1. Introduction
Understanding the long-term dynamics of infectious diseases in populations subject to environmental uncertainty is a central problem in mathematical epidemiology [
1]. Traditional deterministic models, while foundational, are insufficient for capturing the complex reality that transmission mechanisms often vary due to a multitude of unpredictable factors, including seasonal cycles, behavioral responses, environmental changes, and public health interventions [
2]. To incorporate this variability,
stochastic epidemic models have been developed, wherein randomness is typically introduced by perturbing key parameters (most notably the transmission rate) through additive white noise or time-dependent deterministic functions [
3]. While such models represent a step toward realism, they often fall short because they fail to capture important features such as heavy-tailed fluctuations, long memory effects, and sudden extreme events that are frequently observed in real epidemic data [
4,
5].
In classical stochastic models, transmission rates are often perturbed by additive Gaussian white noise, resulting in dynamics of the form
, where
is standard Brownian motion [
6]. This formulation implies that the transmission rate can grow without bound or become negative, an evident violation of biological feasibility [
7]. Even if restricted artificially to the positive domain, such models ignore the empirical observation that
transmission rates fluctuate around natural baseline levels, shaped by long-term behavioral and ecological equilibria [
8]. For instance, contact rates may increase during flu season but gradually decline due to awareness campaigns or adaptive behaviors such as social distancing [
9].
Additive noise models typically assume independent increments in perturbations, meaning that they lack temporal memory [
10]. In real epidemics, however, changes in transmission are often
temporally correlated [
11]. For example, a surge in infection may prompt sustained behavioral changes, such as mask-wearing or lockdowns, leading to long-term reductions in contact rates [
12]. Likewise, seasonal environmental factors like temperature and humidity exert
persistent effects on transmission dynamics [
13]. Thus, models that treat perturbations as memoryless may misrepresent the autocorrelated structure of real-world transmission [
14].
The most significant mathematical limitation of traditional stochastic models with additive noise is the lack of stationary behavior in the perturbed parameters [
15]. When the transmission rate evolves as a Brownian motion or a non-reverting diffusion, its distribution spreads over time, and no stationary distribution exists [
16]. This renders
long-term analysis intractable, precluding the use of ergodic theorems and invariant measure theory to study endemic equilibria or extinction thresholds [
17].
To overcome these limitations, we replace the classical modeling of stochastic transmission with
mean-reverting stochastic differential equations of the Ornstein–Uhlenbeck type [
18]. Specifically, we assume that the transmission rate
evolves according to
where
denotes the speed of reversion,
is the baseline transmission level, and
scales the stochastic perturbation. This equation captures the essential epidemiological characteristic that, while transmission fluctuates in response to exogenous shocks, it tends to return to a characteristic level in the absence of sustained forcing [
19].
From a mathematical standpoint, modeling the transmission rate
using mean-reverting stochastic differential equations with multiplicative noise offers several significant advantages [
20]. First, under appropriate parameter conditions, the process
remains strictly positive almost surely, which ensures biological consistency by preventing negative transmission rates [
21]. Second, such processes admit explicit stationary distributions that allow for a rigorous analysis of long-term behavior [
22]. Third, the ergodic nature of this process guarantees convergence in distribution to the stationary law, enabling the application of ergodic theorems [
23,
24,
25,
26].
To elucidate the advantages of incorporating mean-reverting stochastic transmission rates, we investigate how environmental and behavioral variability influences the long-term dynamics of a stochastic SVIR (susceptible–vaccinated–infected–recovered) epidemic model [
27,
28,
29]. The model is formulated on a complete filtered probability space
, where the filtration
satisfies the usual conditions of right-continuity and completeness. The state variables
,
,
, and
denote the respective sizes of the susceptible, vaccinated, infected, and recovered populations at time
t, and evolve as
-valued càdlàg,
-adapted processes. The stochastic evolution of the epidemic is then described by the following system of coupled stochastic differential equations:
Here, is the constant recruitment rate into the population. A proportion of new individuals enter the susceptible class, while the remaining are vaccinated at birth. The parameter is the natural mortality rate. The disease-induced death rate and recovery rate are given by and , respectively. Infection transmission is modeled through mass-action incidence, with time-varying stochastic transmission rates and for the susceptible and vaccinated classes, respectively.
