In this paper, we considered the following stochastic version of the model
accounting for parametric random disturbances in the flux
:
Here,
is a white Gaussian noise of intensity
. Thus, in system (
2), we have
Under stochastic perturbations, the random solutions of system (
2) leave the attractors from which they started and form some probability distribution. For weak noise, random solutions are localised near the initial deterministic attractors. In
Figure 3, for
,
= 0.02, we show in green system (
2) solutions starting at the equilibrium
(red dot) and the cycle
C (brown). As can be seen, in both cases, the dispersion of random solutions is quite non-uniform. For a quantitative description of this dispersion, we apply the stochastic sensitivity technique [
28,
31].
3.1. Stochastic Sensitivity of Attractors
In understanding the internal mechanisms of the various noise-induced deformations of calcium oscillations, a stochastic sensitivity analysis of the coexisting attractors plays an important role.
Stochastic sensitivity theory was initiated by studies on the quasipotential approximation of nonlinear stochastic differential equations. Under the influence of random perturbations, around the attractors of the system, a probability distribution is formed, the geometrical properties of which are determined by the spectral characteristics of the corresponding stochastic sensitivity matrix. Using these spectral characteristics, one can construct a confidence domain around the attractor that approximates the dispersion of the random states: an ellipsoid around the equilibrium and a torus around the cycle [
36]. For discrete-time systems, the theory of stochastic sensitivity has been developed for the cases of quasiperiodic and chaotic attractors (see, e.g., [
37]). This theory and the confidence domain method have been successfully applied to studies of the constructive role of noise in mathematical models of neural activity [
32,
38], population dynamics [
34,
39], climatology [
33], pattern formation [
40], etc.
Let us briefly discuss the stochastic sensitivity technique for equilibria and cycles of the two-dimensional continuous-time model (
2) of calcium stochastic dynamics. In this case, the stochastic sensitivity of the stable equilibrium
is determined by the
-matrix
W, which is a unique solution of the matrix equation
due to the stability of the equilibrium. Here,
F is the Jacobi matrix of the initial deterministic system at point
M, and
G is the matrix characterizing random disturbances. For system (
2), matrix
G is written as
Let
be eigenvalues and
be normalized eigenvectors of the stochastic sensitivity matrix
W. The geometrical arrangement of system (
2)’s random solutions around the equilibrium
is described by the confidence ellipse
Here,
are coordinates of this ellipse in the basis of eigenvectors
of the matrix
W with the point
M as an origin, and
is a fiducial probability. Note that eigenvalues
and
define the eccentricity of the confidence ellipse. In
Figure 4, plots of eigenvalues
of the stochastic sensitivity matrices are shown for the equilibrium
(
a) and the equilibrium
(
b). As parameter
p approaches the bifurcation values
and
, stochastic sensitivity increases unboundedly. In
Figure 3, the confidence ellipse is shown around
for
.
The stochastic sensitivity
of the
T-periodic solution
which determines the stable cycle
C in the two-dimensional deterministic system is a solution of the boundary problem
where
Here,
is a normalized vector that is orthogonal to cycle
C at the current point
. The geometrical arrangement of system (
2) random solutions around cycle
C is described by the confidence band. The boundaries of this band are written as:
Characterizing the stochastic sensitivity of the cycle as a whole, we use the stochastic sensitivity factor
In
Figure 5a, stochastic sensitivity function
is plotted for
(green),
(blue), and
(magenta). As can be seen, the stochastic sensitivity of cycles under consideration is highly nonuniform. In
Figure 5b, the stochastic sensitivity factor
is plotted versus parameter
p. This function tends to infinity as parameter
p approaches bifurcation values
and
. In
Figure 3, the confidence band around the deterministic cycle
C is shown for
. As can be seen, this band adequately reflects non-uniformity of dispersion of random solutions along the cycle.
It should be noted that the stochastic sensitivity technique provides a quantitative description of the response of calcium oscillations to random perturbations. The confidence domain method allows one to parametrically study complex deformations of calcium dynamics in multistable systems.
