Hierarchical Multiattention Temporal Fusion Network for Dual-Task Atrial Fibrillation Subtyping and Early Risk Prediction
Abstract
1. Introduction
- Advancing dual-task AF modeling. This study reframes AF analysis by unifying subtype classification and short-horizon risk prediction into a single end-to-end HMA-TFN framework. This dual-task formulation contributes a new paradigm for simultaneously addressing diagnostic classification and proactive risk assessment, thereby enriching AF detection and short-horizon risk strategies.
- Hierarchical multiattention. By coordinating attention across lead, morphology, and rhythm levels, this work contributes a hierarchical mechanism that mirrors clinical reasoning—from multilead comparisons to waveform inspection and rhythm analysis. The demonstrated monotonic gains highlight the scientific value of progressive, synergistic feature integration over isolated attention approaches.
2. Methodology
2.1. Datasets
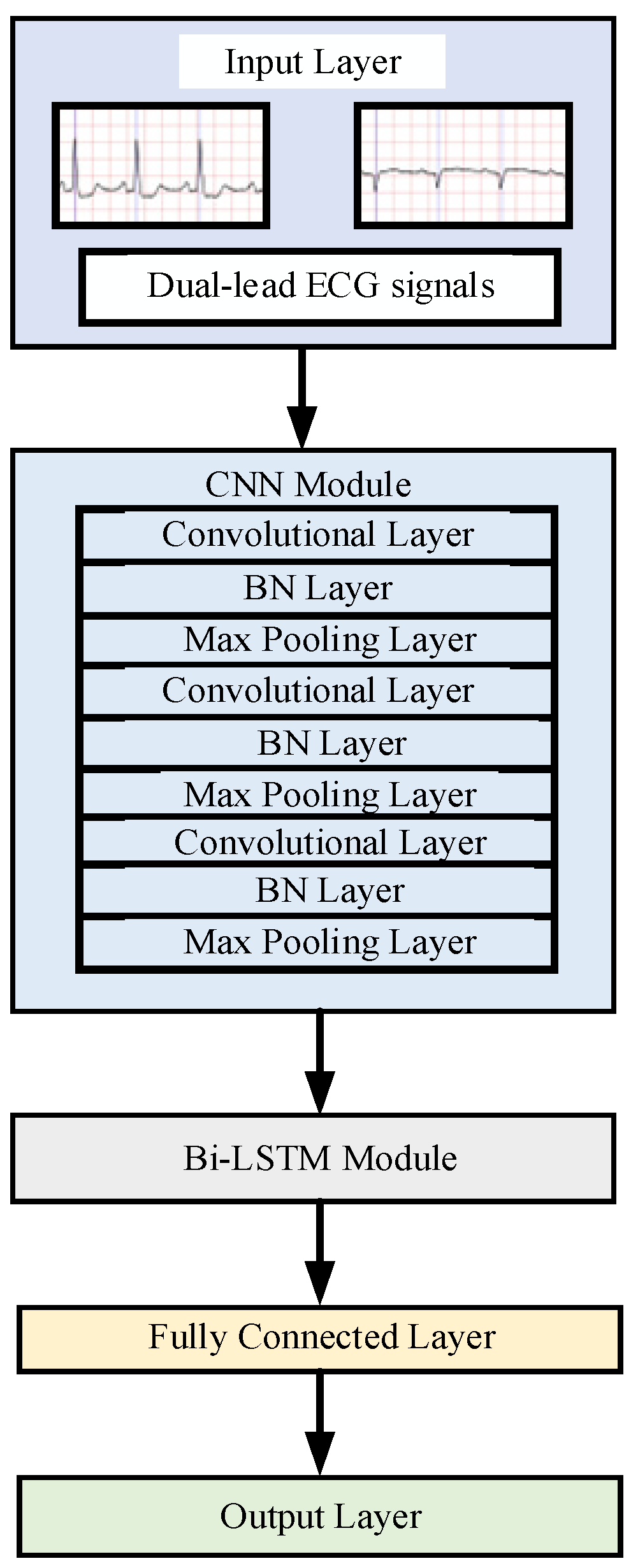
2.2. Baseline Model Construction
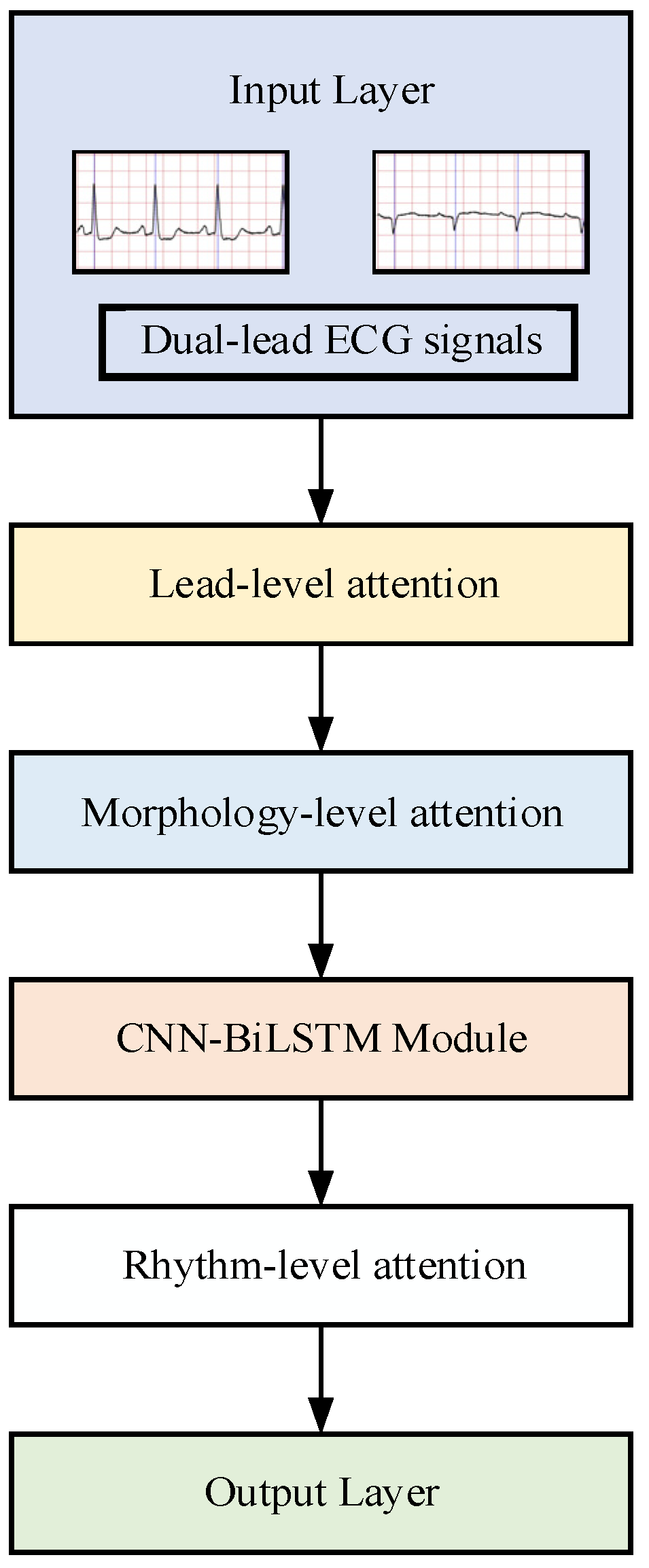
2.3. Lead-Level Attention
2.4. Morphology-Level Attention
2.5. Rhythm-Level Attention
2.6. Hierarchical Multiattention Temporal Fusion Network Construction
- Lead-level attention dynamically weights two-lead ECG signals through channel-wise attention gates.
- Morphology-level attention integrates CondConv generated kernels to emphasize waveform-specific features (P-wave suppression and F-wave enhancement).
- Rhythm-level attention applies multihead temporal self-attention to model arrhythmic patterns.
