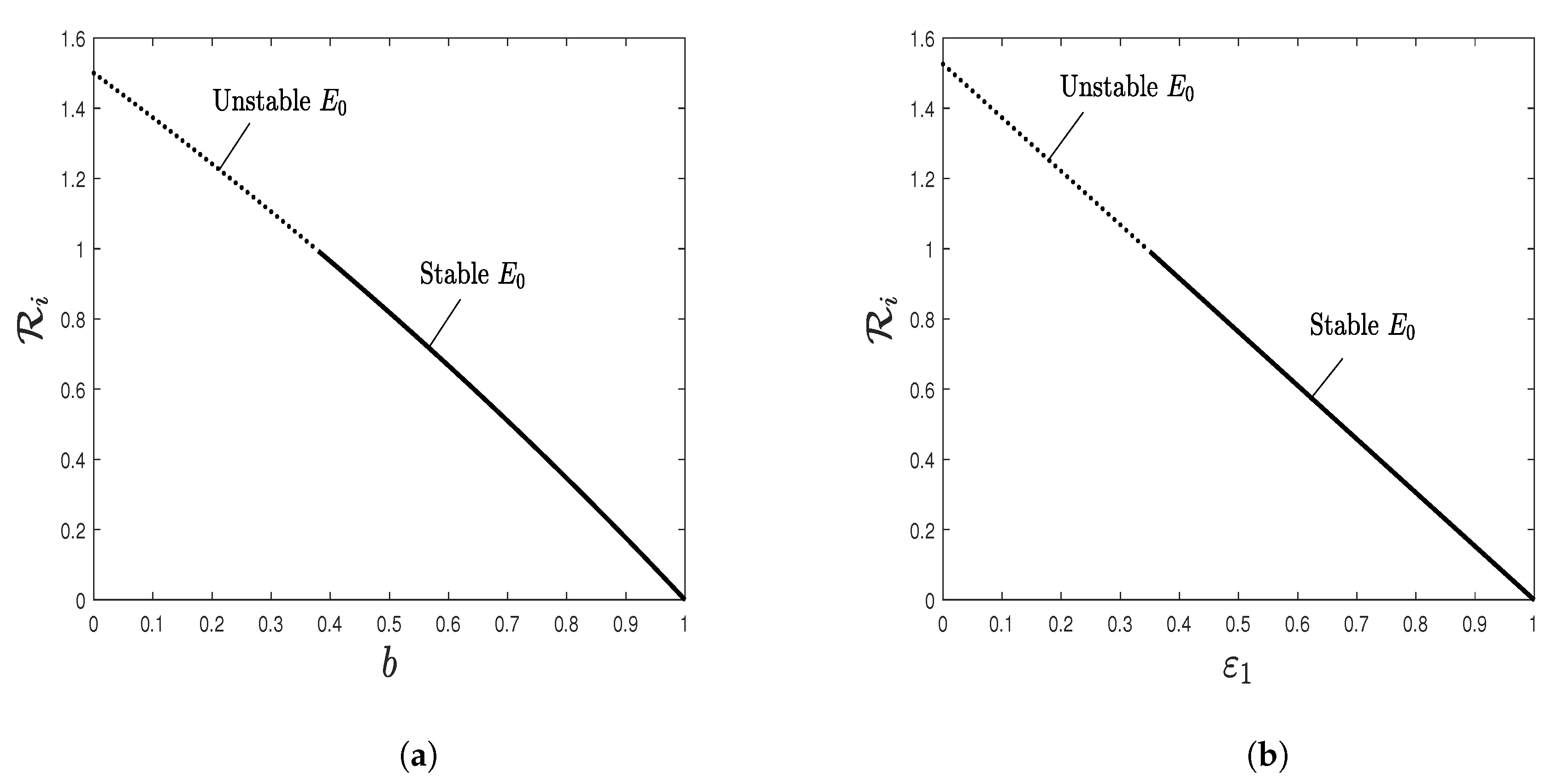
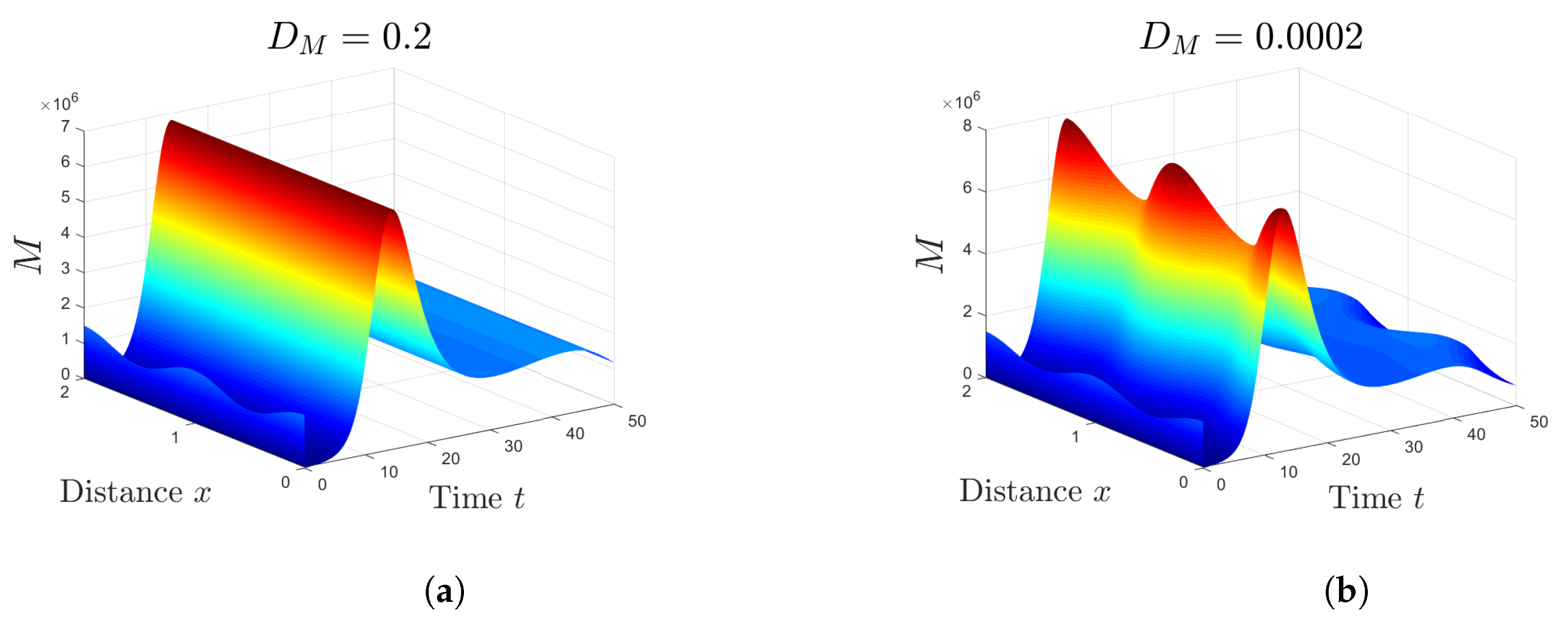
5.2. Effect of Isoleucine Starvation and Drugs on the Malaria Dynamics
6. Discussion
- (a)
- The disease-free equilibrium is usually defined and globally asymptotically stable if . This point corresponds to the elimination of malaria infection.
- (b)
- The immune-free equilibrium is defined if , and it is globally asymptotically stable if and . At this point, the malaria parasite P. falciparum succeeds in establishing the infection when the immune responses are not active.
- (c)
- The antibody-activated equilibrium is defined if , and it is globally asymptotically stable if . At this point, the antibody immune response is activated to attack the free blood merozoites.
- (d)
- The CTL-activated equilibrium is defined if , and it is globally asymptotically stable if . At this point, the CTL immune response is activated to kill the schizont infected red blood cells.
- (e)
- The CTL/antibody coexistence equilibrium is defined and globally asymptotically stable if and . Here, the malaria infection stimulates the CTL and antibody immune responses to fight the infection.