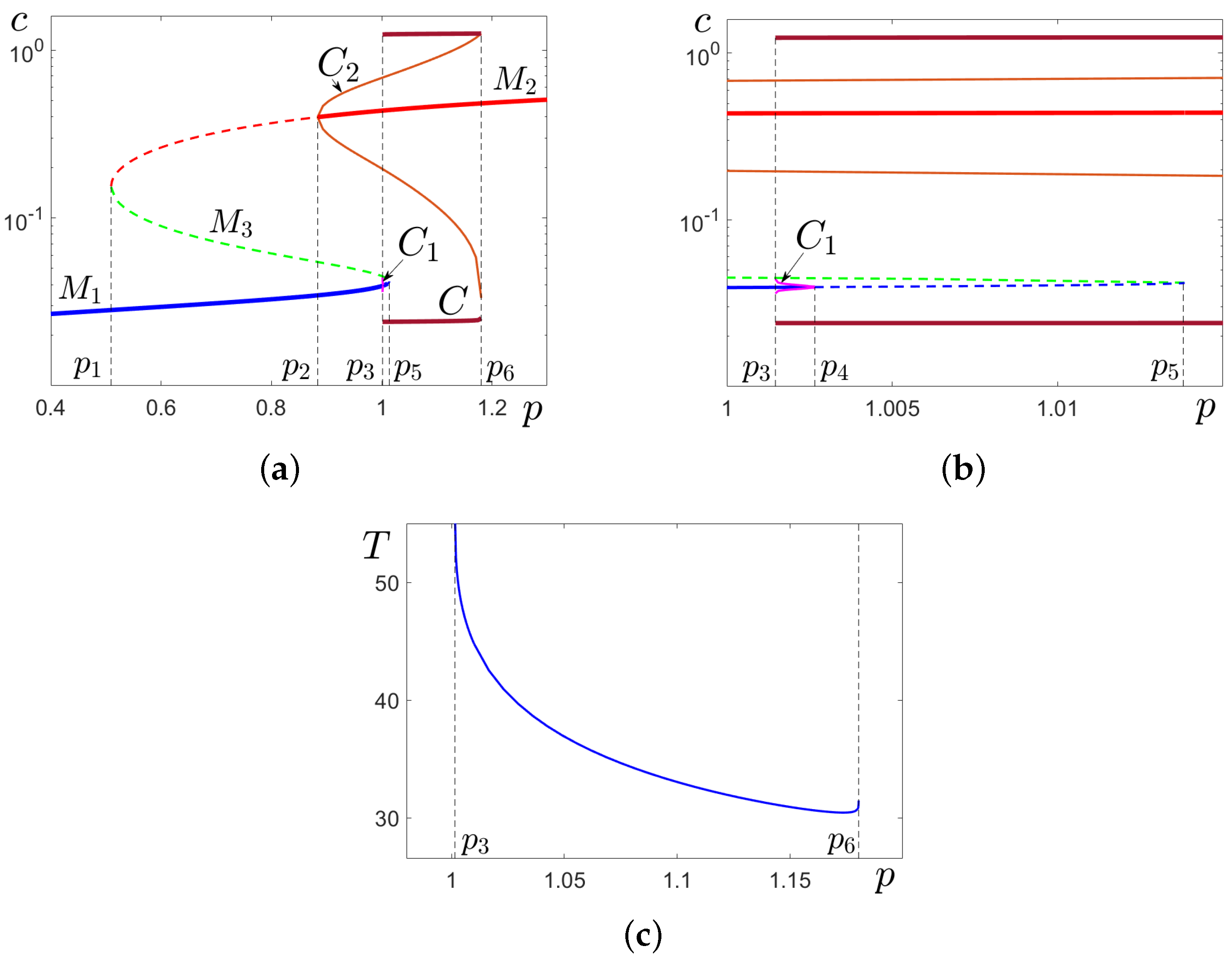
3.2. Noise-Induced Effects in the Tristability Zone
Here, we consider a parametric tristability zone when the concentration
p of the inositol triphosphate
belongs to the range
. In this zone, system (
1) shows the coexistence of the stable equilibria
,
and the stable limit cycle
C. Recall that basins of
,
are separated from the basin of
C by orbits of unstable cycles
, respectively (see
Figure 1 and
Figure 2). In this multistability zone, system (
2) exhibits rather complex scenarios of stochastic transformations.
Let us first compare the effect of noise on the equilibrium
and the limit cycle
C. These attractors are very close to each other, and noise-induced transformations occur at very small noise. In
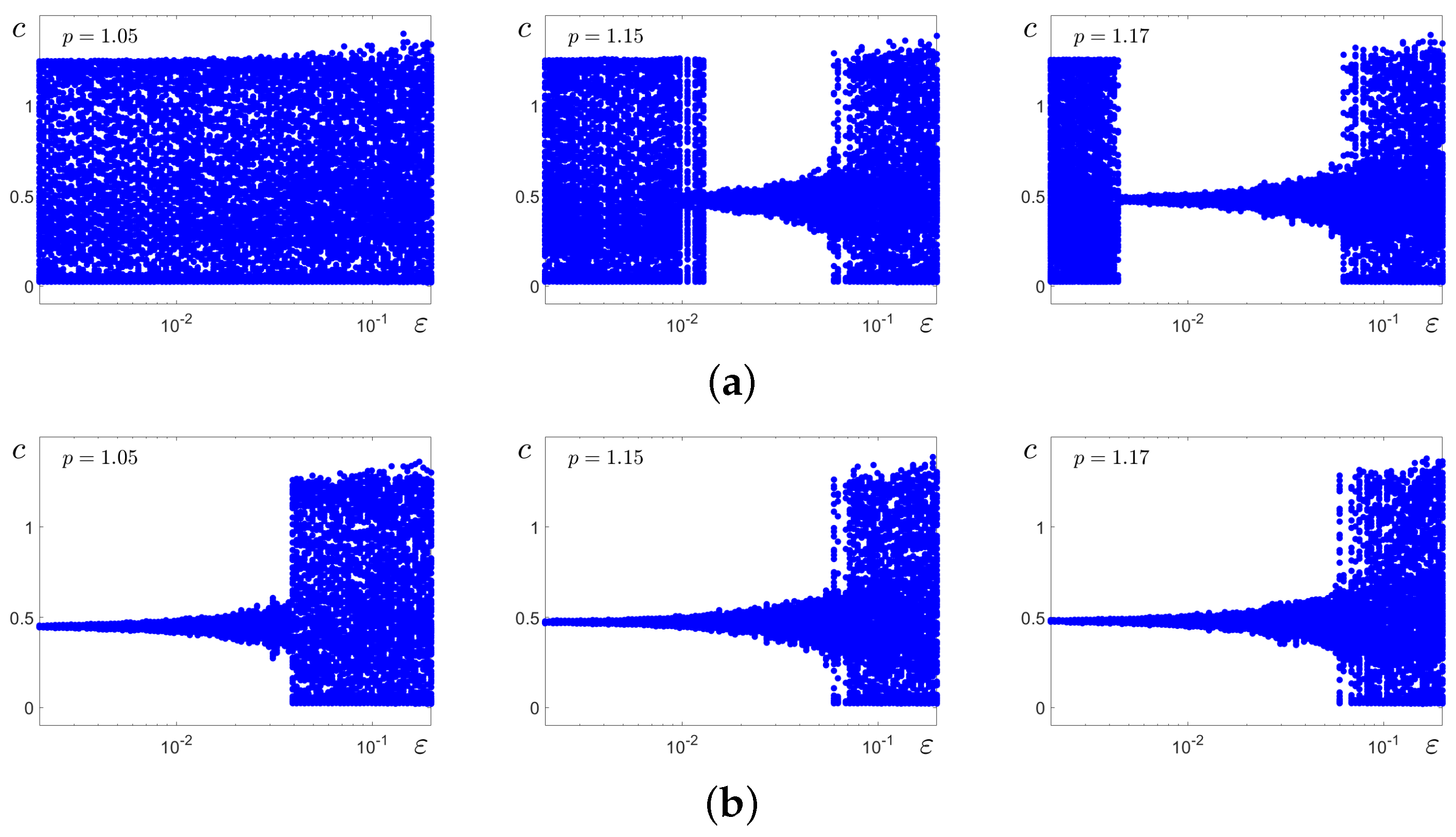
Figure 6, for three values of
p, we show changes in the dispersion of random states for system (
2)’s solutions starting at the stable equilibrium
(
Figure 6a) and cycle
C (
Figure 6b). In
Figure 6a, one can see how the regime of small-amplitude stochastic oscillations (SASOs) near