3. Experimental Results and Analysis
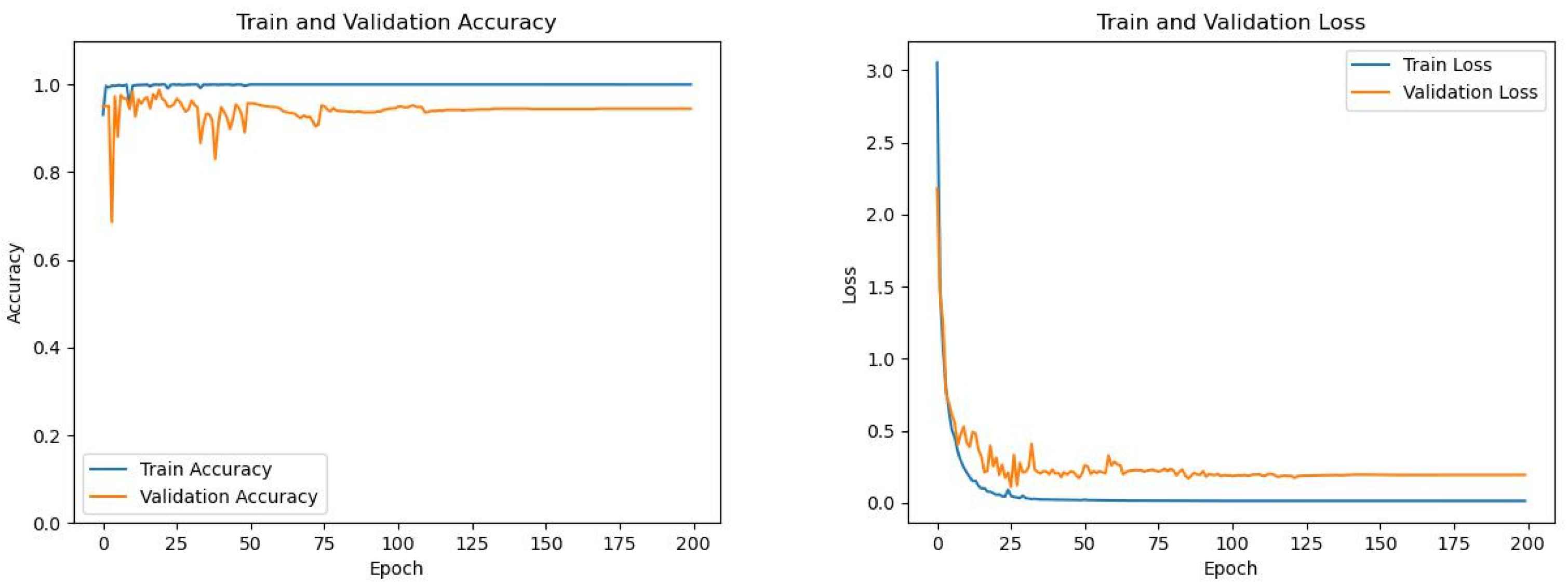
3.1. Basic Setting
3.2. Model Evaluation Metrics
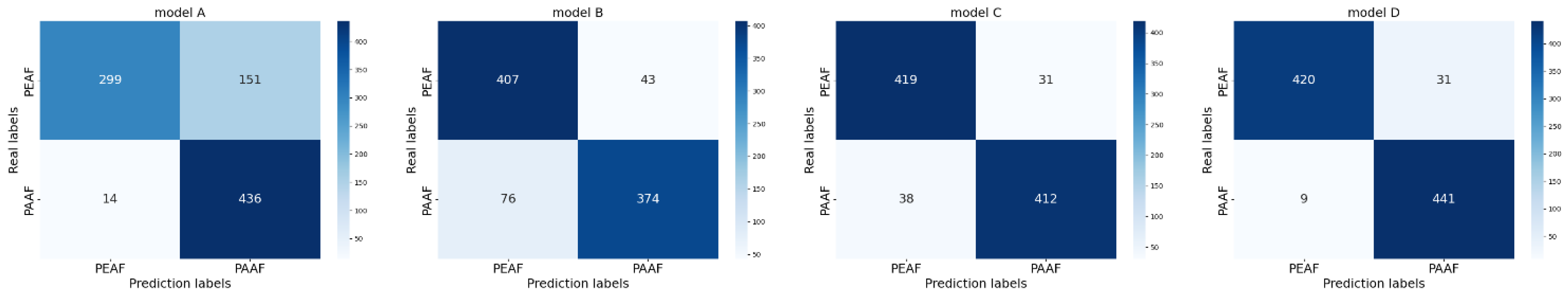
3.3. Classification of Paroxysmal Atrial Fibrillation and Persistent Atrial Fibrillation
3.4. Early Prediction of Paroxysmal Atrial Fibrillation
3.5. Visualization Analysis of Attention Weights
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- The Writing Committee of the Report on Cardiovascular Health and Diseases in China. Report on cardiovascular health and diseases in China 2022: An updated summary. Chin. J. Interv. Cardiol. 2023, 31, 1004–8812. [Google Scholar] [CrossRef]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.-A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef] [PubMed]
- Svennberg, E.; Tjong, F.; Goette, A.; Akoum, N.; Di Biase, L.; Bordachar, P.; Boriani, G.; Burri, H.; Conte, G.; Deharo, J.C.; et al. How to use digital devices to detect and manage arrhythmias: An EHRA practical guide. Europace 2022, 24, 979–1005. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.E.; Sagris, D.; Hill, A.; Lip, G.Y.H.; Abdul, R.A.H. Atrial fibrillation and stroke. Expert Rev. Cardiovasc. Ther. 2023, 21, 35–56. [Google Scholar] [CrossRef]
- Feng, K.; Fan, Z. A novel bidirectional LSTM network based on scale factor for atrial fibrillation signals classification. Biomed. Signal Process. Control 2023, 76, 1746–8094. [Google Scholar] [CrossRef]
- Makhir, A.; Alaoui, H.E.; Jilbab, A.; Bellarbi, L. Classification of Atrial Fibrillation and Cardiac Arrhythmias by a CNN-BiLSTM Hybrid Model with DWT Preprocessing. In Proceedings of the 2024 4th International Conference on Innovative Research in Applied Science, Engineering and Technology (IRASET), Fez, Morocco, 16–17 May 2024; pp. 1–5. [Google Scholar] [CrossRef]
- Argha, A.; Hamid, A.R.; Martin, B. A Novel Deep Ensemble Method for Selective Classification of Electrocardiograms. IEEE Trans. Biomed. Eng. 2025, 72, 833–842. [Google Scholar] [CrossRef]
- Zhou, Z.; Chen, Y.; Zhang, F.; Liu, H.; Karimi, H.R.; Cao, J. Multimodal prediction of catheter ablation outcomes in patients with persistent atrial fibrillation. Neural Netw. 2025, 191, 107835. [Google Scholar] [CrossRef]
- Wang, Y.N.; Liu, S.; Jia, H.J. A two-step method for paroxysmal atrial fibrillation event detection based on machine learning. Math. Biosci. Eng. 2022, 19, 9877–9894. [Google Scholar] [CrossRef]
- Laith, A.; Yaman, J.; Zaid, A.F.; Emmanuel, O.; Justin, L.; Jana, A. A machine learning–based risk prediction model for atrial fibrillation in critically ill patients. Heart Rhythm. O2 2025, 6, 652–660. [Google Scholar] [CrossRef]
- Gliner, V.; Yaniv, Y. An SVM approach for identifying atrial fibrillation. Physiol. Meas. 2018, 39, 1361–6579. [Google Scholar] [CrossRef]
- Wu, X.; Zheng, Y.; Chu, C.-H.; He, Z. Extracting deep features from short ECG signals for early atrial fibrillation detection. Artif. Intell. Med. 2020, 109, 101896. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Rajpal, N.; Mehta, R. An empiric analysis of wavelet-based feature extraction on deep learning and machine learning algorithms for arrhythmia classification. Int. J. Interact. Multimed. Artif. Intell. 2021, 6, 25–34. [Google Scholar] [CrossRef]