Author Contributions
Funding
Conflicts of Interest
References
- WHO. World Malaria Report 2019; License: CC BY-NC-SA 3.0 IGO; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Chen, H.; Wang, W.; Fu, R.; Luo, J. Global analysis of a mathematical model on malaria with competitive strains and immune responses. Appl. Math. Comput. 2015, 259, 132–152. [Google Scholar] [CrossRef]
- Khoury, D.; Aogo, R.; Randriafanomezantsoa-Radohery, G.; McCaw, J.; Simpson, J.; McCarthy, J.; Haque, A.; Cromer, D.; Davenport, M. Within-host modeling of blood-stage malaria. Immun. Rev. 2018, 285, 168–193. [Google Scholar] [CrossRef]
- Niger, A.; Gumel, A. Immune response and imperfect vaccine in malaria dynamics. Math. Popul. Stud. 2011, 18, 55–86. [Google Scholar] [CrossRef]
- Agusto, F.; Leite, M.; Orive, M. The transmission dynamics of a within-and between-hosts malaria model. Ecol. Complex. 2019, 38, 31–55. [Google Scholar] [CrossRef]
- Song, T.; Wang, C.; Tian, B. Mathematical models for within-host competition of malaria parasites. Math. Biosci. Eng. 2019, 16, 6623–6653. [Google Scholar] [CrossRef] [PubMed]
- Zaloumis, S.; Humberstone, A.; Charman, S.; Price, R.; Moehrle, J.; Gamo-Benito, J.; McCaw, J.; Jamsen, K.; Smith, K.; Simpson, J. Assessing the utility of an anti-malarial pharmacokinetic-pharmacodynamic model for aiding drug clinical development. Malaria J. 2012, 11, 303. [Google Scholar] [CrossRef] [PubMed]
- Nowak, M.A.; May, R.M. Virus Dynamics: Mathematical Principles of Immunology and Virology; Oxford University: Oxford, UK, 2000. [Google Scholar]
- Ren, X.; Tian, Y.; Liu, L.; Liu, X. A reaction-diffusion within-host HIV model with cell-to-cell transmission. J. Math. Biol. 2018, 76, 1831–1872. [Google Scholar] [CrossRef] [PubMed]
- Elaiw, A.M.; Hobiny, A.D.; Al Agha, A.D. Global dynamics of reaction-diffusion oncolytic M1 virotherapy with immune response. Appl. Math. Comput. 2020, 367, 1–21. [Google Scholar] [CrossRef]
- Elaiw, A.M.; AlShamrani, N.H. Stability of an adaptive immunity pathogen dynamics model with latency and multiple delays. Math. Meth. Appl. Sci. 2018, 41, 6645–6672. [Google Scholar] [CrossRef]
- Miao, H.; Teng, Z.; Abdurahman, X.; Li, Z. Global stability of a diffusive and delayed virus infection model with general incidence function and adaptive immune response. Comput. Appl. Math. 2017, 37, 3780–3805. [Google Scholar] [CrossRef]
- Elaiw, A.M.; Elnahary, E.K. Analysis of general humoral immunity HIV dynamics model with HAART and distributed delays. Mathematics 2019, 7, 157. [Google Scholar] [CrossRef]
- Elaiw, A.M.; Alshehaiween, S.F.; Hobiny, A.D. Global properties of delay-distributed HIV dynamics model including impairment of B-cell functions. Mathematics 2019, 7, 837. [Google Scholar] [CrossRef]
- Elaiw, A.M.; Alshehaiween, S.F. Global stability of delay-distributed viral infection model with two modes of viral transmission and B-cell impairment. Math. Methods Appl. Sci. 2020. [Google Scholar] [CrossRef]
- Elaiw, A.M.; AlShamrani, N.H. Stability of a general adaptive immunity virus dynamics model with multi-stages of infected cells and two routes of infection. Math. Methods Appl. Sci. 2020, 43, 1145–1175. [Google Scholar] [CrossRef]
- Anderson, R.; May, R.; Gupta, S. Non-linear phenomena in host-parasite interactions. Parasitology 1989, 99, S59–S79. [Google Scholar] [CrossRef]
- Anderson, R. Complex dynamic behaviours in the interaction between parasite populations and the host’s immune system. Int. J. Parasitol. 1998, 28, 551–566. [Google Scholar] [CrossRef]
- Saul, A. Models for the in-host dynamics of malaria revisited: Errors in some basic models lead to large over-estimates of growth rates. Parasitology 1998, 117, 405–407. [Google Scholar] [CrossRef]
- Gravenor, M.; Lloyd, A. Reply to: Models for the in-host dynamics of malaria revisited: Errors in some basic models lead to large over-estimates of growth rates. Parasitology 1998, 171, 409–410. [Google Scholar] [CrossRef][Green Version]