abruptly transforms into the large-amplitude stochastic oscillation (LASO) mode. More complex, three-stage scenarios are shown in
Figure 6b. For weak noise, random solutions starting at cycle
C slightly fluctuate around it. When
exceeds some threshold, random solutions leave the basin of the cycle and begin to oscillate near
with small amplitudes. Such a SASO regime is observed in some
window. As the noise is further increased, this mode breaks down and the system enters the LASO mode again. It should be noted that the size of the
window of SASOs decreases, and this window disappears with the increase in the parameter
p (see
Figure 6b).
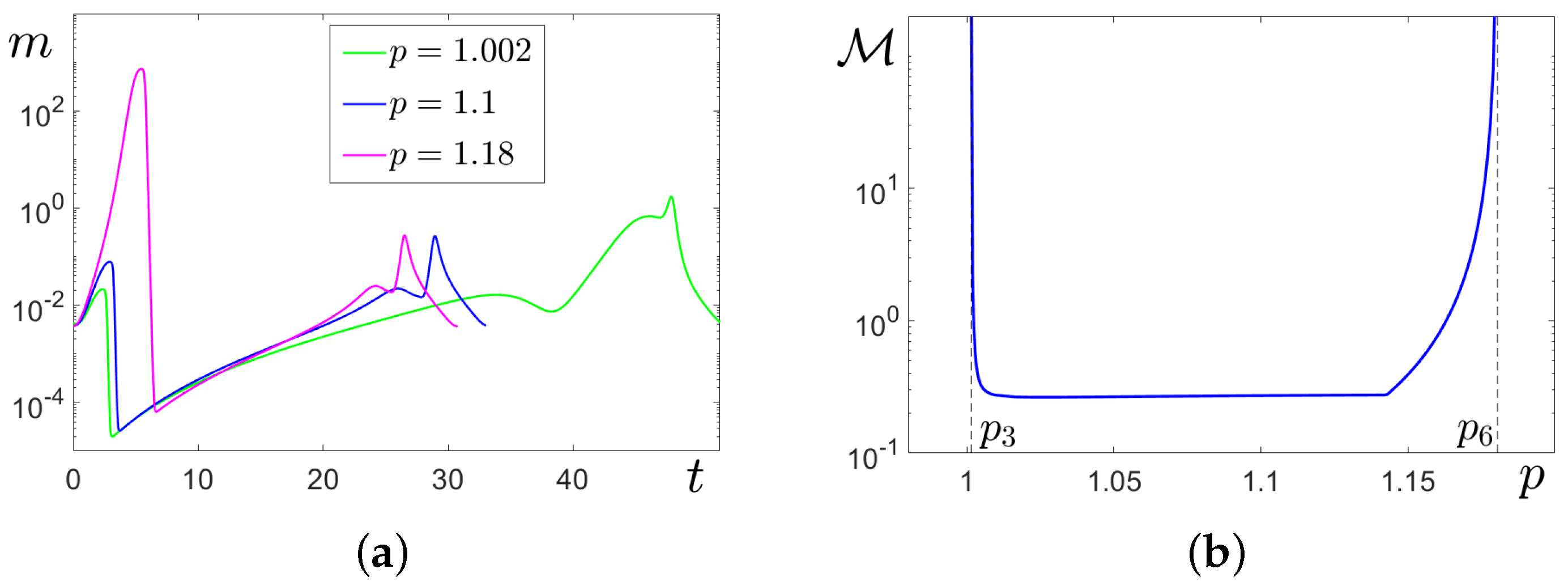
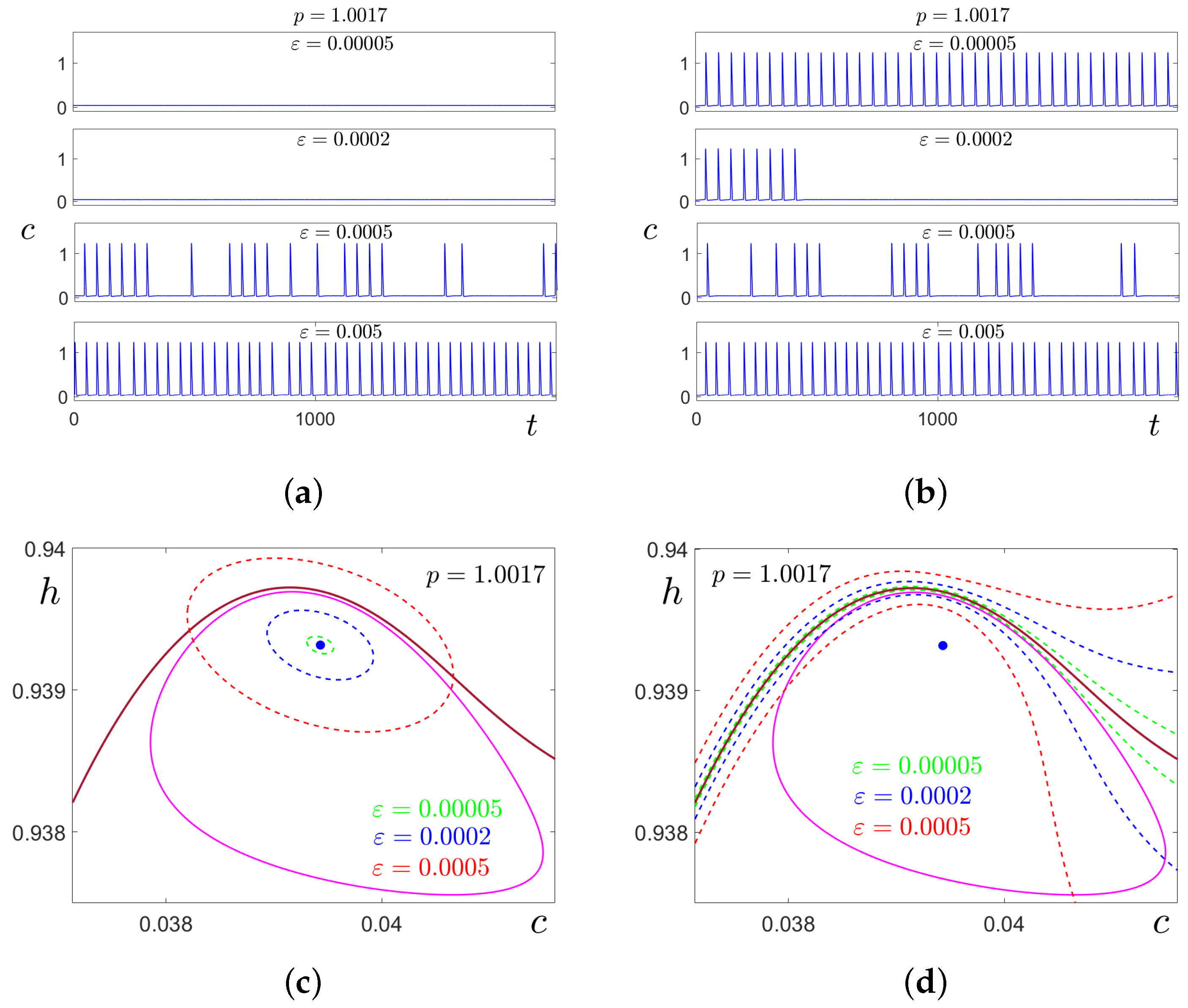
In
Figure 7a,b, these transformations are illustrated for
by time series of stochastic solutions starting at
(
Figure 7a) and at cycle
C (
Figure 7b). Here, the results of stochastic forcing for four values of the noise intensity
are compared. At
, the random solutions oscillate slightly around the deterministic attractors where they started. For
, this behaviour is preserved for solutions starting at
, but solutions starting at