- Wang, J.; Wang, P.; Wang, S. Automated detection of atrial fibrillation in ECG signals based on wavelet packet transform and correlation function of random process. Biomed. Signal Process. Control 2019, 55, 101662. [Google Scholar] [CrossRef]
- Zvuloni, E.; Levi, N.; Marai, I.; Shenhar, Y.; Suleiman, M.; Amit, G. Machine Learning Analysis of Pre- and Post-Ablation Electrocardiograms for Prediction of Atrial Fibrillation Recurrence. J. Cardiovasc. Electrophysiol. 2023, 34, 567–575. [Google Scholar] [CrossRef]
- Fu, L.; Lu, B.; Nie, B.; Peng, Z.; Liu, H.; Pi, X. Hybrid Network with Attention Mechanism for Detection and Location of Myocardial Infarction Based on 12-Lead Electrocardiogram Signals. Sensors 2020, 20, 1020. [Google Scholar] [CrossRef]
- Raghunath, S.; Pfeifer, J.M. Deep Neural Networks Can Predict New Onset Atrial Fibrillation From the 12-Lead Electrocardiogram and Help Identify Those at Risk of AF-Related Stroke. Circulation 2021, 143, 1287–1298. [Google Scholar] [CrossRef]
- Wang, X.; He, Z.S.; Lin, Z.J.; Han, Y. PA2Net: Period-Aware Attention Network for Robust Fetal ECG Detection. IEEE Trans. Instrum. Meas. 2022, 71, 2513812. [Google Scholar] [CrossRef]
- Yildirim, O.; Talo, M.; Ciaccio, E.J. Accurate deep neural network model to detect cardiac arrhythmia on more than 10,000 individual subject ECG records. Comput. Methods Programs Biomed. 2020, 197, 105740. [Google Scholar] [CrossRef]
- Taniguchi, H.; Takata, T.; Takechi, M. Explainable Artificial Intelligence Model for Diagnosis of Atrial Fibrillation Using Holter Electrocardiogram Waveforms. Int. Heart J. 2021, 62, 534–539. [Google Scholar] [CrossRef]
- Khurshid, S.; Friedman, S.; Reeder, C. ECG-based deep learning and clinical risk factors to predict atrial fibrillation. Circulation 2022, 145, 122–133. [Google Scholar] [CrossRef]
- Elias, E.; Maede, K.; Mohammadamin, J. Prediction of Paroxysmal Atrial Fibrillation: A Machine Learning Based Approach Using Combined Feature Vector and Mixture of Expert Classification on HRV Signal. Comput. Methods Programs Biomed. 2018, 165, 53–67. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, G.; Jensen, S.H.; Poulsen, E.S.; Puthusserypady, S. Atrial fibrillation detection using heart rate variability and atrial activity: A hybrid approach. Expert Syst. Appl. 2021, 169, 114452. [Google Scholar] [CrossRef]
- Godwin, M.; Ester, N.; Jaeseok, Y. Enhancing atrial fibrillation classification from single-lead electrocardiogram signals using attention-based networks and generative adversarial networks with density-based clustering. Eng. Appl. Artif. Intell. 2025, 133, 108607. [Google Scholar] [CrossRef]
- Wu, X.; Yan, M.; Wang, R.; Xie, L. Multiscale feature enhanced gating network for atrial fibrillation detection. Comput. Methods Programs Biomed. 2025, 261, 108606. [Google Scholar] [CrossRef]