The dynamics of
and
are governed by stochastic differential equations of the form (6) and (7), incorporating a mean-reverting drift and multiplicative Brownian noise. The deterministic drift terms
(
) model the mean-reversion mechanism, where the transmission rates
tend to revert to baseline levels
over time in the absence of noise. Here,
is a constant that governs the speed of mean reversion. The diffusion terms
capture continuous stochastic fluctuations arising from environmental or behavioral variability, with
and
representing independent standard Brownian motions.
Figure 1 illustrates the mean-reverting behavior driven by the deterministic pull term
and the stochastic multiplicative noise
.
Building upon earlier studies of SVIR epidemic models, the authors in [
30] investigated a stochastic SVIR framework incorporating saturated incidence rates and logistic population growth. They established sufficient conditions for disease extinction and ergodicity. Subsequently, ref. [
31] introduced an extended version of the SVIR model and examined the conditions under which the disease persists or dies out. In [
32], the model was further generalized to account for transport-induced infections, media influence, and Lévy noise. The authors derived rigorous criteria for both extinction and persistence in this context. Additionally, ref. [
33] explored the role of heterogeneous vaccination strategies in disease transmission by formulating a discrete-time Markov chain SVIR model. They derived the epidemic threshold and investigated various probabilistic characteristics of the system.
Compared to the aforementioned recent studies, the key innovation of our work lies in the rigorous formulation and analysis of a bivariate SVIR model incorporating ergodic stochastic transmission rates governed by inverse gamma distributions. By modeling the time-varying transmission rates and as solutions to geometric mean-reverting stochastic differential equations with multiplicative noise, we show that (under some meaningful conditions) each rate admits a unique stationary distribution. We identify this invariant distribution as the inverse gamma law with explicitly computable shape and scale parameters. This provides a fully tractable stochastic structure of the transmission process, enabling the exact characterization of the stationary effective reproduction number . Unlike classical models that rely on fixed or mean-field estimates of , our formulation captures intrinsic stochastic fluctuations and their long-term epidemiological impact. Moreover, we derive an analytically computable expression for , offering a novel probabilistic threshold condition for disease persistence based on the convolution of inverse gamma laws.
The paper is organized as follows.
Section 2 establishes foundational results by presenting key lemmas and exploring the asymptotic behavior of the stochastic transmission processes
, thereby setting the stage for the subsequent analysis. In
Section 3, we develop the main theoretical contributions, including a novel classification framework for the long-term dynamics of the SVIR model driven by mean-reverting stochastic transmission rates.
Section 4 offers numerical simulations that illustrate and validate the theoretical findings. Finally,
Section 5 concludes the paper with a summary of the key insights, a discussion of limitations, and suggestions for future research directions.
2. Asymptotic Properties of Mean-Reverting Stochastic Transmission Rates
First, the processes
and
admit unique strong solutions that remain strictly positive for all
due to the multiplicative noise structure and linear drift (see Theorem 4.4 in [
34]). Given the continuity and local Lipschitz properties of the drift coefficients in the equations for
, and the fact that the transmission rates
are well-behaved adapted stochastic processes, the full system admits a unique strong solution up to an explosion time. Moreover, standard arguments using linear boundedness of the drift and the non-negativity of initial conditions imply that the solution remains in
and is non-explosive for all finite time
. Therefore, the full system is well-posed and models a biologically meaningful evolution of the disease dynamics under stochastic transmission rates.
Now, we present the explicit analytical expressions for the solutions of the processes and .
Lemma 1. Let (i = 1, 2) satisfy the stochastic differential Equations (2) and (5). Then, the unique strong solution is given by Proof. Let us introduce the stochastic integrating factor
and define the product process
. By Itô’s product rule, we have
Using Itô’s formula applied to
, we compute
and, recalling the dynamics of
, the differential becomes
Summing all three contributions yields
Observe that the stochastic integrals cancel exactly, and the drift terms simplify
so that
This is a deterministic differential equation for
, which integrates directly to give
Substituting back the definition of
, we obtain
which gives the explicit representation of the solution. Writing out
explicitly yields
which completes the derivation of the explicit solution for
. □
In the next two lemmas, we investigate the analytical structure and moment dynamics of the mean-reverting stochastic processes . We begin by deriving their explicit solutions and conditional expectations, and then proceed to analyze the conditional second moment , which plays a crucial role in characterizing the asymptotic behavior and variance of the processes.