C exhibit a unidirectional transition
. At
, for both cases, the noise induces two-way transitions
, and one can see mixed-mode stochastic oscillations with alternation of SASOs near
and LASOs of spike form near
C. With a further increase in noise, the frequency of spikes grows (see bottom panels for
).
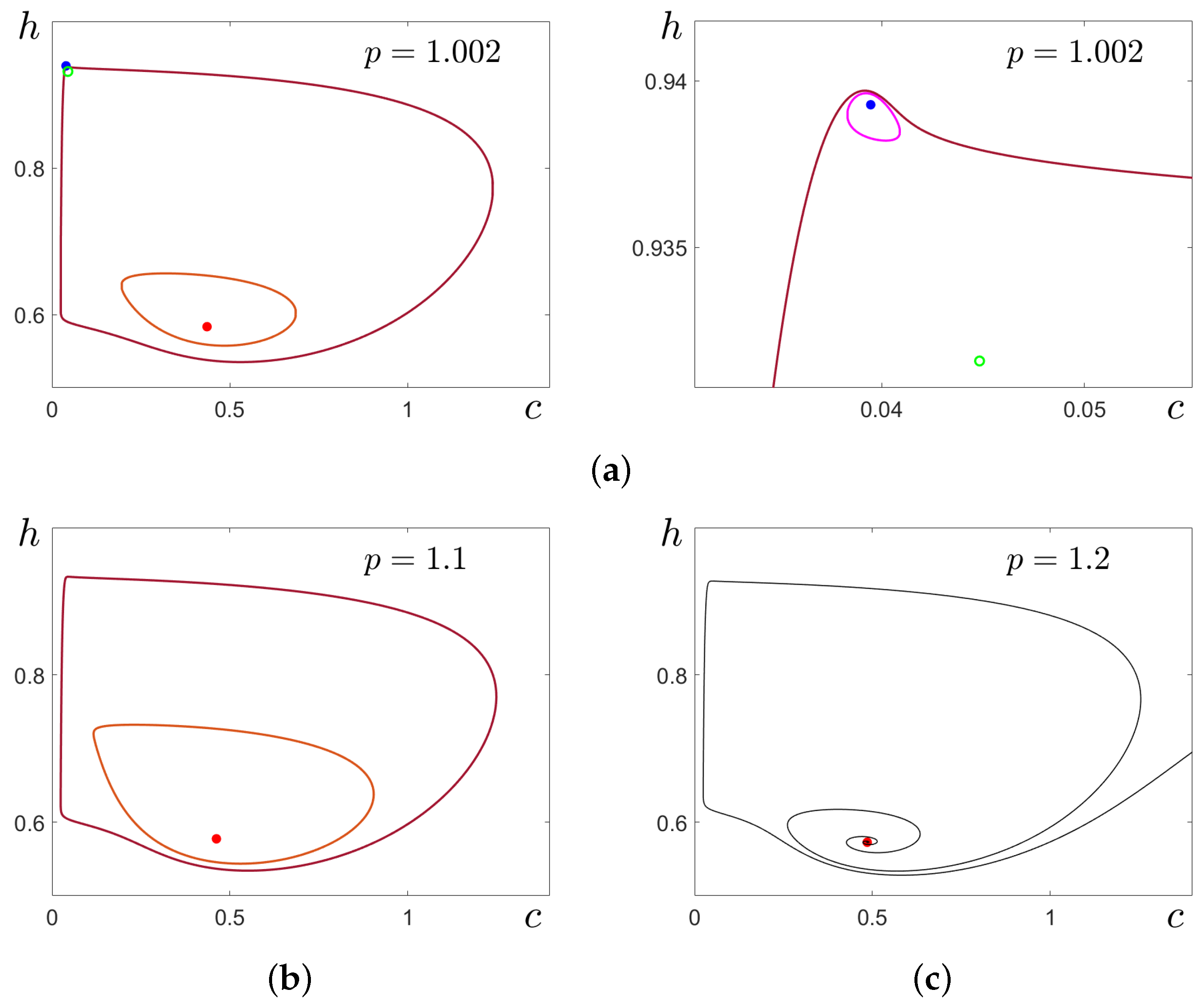
To analyse these transitions parametrically, one can apply the stochastic sensitivity technique and method of confidence domains presented in
Section 3.1. In
Figure 7c,d, for
, an enlarged fragment of the phase plane is presented. Here, the stable equilibrium
is shown by a blue dot, the stable cycle
C is plotted in brown, and the unstable cycle
separating the basins of these attractors is shown in magenta. For three values of noise intensity, confidence ellipses (dashed) around
are shown in
Figure 7c, and confidence bands (dashed) around cycle
C are shown in
Figure 7d by the same colours. As can be seen in
Figure 7c, for
and
, confidence ellipses entirely belong to the basin of
. This arrangement means that random trajectories starting at
are located near
. For
, the confidence ellipse (red) crosses the separatrix