- Rahul, J.; Sharma, L.D. Artificial Intelligence-Based Approach for Atrial Fibrillation Detection Using Normalised and Short-Duration Time-Frequency ECG. Biomed. Signal Process. Control 2022, 71, 103270. [Google Scholar] [CrossRef]
- Xing, Z.; Ma, G.; Wang, L.; Yang, L.; Guo, X.; Chen, S. Toward Visual Interaction: Hand Segmentation by Combining 3-D Graph Deep Learning and Laser Point Cloud for Intelligent Rehabilitation. IEEE Internet Things J. 2025, 12, 21328–21338. [Google Scholar] [CrossRef]
- Xing, Z.; Meng, Z.; Zheng, G.; Ma, G.; Yang, L.; Guo, X.; Tan, L.; Jiang, Y.; Wu, H. Intelligent Rehabilitation in an Aging Population: Empowering Human–Machine Interaction for Hand Function Rehabilitation Through 3D Deep Learning and Point Cloud. Front. Comput. Neurosci. 2025, 19, 1543643. [Google Scholar] [CrossRef]
- Lin, Y.C.; Antonio, L.; Pyotr, G.; Iwona, C.; Elsayed, Z. P Wave Parameters and Indices: A Critical Appraisal of Clinical Utility, Challenges, and Future Research—A Consensus Document Endorsed by the International Society of Electrocardiology and the International Society for Holter and Noninvasive Electrocardiology. Circ. Arrhythmia Electrophysiol. 2022, 15, 427–437. [Google Scholar] [CrossRef]
- LeCun, Y.; Bengio, Y.; Hinton, G. Deep learning. Nature 2015, 521, 436–444. [Google Scholar] [CrossRef]
- He, K.; Zhang, X.; Ren, S.; Sun, J. Deep residual learning for image recognition. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition (CVPR), Las Vegas, NV, USA, 27–30 June 2016; pp. 770–778. [Google Scholar] [CrossRef]
- Liu, S.; Wang, A.; Deng, X.; Yang, C. MGNN: A Multiscale Grouped Convolutional Neural Network for Efficient Atrial Fibrillation Detection. Comput. Biol. Med. 2022, 148, 105863. [Google Scholar] [CrossRef]
- Lee, K.-S.; Jung, S.; Gil, Y.; Son, H.S. Atrial Fibrillation Classification Based on Convolutional Neural Networks. BMC Med. Inform. Decis. Mak. 2019, 19, 1–6. [Google Scholar] [CrossRef]
- Oh, S.L.; Ng, E.Y.K.; Tan, R.S.; Acharya, U.R. Automated Diagnosis of Arrhythmia Using Combination of CNN and LSTM Techniques with Variable Length Heart Beats. Comput. Biol. Med. 2018, 102, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhang, J.; Zhang, Q.; Wei, X. Classification of ECG Signals Based on 1D Convolution Neural Network. In Proceedings of the IEEE 19th International Conference on e-Health Networking, Applications and Services (Healthcom), Dalian, China, 12–15 October 2017; pp. 1–6. [Google Scholar] [CrossRef]
- Rawal, V.; Prajapati, P.; Darji, A. Hardware Implementation of 1D-CNN Architecture for ECG Arrhythmia Classification. Biomed. Signal Process. Control 2023, 85, 104865. [Google Scholar] [CrossRef]
- Ouni, R.; Alhichri, H.; Kharshid, A. Lightweight Residual Convolutional Neural Network for Atrial Fibrillation Detection in Single-Lead ECG Recordings. Eng. J. 2024, 28, 67–80. [Google Scholar] [CrossRef]
- Bahdanau, D.; Cho, K.; Bengio, Y. Neural Machine Translation by Jointly Learning to Align and Translate. Int. Conf. Learn. Represent. 2015, 13, 37–53. [Google Scholar] [CrossRef]