Lemma 2. Let denote the unique solution to Equations (6) and (7). Then, for any , the process admits the following mild (integral) representation: Proof. We define the deterministic integrating factor
which satisfies
. Applying Itô’s product rule to the process
, we have
Integrating both sides from
s to
t, we find
Multiplying both sides by
yields
Evaluating the deterministic integral, we obtain
so the expression simplifies to
as required. To compute the conditional expectation
, we exploit the independence of the Brownian increment
from
. Noting that the stochastic integral is a local martingale with zero mean under conditioning on the past, it follows that
□
Remark 1. The conditional expectation of given its past value decays exponentially to the mean level with rate , consistent with the mean-reverting property of the process.
Lemma 3. Let denote the unique solution to Equations (6) and (7). Assume that and ; then, Proof. We define the integrating factor as
which allows us to express the solution explicitly in the mild form
To compute the conditional second moment, we consider the square
We analyze
, noting that the Brownian increment
. Hence,
is a log-normal random variable and its expectation can be computed using the moment-generating function of the normal distribution:
Substituting this into the expansion of
, taking expectations, and using the independence structure of stochastic integrals with respect to Brownian motion yields the following closed-form expression:
□
Remark 2. The exponential term in (8) captures the interplay between the stochastic forcing (through the noise intensity ) and the deterministic damping (through the rate ). If , the second moment decays exponentially over time, indicating that the damping dominates the noise, and this contributes to the stability and boundedness of the process. Conversely, if , the second moment grows exponentially, suggesting that noise overwhelms damping, leading to potential instability or unbounded growth. We now turn to the asymptotic behavior of the process. It is straightforward to show that the first moment converges to the deterministic equilibrium,
while the second moment tends to
Consequently, the stationary variance is given by
Remark 3. Equations (10) and (11) characterize the long-term fluctuations in around its equilibrium level , with the trade-off between the stabilizing drift strength and the volatility parameter governing the magnitude of the variability. 4. Sensitivity Analysis and Computational Validation of Theoretical Results
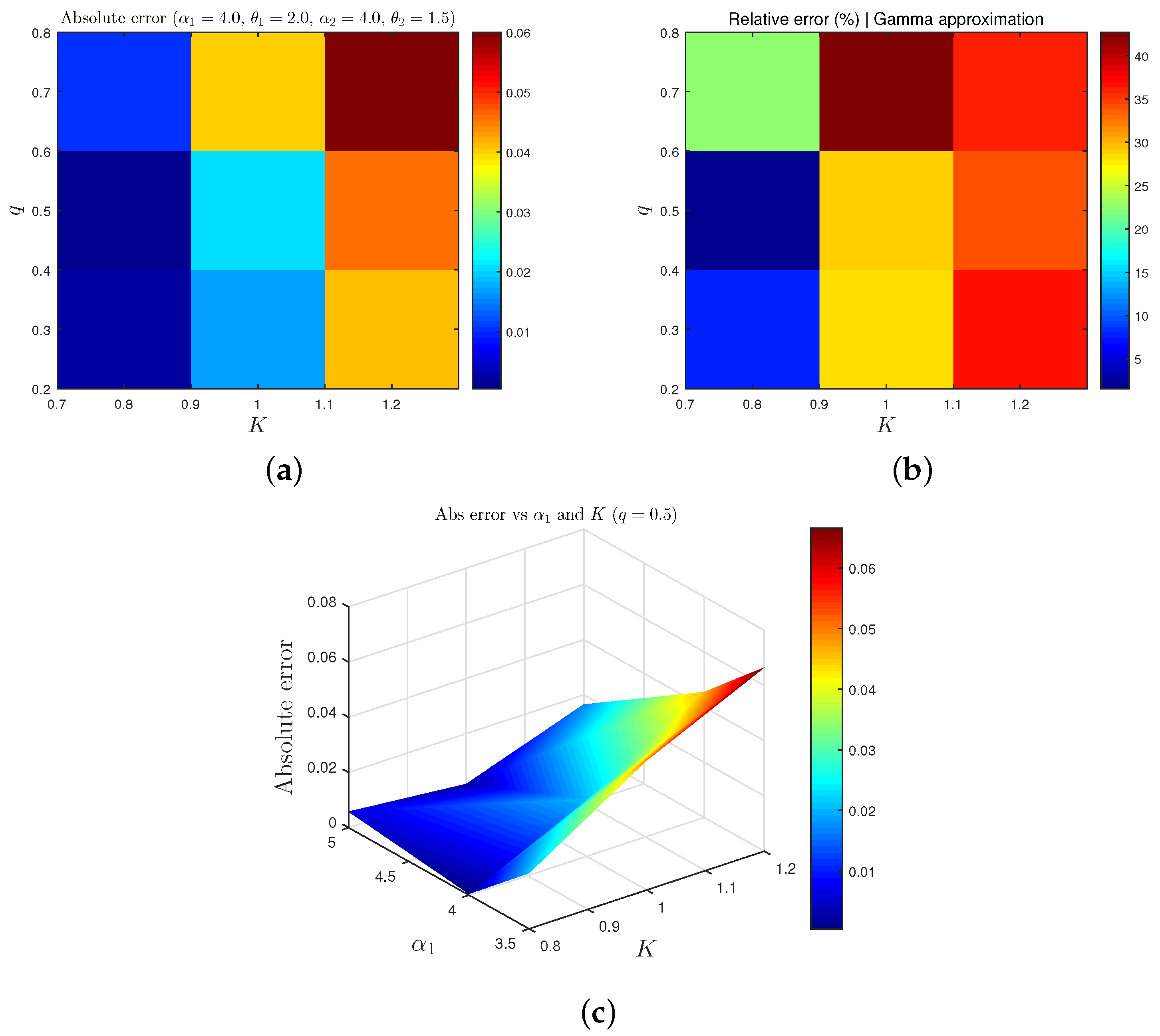