and partially occupies the basin of
C. It means that random trajectories with high probability fall into the basin of
C and exhibit a LASO regime. As can be seen in
Figure 7d, for
, the confidence band lies completely in the basin of
C. For
, the band contains points of the basin of
, while the ellipse does not intersect the separatrix. This mutual arrangement of the ellipse and the band signals unidirectional transitions
, and thus, for
, the “stochastic preference” [
41] of the equilibrium
can be stated. For
, both confidence domains cross the separatrix, so two-way transitions
occur, generating a mixed-type stochastic oscillation regime (see
Figure 7a,b).
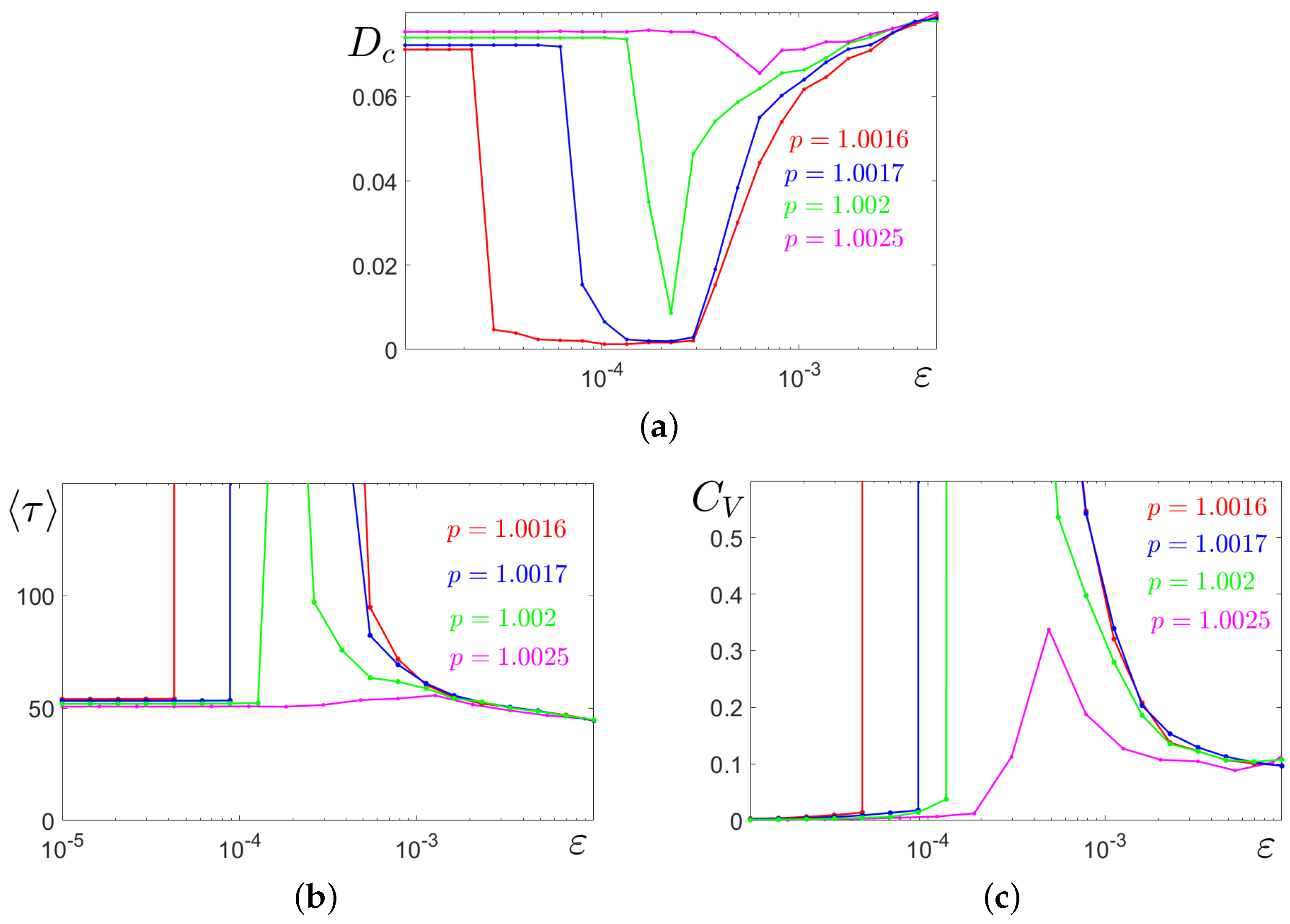
Results of the detailed statistical analysis of noise-induced amplitude and frequency transformations of solutions starting at the cycle
C are represented in
Figure 8 versus the noise intensity for four values of
p which belong to the tristability parametric zone. In
Figure 8a, in plots of variance
, it is well seen how the
windows of SASO regimes narrow and disappear with the increase in
p.
