A Rectified Linear Unit-Based Memristor-Enhanced Morris–Lecar Neuron Model
Abstract
1. Introduction
2. Model
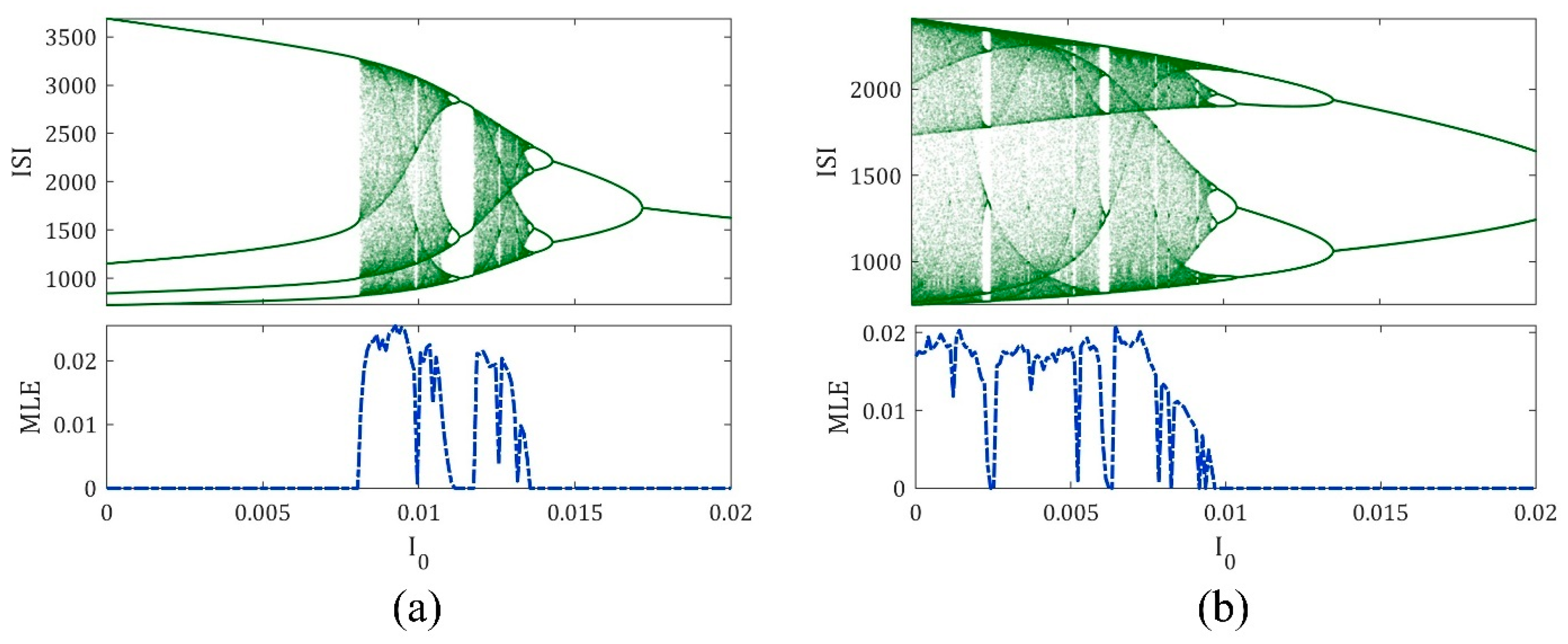
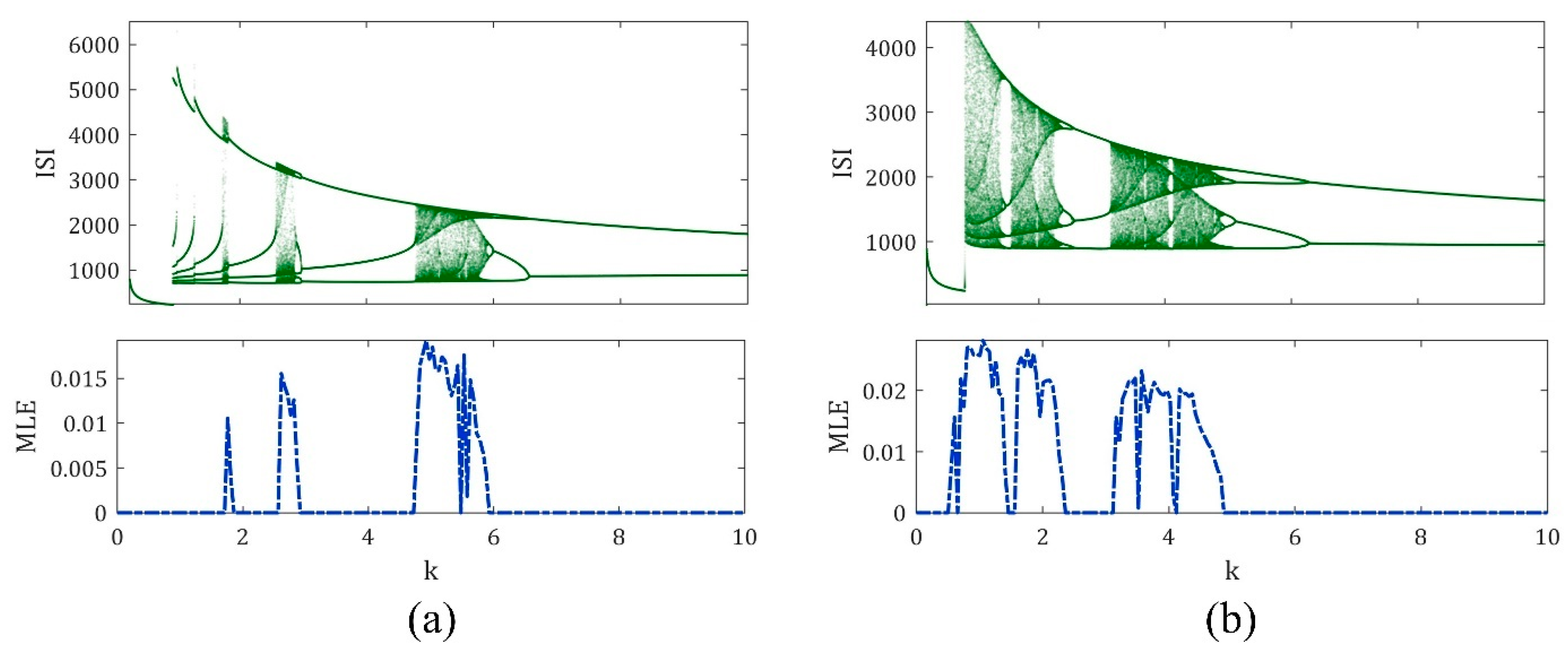
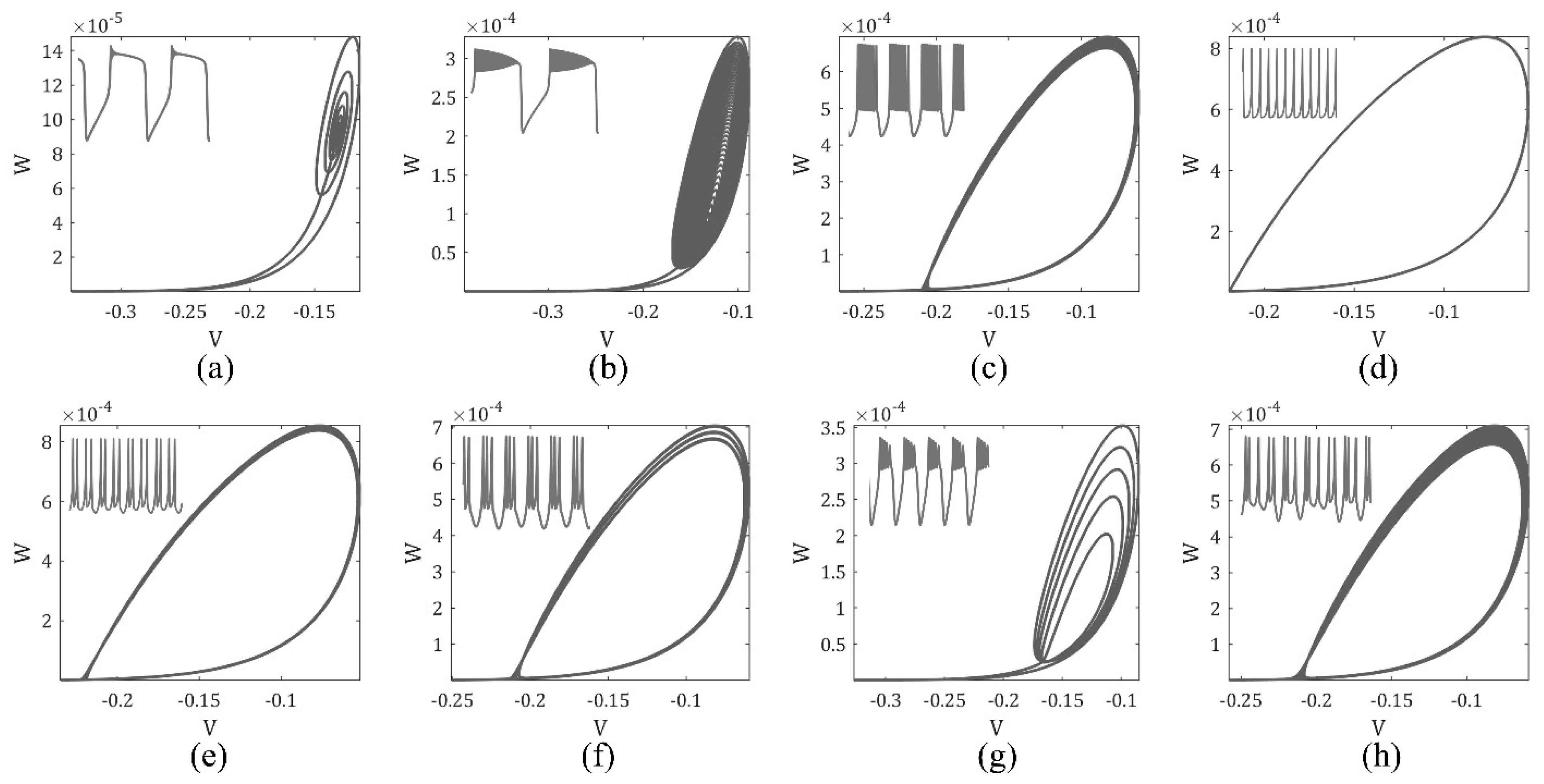
3. The Magnetic Induction Effect on the ML Model Dynamics
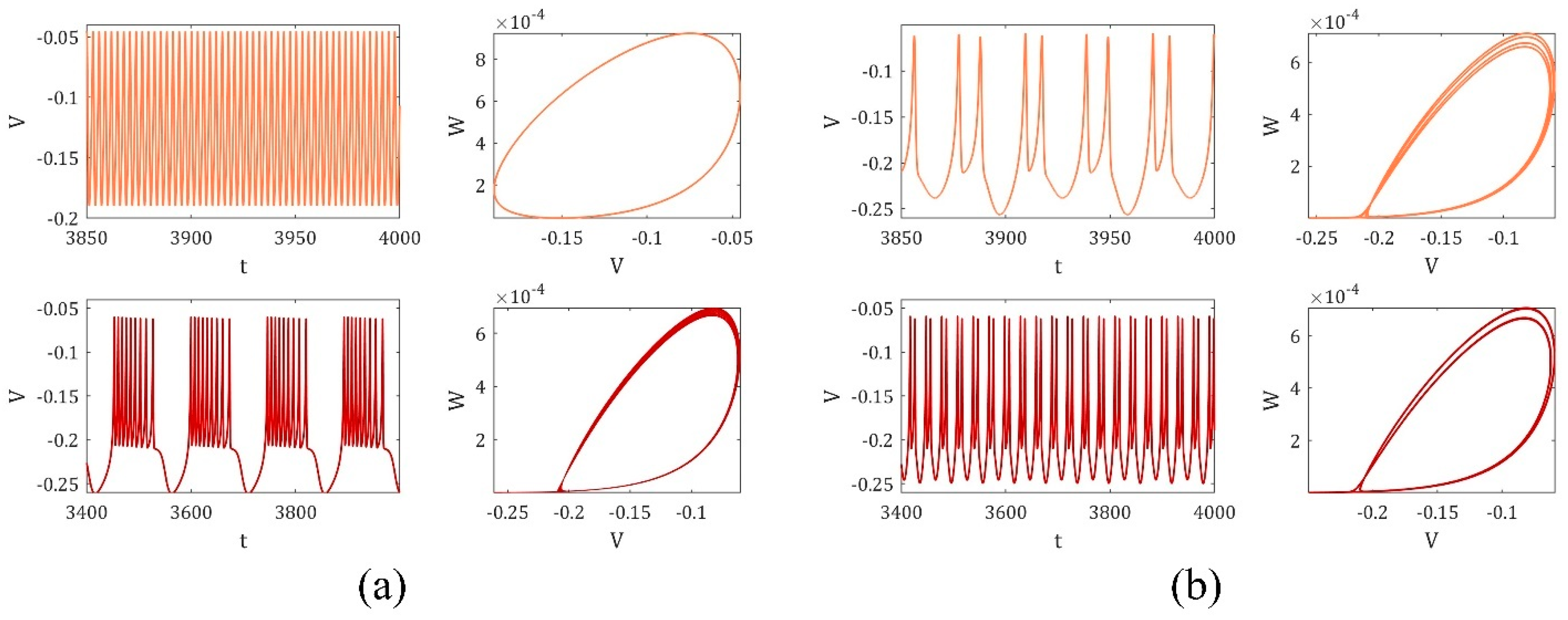
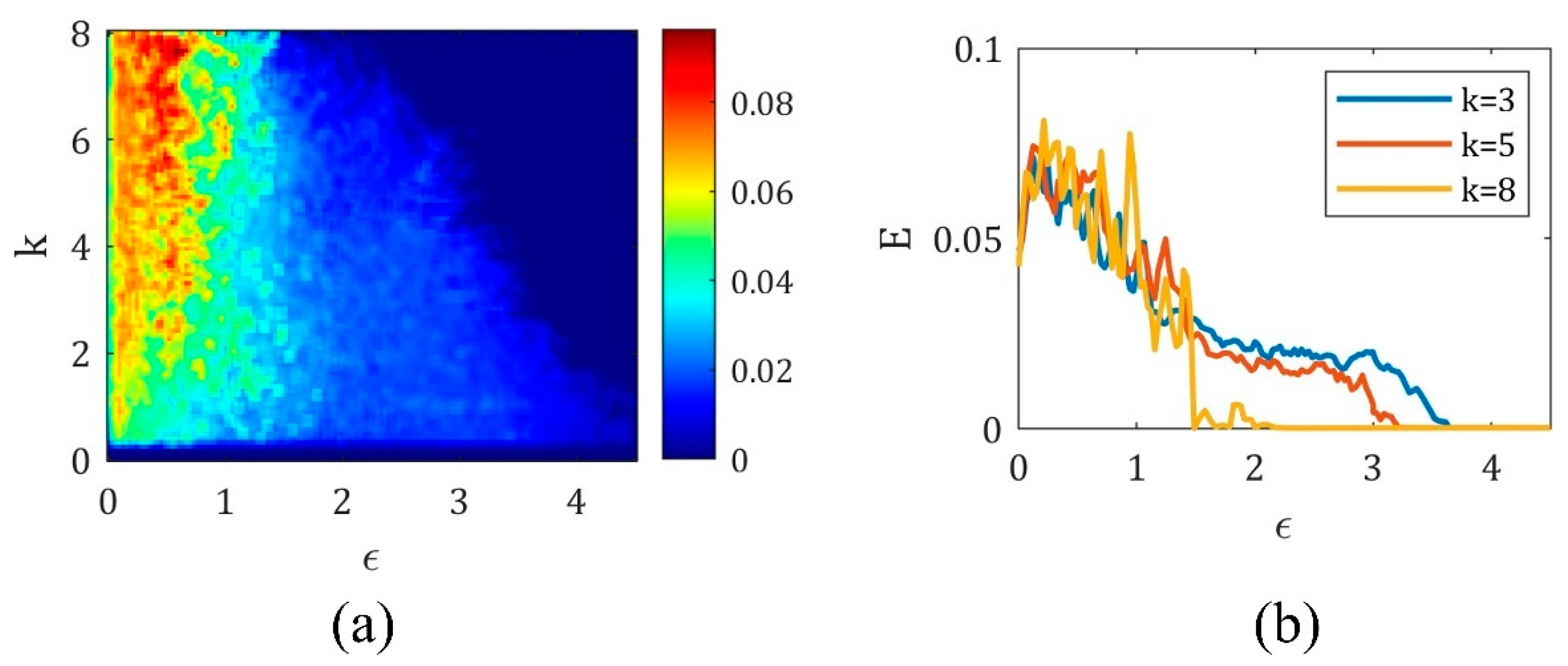