In this section, we focus on a detailed sensitivity analysis of key parameters influencing the probabilities and , which determine the likelihood of epidemic persistence or extinction in the long term. Specifically, we examine how variations in the shape and scale parameters of the inverse gamma distributions associated with the stochastic transmission rates and , as well as the weight parameters q and p, affect the effective reproduction number .
We then proceed to numerically classify the dynamical behavior of the SVIR epidemic model through trajectory simulations, which incorporate the long-term ergodic behavior of the stochastic transmission rates. By simulating numerous sample paths under different parameter configurations, we are able to assess how stochastic fluctuations in the transmission dynamics shape the qualitative evolution of the epidemic.
4.1. Sensitivity Analysis
We begin by analyzing how variations in key parameters influence the probability
, which quantifies the likelihood of epidemic persistence. The surface plots in
Figure 3 illustrate the behavior of
as a function of the shape and scale parameters
and
of the inverse gamma distribution characterizing the stochastic transmission rate
, while keeping the parameters of
and the scaling constant
K fixed. The plots are generated for three representative values of the parameter
, which influence the overall shape of the inverse gamma distribution. The results indicate that increasing the shape parameter or decreasing the scale parameter reduces
, thereby increasing the probability of disease extinction. This behavior aligns with the intuition that a reduced variability or tighter concentration of transmission rates favors epidemic control.
In
Figure 4, we further investigate the extinction probability
by examining its sensitivity to three sets of key parameters. First, we assess the influence of the shape parameters
and
, which are shown to control the concentration of the inverse gamma distributions of
and
. An increase in
results in a more peaked distribution, leading to reduced transmission variability and thus a higher chance of epidemic extinction. Second, we explore the role of the scale parameters
and
, which modulate both the mean and the variance of the transmission rates. Larger values of
lead to greater dispersion, which tends to elevate the likelihood of persistence due to amplified stochastic effects. Finally, we consider the impact of the weighting coefficients
q and
p, which determine the relative contributions of the two stochastic transmission components to the overall effective reproduction number
. Adjusting these weights alters the balance between the two sources of infection pressure and can significantly influence the extinction probability.