- Hoshen, M.; Heinrich, R.; Stein, W.; Ginsburg, H. Mathematical modelling of the within-host dynamics of Plasmodium falciparum. Parasitology 2000, 121, 227–235. [Google Scholar] [CrossRef]
- Iggidr, A.; Kamgang, J.; Sallet, G.; Tewa, J. Global analysis of new malaria intrahost models with a competitive exclusion principle. SIAM J. Appl. Math. 2006, 67, 260–278. [Google Scholar] [CrossRef]
- Saralamba, S.; Pan-Ngum, W.; Maude, R.; Lee, S.J.; Tarning, J.; Lindegårdh, N.; Chotivanich, K.; Nosten, F.; Day, N.; Socheat, D.; et al. Intrahost modeling of artemisinin resistance in Plasmodium falciparum. Proc. Natl. Acad. Sci. USA 2011, 108, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Demasse, R.; Ducrot, A. An age-structured within-host model for multistrain malaria infections. SIAM J. Appl. Math. 2013, 73, 572–593. [Google Scholar] [CrossRef]
- Li, Y.; Ruan, S.; Xiao, D. The within-host dynamics of malaria infection with immune response. Math. Biosci. Eng. 2011, 8, 999–1018. [Google Scholar] [PubMed]
- Tumwiine, J.; Mugisha, J.; Luboobi, L. On global stability of the intra-host dynamics of malaria and the immune system. J. Math. Anal. Appl. 2008, 341, 855–869. [Google Scholar] [CrossRef]
- Hetzel, C.; Anderson, R. The within-host cellular dynamics of bloodstage malaria: Theoretical and experimental studies. Parasitology 1996, 113, 25–38. [Google Scholar] [CrossRef]
- Tewa, J.; Fokouop, R.; Mewoli, B.; Bowong, S. Mathematical analysis of a general class of ordinary differential equations coming from within-hosts models of malaria with immune effectors. App. Math. Comput. 2012, 2018, 7347–7361. [Google Scholar] [CrossRef]
- Tchinda, P.; Tewa, J.; Mewoli, B.; Bowong, S. Mathematical analysis of a general class of intra-host model of malaria with “allee effect“. J. Nonl. Syst. Appl. 2013, 36, 52. [Google Scholar]
- Cai, L.; Tuncer, N.; Martcheva, M. How does within-host dynamics affect population-level dynamics? Insights from an immuno-epidemiological model of malaria. Math. Methods Appl. Sci. 2017, 40, 6424–6450. [Google Scholar] [CrossRef]
- Orwa, T.; Mbogo, R.; Luboobi, L. Mathematical model for the in-host malaria dynamics subject to malaria vaccines. Lett. Biomath. 2018, 5, 222–251. [Google Scholar] [CrossRef]
- Funk, G.; .Jansen, V.A.A.; Bonhoeffer, S.; Killingback, T. Spatial models of virus-immune dynamics. J. Theor. Biol. 2005, 233, 221–236. [Google Scholar] [CrossRef]
- Takoutsing, E.; Temgoua, A.; Yemele, D.; Bowong, S. Dynamics of an intra-host model of malaria with periodic antimalarial treatment. Int. J. Nonl. Sci. 2019, 27, 148–164. [Google Scholar]
- Babbitt, S.; Altenhofen, L.; Cobbold, S.; Istvan, E.; Fennell, C.; Doerig, C.; Llinas, M.; Goldberg, D. Plasmodium falciparum responds to amino acid starvation by entering into a hibernatory state. Proc. Natl. Acad. Sci. USA 2012, 109, E3278–E3287. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, Z. Dynamics of a diffusive HBV model with delayed Beddington-DeAngelis response. Nonl. Anal. Real World Appl. 2014, 15, 118–139. [Google Scholar] [CrossRef]
- Xu, Z.; Xu, Y. Stability of a CD4+ T cell viral infection model with diffusion. Int. J. Biomath. 2018, 11, 1–16. [Google Scholar] [CrossRef]
- Smith, H.L. Monotone Dynamical Systems: An Introduction to the Theory of Competitive and Cooperative Systems; American Mathematical Society: Providence, RI, USA, 1995. [Google Scholar]
- Protter, M.H.; Weinberger, H.F. Maximum Principles in Differential Equations; Prentic Hall: Englewood Cliffs, NJ, USA, 1967. [Google Scholar]
- Henry, D. Geometric Theory of Semilinear Parabolic Equations; Springer: New York, NY, USA, 1993. [Google Scholar]
- Hattaf, K.; Yousfi, N. Global stability for reaction-diffusion equations in biology. Comput. Math. Appl. 2013, 66, 1488–1497. [Google Scholar] [CrossRef]
- Hsu, S. A survey of constructing lyapunov functions for mathematical models in population biology. Taiwan. J. Math. 2005, 9, 151–173. [Google Scholar] [CrossRef]
- Hale, J.K.; Verduyn Lunel, S.M. Introduction to Functional Differential Equations; Springer: New York, NY, USA, 1993. [Google Scholar]