- Yang, B.; Bender, G.; Le, Q.V. CondConv: Conditionally Parameterized Convolutions for Efficient Inference. arXiv 2019. [Google Scholar] [CrossRef]
- Vaswani, A.; Shazeer, N.; Parmar, N.; Uszkoreit, J.; Jones, L.; Gomez, A.N.; Kaiser, L.; Polosukhin, I. Attention Is All You Need. arXiv 2017. [Google Scholar] [CrossRef]
- Sokolova, M.; Lapalme, G. A Systematic Analysis of Performance Measures for Classification Tasks. Inf. Process. Manag. 2009, 45, 427–437. [Google Scholar] [CrossRef]
- Yang, B. Research on a Prediction Method for Atrial Fibrillation Based on LDA Machine Learning. In Proceedings of the 2024 5th International Conference on Big Data & Artificial Intelligence & Software Engineering (ICBASE), Wenzhou, China, 20–22 September 2024; pp. 830–833. [Google Scholar] [CrossRef]
- Li, Y.; Tang, X.; Wang, A.; Tang, H. Probability Density Distribution of Delta RR Intervals for Atrial Fibrillation Detection. Australas. Phys. Eng. Sci. Med. 2017, 40, 707–716. [Google Scholar] [CrossRef]
- Singh, J.P.; Fontanarava, J.; Masse, D.G. Short-term prediction of atrial fibrillation from ambulatory monitoring ECG using a deep neural network. Eur. Heart J.-Digit. Health 2022, 3, 208–217. [Google Scholar] [CrossRef]
- Myrovali, E.; Hristu, V.D.; Tachmatzidis, D. Identifying patients with paroxysmal atrial fibrillation from sinus rhythm ECG using random forests. Expert Syst. Appl. 2023, 213, 118948. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, M.; Yoon, J. Predicting Future Incidences of Cardiac Arrhythmias Using Discrete Heartbeats from Normal Sinus Rhythm ECG Signals via Deep Learning Methods. Diagnostics 2023, 13, 2849. [Google Scholar] [CrossRef] [PubMed]
- Kraft, D.; Rumm, P. Atrial Fibrillation and Atrial Flutter Detection Using Deep Learning. Sensors 2025, 25, 4109. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Wang, L.; Mo, D.; Zhang, Z.; Liang, M. Intelligent algorithms powered smart devices for atrial fibrillation discrimination. Biomed. Signal Process. Control 2025, 103, 107480. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, F.; Jin, Q.; Wang, Y.; Wang, W.; Deng, D. Transcriptome Analysis of Different Life-History Stages and Screening of Male-Biased Genes in Daphnia sinensis. BMC Genom. 2022, 23, 589. [Google Scholar] [CrossRef]
- Duangburong, N.; Limsakul, C. Comparison of ANN and ANFIS Models for AF Diagnosis Using RR Irregularities. Appl. Sci. 2023, 13, 1712. [Google Scholar] [CrossRef]
- Hirsch, O.; Shenfield, A.; Kareem, M.; San, T.R.; Fujita, H.; Acharya, U.R. Automated detection of atrial fibrillation using long short-term memory network with RR interval signals. Comput. Biol. Med. 2018, 102, 327–335. [Google Scholar] [CrossRef]
- Parsi, Y.S.; Lee, S.C.; Choi, W.I.; Kim, D.H. Prediction of atrial fibrillation from normal ECG using artificial intelligence in patients with unexplained stroke. Eur. Heart J. 2020, 41, 348. [Google Scholar] [CrossRef]
- Tzou, H.A.; Lin, S.F.; Chen, P.S. Paroxysmal atrial fibrillation prediction based on morphological variant P-wave analysis with wideband ECG and deep learning. Comput. Methods Programs Biomed. 2021, 211, 106396. [Google Scholar] [CrossRef]