Collectively, these results highlight the nuanced interplay between parameter uncertainty and epidemic outcomes. They underscore that even in the presence of randomness, careful parameter tuning (especially of the distributional characteristics of the transmission rates) can enhance disease control efforts.
4.2. Empirical Verification of Stationary Distributions of
Figure 5 provides a comparison between the empirical and theoretical stationary distributions of the stochastic transmission rates
and
, offering strong numerical support for the assumed ergodic inverse gamma behavior. In the left panel, the histogram represents the empirical distribution of
generated from long-run simulations after discarding the transient phase, ensuring convergence to the stationary regime. This histogram closely aligns with the orange curve corresponding to the theoretical inverse gamma probability density function, suggesting that the stochastic differential equation governing
indeed admits an inverse gamma stationary distribution. Similarly, the right panel displays the results for
, where the empirical distribution matches well with the inverse gamma PDF shown in blue, reinforcing the same conclusion.
The parameters , , , and were selected to ensure consistent shape behavior while allowing for variation in scale, which directly influences the tail behavior and variance of the distribution. The strong agreement observed in both panels between the simulated empirical distributions and the theoretical inverse gamma densities confirms not only the ergodicity of the transmission processes but also the structural robustness of the inverse gamma assumption across the considered parameter regimes. This consistency indicates that the inverse gamma law remains a reliable stationary approximation for the stochastic transmission rates, even under varying dynamical and noise conditions.
From a modeling perspective, this result is conceptually important because it demonstrates internal consistency: the long-term behavior of the stochastic transmission rates governed by the chosen mean-reverting dynamics aligns with an analytically tractable and interpretable distribution. While this does not constitute empirical validation, it offers theoretical justification for adopting the inverse gamma law as a stationary approximation, which, in turn, enables explicit analytical expressions for quantities like the effective reproduction number . This analytical tractability is essential for conducting rigorous probabilistic and sensitivity analyses within the model framework.
Figure 6 explores the influence of the diffusion coefficient
on the stationary behavior of the stochastic transmission rate
. Using fixed values
and
, we simulate three distinct cases that vary only by the diffusion intensity
, thereby isolating its role in determining the existence and shape of the stationary distribution.
In the first scenario, , yielding , the theoretical conditions for the existence of a stationary inverse gamma distribution are satisfied. The resulting histogram aligns well with the corresponding inverse gamma density, confirming the theoretical prediction. In the second case, , where , the process remains within the stationary regime, and the empirical distribution again matches the inverse gamma density, though the distribution exhibits increased variance and heavier tails due to the higher diffusion intensity.
In contrast, the third case with leads to , violating the theoretical condition for stationarity. As expected, the empirical histogram shows clear divergence from the inverse gamma shape, indicating that the process may not admit a stationary distribution in this regime. The absence of a matching theoretical curve highlights the loss of ergodicity and the onset of instability in the dynamics of .
Figure 7 examines how changes in the diffusion coefficient
impact the stationary distribution of the stochastic transmission rate
under the fixed parameters
and
. We consider three representative cases by varying
in order to assess whether the process remains within the stationary inverse gamma regime.
In the first case, , corresponding to , the theoretical condition for the existence of an inverse gamma stationary distribution is satisfied (see Theorem 1). The close agreement between the simulated histogram and the corresponding theoretical density confirms that the process has reached its stationary regime. This numerical evidence supports the ergodicity of the transmission rate process and illustrates the model’s capacity to capture stable long-term stochastic dynamics in the transmission rate, consistent with the theoretical predictions.
For the second case, , the squared diffusion intensity is , which still falls below the critical threshold . Here again, the empirical histogram aligns well with the inverse gamma density, though with a greater spread and heavier tails, as expected due to increased stochastic fluctuations. This supports the robustness of the stationary regime under moderate diffusion.
However, in the third case, where and hence , the theoretical condition for stationarity is violated. The resulting histogram deviates significantly from the inverse gamma shape, indicating that the process likely lacks a stationary distribution in this regime. The loss of convergence and increasing variability reflect a breakdown in the balance between drift and diffusion, pushing the system beyond the bounds of ergodic stability.