- Khalil, H.K. Nonlinear Systems; Prentice-Hall: New Jersey, NJ, USA, 1996. [Google Scholar]
- Bakker, N.; Eling, W.; De Groot, A.; Sinkeldam, E.; Luyken, R. Attenuation of malaria infection, paralysis and lesions in the central nervous system by low protein diets in rats. Acta Trop. 1992, 50, 285–293. [Google Scholar] [CrossRef]
- Edirisinghe, J.; Fern, E.; Targett, G. Resistance to superinfection with Plasmodium berghei in rats fed a protein-free diet. Trans. R Soc. Trop. Med. Hyg. 1982, 76, 382–386. [Google Scholar] [CrossRef]
- Sun, H.; Wang, J. Dynamics of a diffusive virus model with general incidence function, cell-to-cell transmission and time delay. Comput. Math. Appl. 2019, 77, 284–301. [Google Scholar] [CrossRef]
- Elaiw, A.M.; Raezah, A.A. Stability of general virus dynamics models with both cellular and viral infections and delays. Math. Methods Appl. Sci. 2017, 40, 5863–5880. [Google Scholar] [CrossRef]
- Elaiw, A.M.; AlShamrani, N.H. Global stability of a delayed adaptive immunity viral infection with two routes of infection and multi-stages of infected cells. Commun. Nonl. Sci. Num. Simul. 2020, 86, 1–27. [Google Scholar] [CrossRef]
- Elaiw, A.M.; AlAgha, A.D. Analysis of a Delayed and Diffusive Oncolytic M1 Virotherapy Model with Immune Response; Nonlinear Analysis: Real World Applications; Article 103116; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar] [CrossRef]
- Luo, J.; Wang, W.; Chen, H.; Fu, R. Bifurcations of a mathematical model for HIV dynamics. J. Math. Anal. Appl. 2016, 434, 837–857. [Google Scholar] [CrossRef]

| Parameter | Description | Value | Reference |
|---|---|---|---|
| Recruitment rate of red blood cells | cell mLday | [27] | |
| Death rate of uninfected red blood cells | day | [27] | |
| Infection rate of red blood cells | Varied | – | |
| Progression rate from rings to trophozoites | day | [31] | |
| Progression rate from trophozoites to schizonts | day | [31] | |
| Number of merozoites released per schizont infected cell | 16 | [17] | |
| Killing rate of infected cells by CTLs | mL cellday | [27] | |
| Removal rate of free merozoites by antibodies | mL cellday | [27] | |
| Stimulation rate of CTL immune response | Varied | – | |
| Stimulation rate of antibody immune response | Varied | – | |
| Death rate of ring infected red blood cells | day | [31] | |
| Death rate of trophozoite infected red blood cells | day | [31] | |
| Death rate of schizont infected red blood cells | day | [31] | |
| Death rate of free merozoites | 48 day | [27] | |
| Decay rate of CTLs | day | [17] | |
| Decay rate of antibodies | day | [17] | |
| Efficacy of blood-stage treatment | Varied | – | |
| b | Efficacy of isoleucine starvation | Varied | – |
| Efficacy of blood-stage treatment | Varied | – | |
| Diffusion coefficient of uninfected red blood cells | Assumed | ||
| Diffusion coefficient of ring infected red blood cells | Assumed | ||
| Diffusion coefficient of trophozoite infected red blood cells | Assumed | ||
| Diffusion coefficient of schizont infected red blood cells | Assumed | ||
| Diffusion coefficient of free merozoites | Assumed | ||
| Diffusion coefficient of CTLs | Assumed | ||
| Diffusion coefficient of antibodies | Assumed |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elaiw, A.; Al Agha, A. Global Analysis of a Reaction-Diffusion Within-Host Malaria Infection Model with Adaptive Immune Response. Mathematics 2020, 8, 563. https://doi.org/10.3390/math8040563
Elaiw A, Al Agha A. Global Analysis of a Reaction-Diffusion Within-Host Malaria Infection Model with Adaptive Immune Response. Mathematics. 2020; 8(4):563. https://doi.org/10.3390/math8040563
Chicago/Turabian StyleElaiw, Ahmed, and Afnan Al Agha. 2020. "Global Analysis of a Reaction-Diffusion Within-Host Malaria Infection Model with Adaptive Immune Response" Mathematics 8, no. 4: 563. https://doi.org/10.3390/math8040563
APA StyleElaiw, A., & Al Agha, A. (2020). Global Analysis of a Reaction-Diffusion Within-Host Malaria Infection Model with Adaptive Immune Response. Mathematics, 8(4), 563. https://doi.org/10.3390/math8040563