- Petmezas, G.; Haris, K.; Stefanopoulos, L.; Kilintzis, V.; Tzavelis, A.; Rogers, J.A.; Katsaggelos, A.K.; Maglaveras, N. Automated Atrial Fibrillation Detection Using a Hybrid CNN-LSTM Network on Imbalanced ECG Datasets. Biomed. Signal Process. Control 2021, 63, 102194. [Google Scholar] [CrossRef]
- Liu, L.; Liu, F.; Ren, X.; Li, Y.; Han, B.; Zhang, L.; Wei, S. Predicting spontaneous termination of atrial fibrillation based on dual path network and feature selection. Biomed. Signal Process. Control 2024, 88, 105606. [Google Scholar] [CrossRef]







| Task | Data Source | Sample Type | Label | Patients | Samples | Length (Points) | Split (Train/Val/Test) |
|---|---|---|---|---|---|---|---|
| PAAF vs. PEAF Classification | |||||||
| LTAFDB | PAAF | PAAF | 31 | 4500 | 960 (7.5 s) | 7200/900/900 | |
| LTAFDB | PEAF | PEAF | 16 | 4500 | 960 (7.5 s) | ||
| Early Prediction (≤30 min pre-AF) | |||||||
| LTAFDB | Normal rhythm ≤ 30 min pre-AF | N’ | 23 | 4820 | 960 (7.5 s) | 14,080/1760/1760 | |
| MBAFDB | Normal rhythm ≤ 30 min pre-AF | N’ | 19 | 3980 | 960 (7.5 s) | ||
| MBNSRDB | Healthy sinus rhythm | N | 18 | 8800 | 960 (7.5 s) | ||
| Clinical Test | |||||||
| FZU-FPH | Normal rhythm ≤ 30 min pre-AF | N’ | - | 176 | Variable | Test only | |
| FZU-FPH | Normal rhythm > 30 min pre-AF | N | - | 176 | Variable | ||
| Layers | Type | Kernel | Channel | Strid | Activation | Output |
|---|---|---|---|---|---|---|
| 1 | Input | / | / | / | / | [960, 1, 2] |
| 2 | Conv | 7 1 | 32 | 1 1 | ReLU | [960, 1, 32] |
| 3 | Pooling | 4 1 | / | 2 1 | / | [480, 1, 32] |
| 4 | Conv | 5 1 | 64 | 1 1 | ReLU | [480, 1, 64] |
| 5 | Pooling | 2 1 | / | 1 1 | / | [240, 1, 64] |
| 6 | Conv | 3 1 | 128 | 1 1 | ReLU | [240, 1, 128] |
| 7 | Bi-LSTM | 128 | Tanh | [240, 256] | ||
| 8 | FC | 64 | ReLU | [32] | ||
| 9 | Output | 2 | SoftMax | [2] |
| Prediction | |||
|---|---|---|---|
| Positive Class | Negative Class | ||
| Label | Positive Class | True Positive (TP) | False Negative sample (FN) |
| Negative Class | False Positive sample (FP) | True Negative sample (TN) | |
| Baseline Model | Lead-Level Attention | Morphology-Level Attention | Rhythm-Level Attention | |
|---|---|---|---|---|
| Model A | ✓ | / | / | / |
| Model B | ✓ | ✓ | / | / |
| Model C | ✓ | ✓ | ✓ | / |
| Model D | ✓ | ✓ | ✓ | ✓ |
| Model | Accuracy | Precision | Recall | |
|---|---|---|---|---|
| Model A | 81.66% | 84.90% | 81.66% | 81.23% |
| Model B | 86.77% | 86.97% | 86.77% | 86.75% |
| Model C | 92.33% | 92.34% | 92.33% | 92.33% |
| Model D (HMA-TFN) | 95.66% | 95.76% | 95.66% | 95.66% |
| Approach | Methods | Features Used | Accuracy | Precision | Recall | |
|---|---|---|---|---|---|---|
| Yang [42] | LDAA | Sparse representation of atrial activity spectrum | 88.82% | / | 95.24% | / |
| Li [43] | SVM | RR interval-related features | 91.23% | 94.23% | 88.96% | 91.52% |
| Singh [44] | ANFIS | RR interval-derived SAV | 89.33% | 87.22% | 89.33% | 88.26% |
| Myrovali [45] | SAFE Score | Clinical/laboratory parameters | 83% | 80% | ||
| Kim [46] | CNN-LSTM Ensemble | Pattern transition features | 91.26% | 82.21% | 95.79% | |