To analyse the frequency characteristics of complex stochastic spike oscillations, we use the statistics of interspike intervals
: the mean values
(
Figure 8b) and the coefficient of variation
Figure 8c). For weak noise, when stochastic trajectories are close to the deterministic cycle, the coefficient
is almost zero, so stochastic oscillations are almost coherent. A sharp jump up and down in the plot of
well localises the boundaries of the
interval of the SASO regime. In the
zone of the mixed-mode stochastic oscillations, for
, the frequency of spikes grows with increasing
(see also the time series in
Figure 7b).
Let us fix
and consider the effect of noise on the equilibrium
and the limit cycle
C. In
Figure 9a, we show changes in the dispersion of random states for system (
2) solutions starting at the stable equilibrium
. Here, an abrupt noise-induced transition
is well seen. In
Figure 9b, time series of stochastic solutions starting at
are plotted for three values of
. For
, these solutions demonstrate a SASO regime near
. For
, and the stochastic transition from SASO to LASO with spike oscillations is observed. For
, one can see the generation of mixed-mode oscillations with alternation of spikes and oscillations near
. In
Figure 9c, for the same values of
, oscillatory regimes are shown for solutions starting at cycle
C. Here, the only transition from spike oscillations to mixed-mode oscillations is observed.
Consider how the confidence domains method works in the analysis of these transformations. In
Figure 9d,e, for
, the stable equilibrium
is shown by a red dot, the stable cycle
C is plotted in brown, and the unstable cycle
separating the basins of these attractors is shown in light brown. For three values of noise intensity, confidence ellipses (dashed) around
are shown in
Figure 9d, and confidence bands (dashed) around the cycle
C are shown against the enlarged fragment of the phase plane in
Figure 9e by the same colours. For
, the confidence ellipse belongs to the basin of
and the confidence band entirely lies in the basin of
C. Thus, noise-induced transitions between
and
C do not occur. For
, the confidence ellipse (blue) partially occupies the basin of
C while the confidence band lies within the basin of
C. This geometrical arrangement signals the one-way noise-induced transition
, and therefore, the “stochastic preference” of the cycle
C. Recall that in the pair
and
C discussed above, the stochastic preference is for the equilibrium
. For
, both ellipses and bands partially occupy basins of opposite attractors. This signals the bidirectional stochastic transitions, and hence the formation of a mixed-mode regime (see the time series in
Figure 9b,c).
The results of the statistical analysis of changes in the amplitude–frequency characteristics of stochastic system (
2)’s solutions starting at the stable limit cycle (blue) and stable equilibrium
(red), caused by increasing noise, are shown in
Figure 10. Here, jumps in red lines mark transitions from the equilibrium to the cycle. As the noise is further increased, the distributions are mixed, and the characteristics merge.
To summarize, in the tristability parameter zone, a wide variety of stochastic regimes is observed. For weak noise, we have the coexistence of noisy equilibrium noisy equilibrium , and noisy cycle C. Biologically, this means that depending on the initial data, calcium can either be in a noisy oscillation mode with a high concentration (near ), a low concentration (near ), or oscillate with a large amplitude of concentration change (along C).
With increasing noise, system (
2) exhibits a unidirectional transition
, which can be biologically interpreted as the suppression of calcium oscillations by noise, and a unidirectional transition
, which can be interpreted as the generation of calcium oscillations by noise. With a further increase in noise, more complex, mixed-mode regimes with alternating large-amplitude spike oscillations and small-amplitude stochastic fluctuations near
or
are observed. Thus, in the tristability case, random noise can qualitatively deform the character of calcium oscillations.
3.3. Noise-Induced Effects in the Bistability Zone
Consider now the behaviour of stochastic system (
2) in the bistability zone
, where the stable equilibrium
coexists with the limit cycle
C. Their basins are separated by the orbit of the unstable cycle
(see the bifurcation diagram in
Figure 1 and the phase portrait in
Figure 2b for
, as an example).
Let us compare for this bistability zone how noise affects the equilibrium
and the limit cycle
C. In
Figure 11, for three values of
p with
, we show changes in the dispersion of random states for system (
2)’s solutions starting at cycle
C (
Figure 11a) and equilibrium
(
Figure 11b).