4.3. Stochastic Dynamics and Sample Trajectories of the SVIR Model
Figure 8 illustrates representative trajectories of the stochastic SVIR model defined by Equations (2)–(5), where the transmission rates
and
evolve as stochastic processes governed by inverse gamma-driven dynamics according to Equations (6) and (7). The simulation uses the following parameter values:
,
,
,
,
,
,
,
,
, and
. These choices ensure that both
and
admit stationary inverse gamma distributions, preserving the ergodicity of the transmission mechanisms. The sample paths of the SVIR compartments over time reflect the dynamic interplay between vaccination, infection, and recovery processes under stochastic fluctuations. Despite the noise in the transmission rates, the trajectories exhibit persistent epidemic behavior, with infected individuals remaining present over a long time horizon. Quantitatively, the estimated probability that the effective reproduction number exceeds one,
, is computed as approximately
. This high probability suggests that, under the given parameter configuration, the system is likely to sustain the epidemic in the long run, consistent with the observed persistence in the sample paths.
Figure 9 presents representative trajectories of the SVIR epidemic model governed by the stochastic system (2)–(5), with t the following parameter configuration:
,
,
,
,
,
,
,
,
, and
. These settings maintain the ergodicity and stationarity of the transmission processes, allowing for a well-defined analysis of long-term dynamics. The trajectories reveal a clear trend toward the extinction of the epidemic, with the number of infected individuals gradually declining and eventually approaching zero over time. This behavior is consistent with a low reproduction potential of the disease under the influence of the chosen stochastic transmission structure. This is further corroborated by the numerical estimate of the probability
, which is approximately
. This high extinction probability strongly indicates that the epidemic is unlikely to persist in the long run under the current parameter regime. The decline in
is primarily driven by the reduced scaling factor
, which effectively lowers the overall transmission intensity despite the inherent stochasticity in
and
.
5. Conclusions
In this study, we carried out a probabilistic analysis of a stochastic SVIR epidemic model, where the transmission rates and evolve according to ergodic stochastic differential equations with inverse gamma stationary distributions. Our primary objective was to understand how stochastic fluctuations and heterogeneity in disease transmission influence the long-term dynamics of the epidemic, particularly in determining whether the disease persists or dies out.
From a theoretical standpoint, we derived sufficient conditions for the existence of stationary distributions of the stochastic transmission processes. Specifically, we showed that the processes and admit stationary inverse gamma distributions when the diffusion coefficients satisfy and for . We analytically characterized these stationary distributions and demonstrated that, under such conditions, the effective reproduction number becomes a well-defined random variable whose distribution governs the probabilistic thresholds of epidemic persistence or extinction. Furthermore, we provided approximations for and , highlighting their dependence on the inverse gamma parameters and the mixing weights q and p used to combine the two transmission pathways.
The theoretical findings were supported by some numerical examples. Monte Carlo simulations of the stochastic processes demonstrated that the empirical distributions of and , computed over long time horizons, closely match their theoretical inverse gamma stationary distributions. This numerical agreement provides strong evidence of the ergodic behavior predicted by the theoretical analysis, confirming that the processes converge in distribution to their respective invariant measures. Sensitivity analyses using three-dimensional surface plots demonstrated that increasing the shape parameters or reducing the scale and diffusion parameters , results in a lower variance of , enhancing predictability and increasing the probability of extinction. Conversely, larger values of or amplify transmission variability, raising the risk of disease persistence.
In addition, we showed that the overall transmission strength, modulated by the parameter A, plays a crucial role in shaping epidemic outcomes. Simulations of the full SVIR model revealed contrasting scenarios: for a high input rate (), the probability of persistence was approximately 0.7538, indicating a likely outbreak. In contrast, for (), the extinction probability increased to 0.9368, suggesting effective epidemic control.
In summary, our findings establish that modeling transmission rates as stochastic processes with inverse gamma stationary distributions offers a nuanced and adaptable framework for capturing epidemic dynamics under uncertainty. Both rigorous theoretical analysis and numerical simulations underscore the pivotal role of transmission variability in influencing disease outcomes, including persistence and extinction scenarios.
Future work may extend this model to account for time-varying vaccination strategies, spatial heterogeneity, and contact network structures. Additionally, integrating real epidemic data through advanced statistical inference techniques could further enhance the model’s applicability to real-world public health planning and epidemic forecasting.