| Kraft [47] | 1D ConvNeXt V2 | Single-lead ECG signals | 98.6% | |||
| Xie [48] | CNN | Printed 12-lead ECG during sinus rhythm | 78.6% | 87.5% | 66.7% | 82.4% |
| Wang [49] | LR + RF | Multi-omics data | 89.2% | 83% | 80% | - |
| Raghunath [17] | Logistic Regression | Cortical biomarkers | 88% | |||
| Duangburong [50] | ANFIS | RR interval-derived SAV | 89.33% | 87.22% | 89.33% | 88.26% |
| Proposed | HMA-TFA | Raw dual-lead ECG signals | 95.77% | 95.78% | 95.78% | 95.77% |
| Model | Accuracy | Precision | Recall | |
|---|---|---|---|---|
| Model A | 90.28% | 90.39% | 90.28% | 90.27% |
| Model B | 92.38% | 93.24% | 92.38% | 92.34% |
| Model C | 94.26% | 94.37% | 94.26% | 94.25% |
| Model D | 95.51% | 95.56% | 95.51% | 95.50% |
| Label | Specificity | Precision | Recall | Accuracy | |
|---|---|---|---|---|---|
| N | 93.18% | 93.33% | 95.45% | 94.37% | 94.31% |
| N’ | 95.45% | 95.34% | 93.18% | 94.24% | |
| Average | 94.31% | 94.34% | 94.31% | 94.30% |
| Approach | Methods | Forecast Period | Accuracy | Precision | Specificity | Recall | |
|---|---|---|---|---|---|---|---|
| Hirsch [51] | RF | 30 beats | 97.40% | / | 96.10% | 95.90% | 87.30% |
| Parsi [52] | SVM | 5 min | 97.70% | / | 96.70% | 98.80% | / |
| Tzou [53] | Lightweight CNN | 5 min | 89.00% | / | 89.00% | 88.00% | 88.00% |
| Elias [22] | Resnet | Between 2 months and 1 week | / | / | 69.33% | 78.33% | / |
| Petmezas [54] | RF, CNN-LSTM, CNN Multihead Attention Model | 2 weeks | / | / | 69.00% | 76.00% | / |
| Lei [55] | RF | / | 93.45% | / | 91.40% | 95.21% | / |
| Cai [17] | CNN-LSTM | 2 weeks | / | 87.37% | / | 83.23% | 84.99% |
| Proposed | HMA-TFA | 30 min | 96.36% | 96.44% | 96.36% | 96.36% | 96.35% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.-H.; Wang, J.-W.; Xie, C.-X.; Lee, Z.-J.; Cai, B.-J.; Chen, T.-Y.; Chen, S.-L.; Chen, C.-A.; Abu, P.A.R.; Yang, T. Hierarchical Multiattention Temporal Fusion Network for Dual-Task Atrial Fibrillation Subtyping and Early Risk Prediction. Mathematics 2025, 13, 2872. https://doi.org/10.3390/math13172872
Wang L-H, Wang J-W, Xie C-X, Lee Z-J, Cai B-J, Chen T-Y, Chen S-L, Chen C-A, Abu PAR, Yang T. Hierarchical Multiattention Temporal Fusion Network for Dual-Task Atrial Fibrillation Subtyping and Early Risk Prediction. Mathematics. 2025; 13(17):2872. https://doi.org/10.3390/math13172872
Chicago/Turabian StyleWang, Liang-Hung, Jia-Wen Wang, Chao-Xin Xie, Zne-Jung Lee, Bing-Jie Cai, Tsung-Yi Chen, Shih-Lun Chen, Chiung-An Chen, Patricia Angela R. Abu, and Tao Yang. 2025. "Hierarchical Multiattention Temporal Fusion Network for Dual-Task Atrial Fibrillation Subtyping and Early Risk Prediction" Mathematics 13, no. 17: 2872. https://doi.org/10.3390/math13172872
APA StyleWang, L.-H., Wang, J.-W., Xie, C.-X., Lee, Z.-J., Cai, B.-J., Chen, T.-Y., Chen, S.-L., Chen, C.-A., Abu, P. A. R., & Yang, T. (2025). Hierarchical Multiattention Temporal Fusion Network for Dual-Task Atrial Fibrillation Subtyping and Early Risk Prediction. Mathematics, 13(17), 2872. https://doi.org/10.3390/math13172872