Figure 8a (left) shows that for
, the LASO regime is preserved when noise intensity increases. For
and
, one can see three-stage scenarios of stochastic transformations. For weak noise, random solutions starting at cycle
C slightly fluctuate around it, demonstrating a LASO regime. When
exceeds some threshold, random solutions leave the basin of cycle
C and begin to oscillate near
with small amplitudes. Such a SASO regime is observed in some
window. With the further increase in noise, this regime breaks down, and system (
2) returns to the LASO mode again. The size of this
window of SASOs increases with the increase in the parameter
p (see
Figure 11a). In
Figure 11b, one can see how the SASO regime near
abruptly transforms into the LASO mode.
In
Figure 12a,b, these transformations are illustrated for
by time series of stochastic solutions starting at cycle
C (
Figure 12a) and equilibrium
(
Figure 12b). Here, we compare the results of stochastic forcing for three values of the noise intensity
. At
, the random solutions oscillate slightly around the deterministic attractors where they started. For
, this behaviour is preserved for solutions starting at
, but solutions starting at
C exhibit a one-way transition
. At
, for both cases, noise induces two-way transitions
, and one can see mixed-mode stochastic oscillations with the alternation of SASOs near
and LASOs of spike form near
C.
Consider now how these stochastic transformations can be predicted analytically by the confidence domains method. In
Figure 12c,d, for
, the stable equilibrium
is shown by the red dot, the stable cycle
C is plotted in brown, and the unstable cycle
separating basins of these attractors is shown in light brown. For three values of noise intensity
, confidence bands (dashed) around
C are shown in
Figure 12c, and confidence ellipses (dashed) around the equilibrium
are shown in
Figure 12d by the same colours. For
, the confidence band entirely lies in the basin of
C, and the confidence ellipse belongs to the basin of
. This means that noise-induced transitions between
C and
do not occur. For
, the confidence band (blue) partially occupies the basin of
C, while the confidence ellipse lies within the basin of
. This geometrical arrangement of confidence domains and the separatrix
signals the one-way noise-induced transition
, and therefore, the “stochastic preference” of the equilibrium
. For
, both ellipses and bands capture basins of opposite attractors. This predicts the onset of bidirectional stochastic transitions, and hence the formation of a mixed-mode regime (see the time series in
Figure 12a,b). Interestingly, the “stochastic preference” in the pair
C and
essentially depends on parameter
p. Indeed, for
(see
Figure 9), we have noise-induced transitions
, while for
(see
Figure 12), we have an opposite transition
.
These results shed light on the intrinsic mechanisms for the formation of unexpected changes in calcium dynamics when large-amplitude oscillations of the
concentration alternate with small-amplitude phases. Let us compare the statistics of the noise-induced amplitude and frequency transformations of solutions starting at cycle
C for four values of parameter
p lying in the bistability parametric zone. In
Figure 13a, in plots of the variance
, it is well seen how
windows of SASO regimes appear and expand with the increase in
p. In
Figure 13b,c, mean values
and coefficient of variation
of interspike intervals
are shown.
3.4. Stochastic Excitement in the Monostability Zone
Consider now a parametric zone
where the stable equilibrium
is a sole attractor of the deterministic system (
1). In this case of monostability, all solutions of system (
1), regardless of the initial data, converge to the point
. However, the convergence character of these solutions may differ significantly. For initial values close enough to
, the solutions immediately tend to
. We call this set of initial data the subcritical zone. For initial values that are farther from equilibrium
than some critical distance, the solutions first move away from
, make a large-amplitude turn, and only then begin to tend to
. We call this set of initial data the supercritical zone. Such a dichotomy is well seen in
Figure 14a for
, where the deterministic phase trajectory is shown in black, and equilibrium
is marked by a red dot. In this figure, we plotted two confidence ellipses for stochastic system (
2) with
(blue) and
(red). The smaller ellipse lies in the subcritical zone, so the random trajectories starting at
are arranged near
. This means that system (
2) for
is in the SASO regime. The larger ellipse captures the points of the supercritical zone, so the random trajectories can move away from
, and in system (
2) with
, the LASO mode is observed.