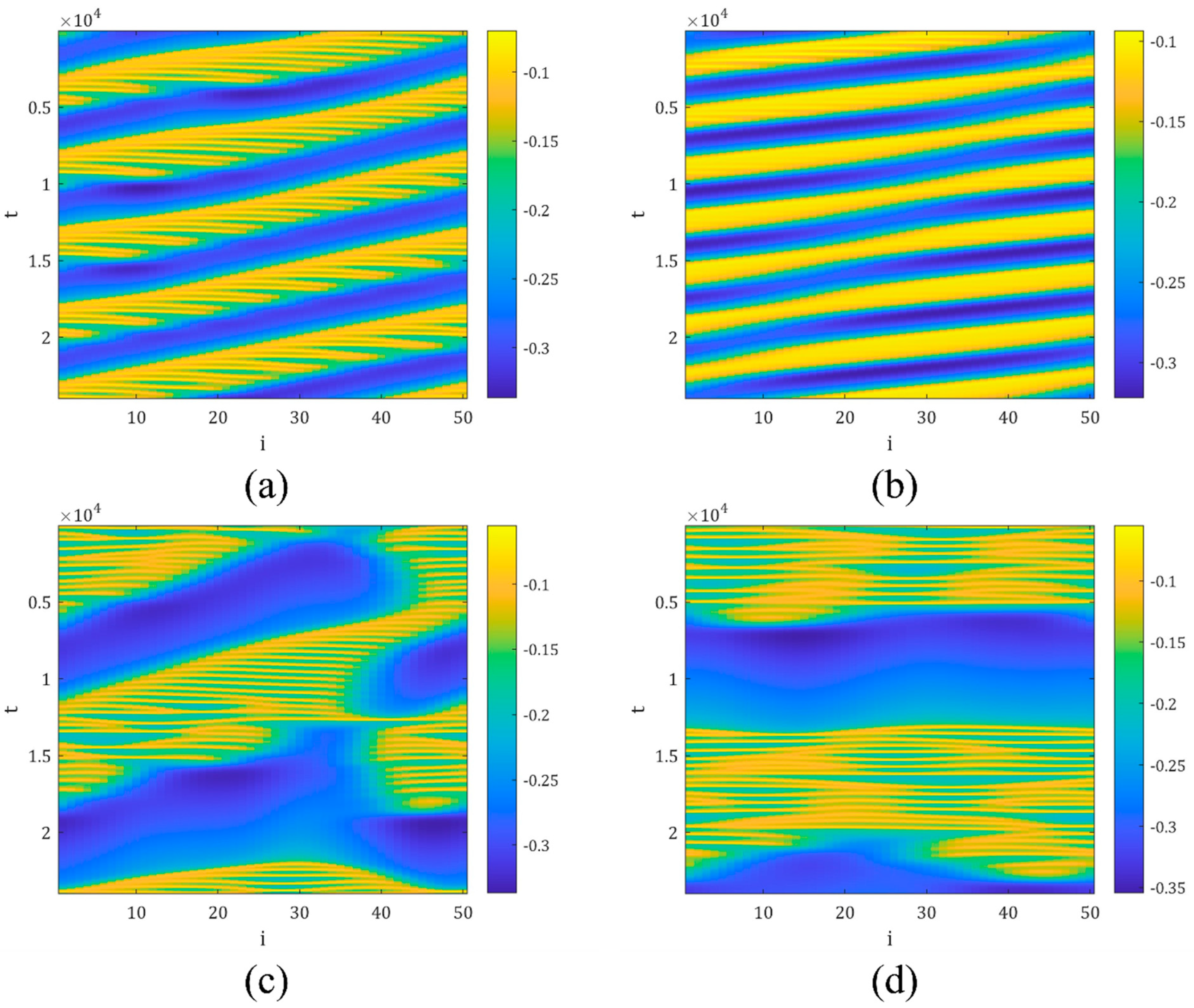
4. The Magnetic Induction Effect on the Synchronization
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ma, J.; Yang, Z.-Q.; Yang, L.-J.; Tang, J. A physical view of computational neurodynamics. J. Zhejiang Univ.-Sci. A 2019, 20, 639–659. [Google Scholar] [CrossRef]