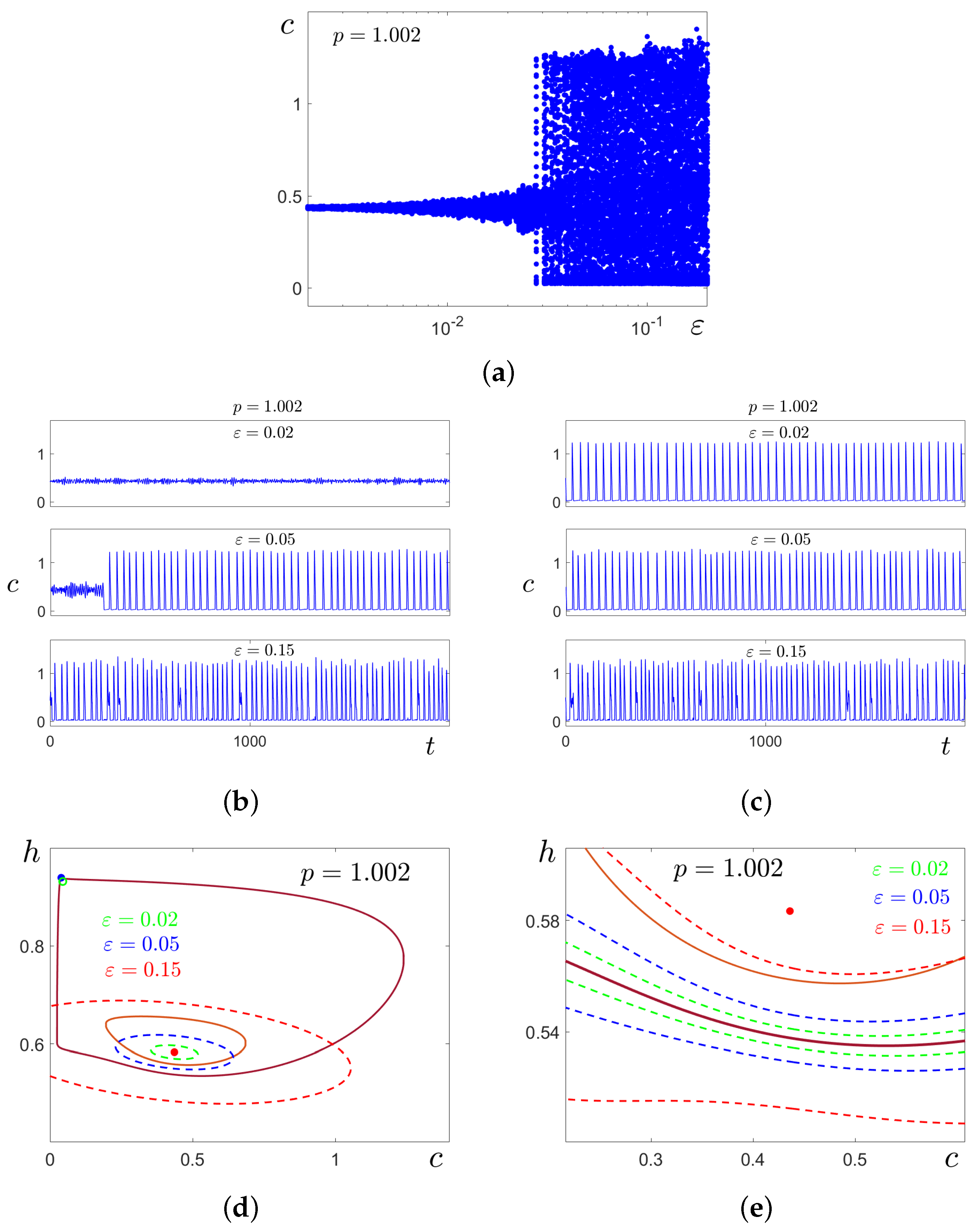
The process of transformation from the SASO regime to the LASO mode with a gradual increase in the noise intensity
is shown in
Figure 14b. In
Figure 14c,d, regimes of SASOs and LASOs in system (
2) with
are illustrated by phase trajectories and time series. Note that in this case, the LASO regime is an alternation of small-amplitude fluctuations near
and large-amplitude spikes (see
Figure 14d). As can be seen, the prediction given by the confidence ellipses agrees well with the results of direct numerical simulations.
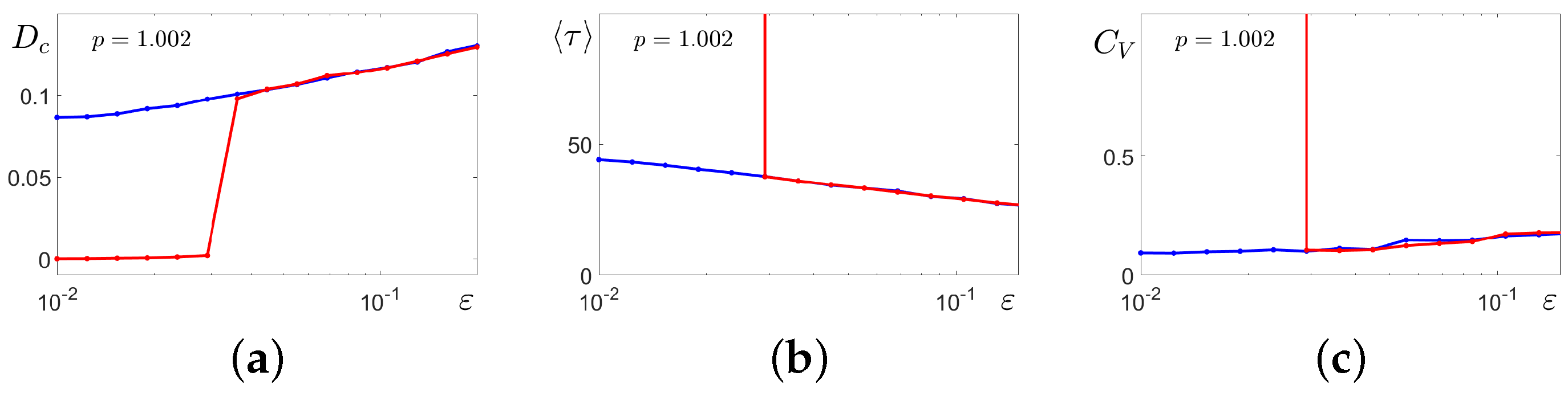
Details of the statistical analysis of amplitude and frequency noise-induced deformations in system (
2) in the parametric monostability zone
are shown in
Figure 15. As can be seen in the plot of the variance in
Figure 15a, the transition from SASOs to LASOs for larger
p requires a stronger noise. The sharp drop in the mean values of the interspike intervals in
Figure 15b localises the onset of the noise-induced excitation of the spike oscillations. Plots of the coefficient of variation
in
Figure 15c indicate that these spike oscillations are rather coherent.
To summarize, even in the monostable case with the single stable equilibrium, the calcium concentration can oscillate with large amplitudes in the presence of random noise. As can be seen, the mathematical confidence domain method based on the stochastic sensitivity technique can successfully predict this important biological phenomenon.