- Hodgkin, A.L.; Huxley, A.F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J. Physiol. 1952, 117, 500. [Google Scholar] [CrossRef] [PubMed]
- FitzHugh, R. Mathematical models of threshold phenomena in the nerve membrane. Bull. Math. Biophys. 1955, 17, 257–278. [Google Scholar] [CrossRef]
- Hindmarsh, J.L.; Rose, R. A model of neuronal bursting using three coupled first order differential equations. Proc. R. Soc. B 1984, 221, 87–102. [Google Scholar]
- Morris, C.; Lecar, H. Voltage oscillations in the barnacle giant muscle fiber. Biophys. J. 1981, 35, 193–213. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Liu, X.; Liu, S. Bifurcation analysis of a Morris–Lecar neuron model. Biol. Cybern. 2014, 108, 75–84. [Google Scholar] [CrossRef]
- Tsumoto, K.; Kitajima, H.; Yoshinaga, T.; Aihara, K.; Kawakami, H. Bifurcations in Morris–Lecar neuron model. Neurocomputing 2006, 69, 293–316. [Google Scholar] [CrossRef]
- Izhikevich, E.M. Neural excitability, spiking and bursting. Int. J. Bifurc. Chaos 2000, 10, 1171–1266. [Google Scholar] [CrossRef]
- Bao, B.; Yang, Q.; Zhu, L.; Bao, H.; Xu, Q.; Yu, Y.; Chen, M. Chaotic bursting dynamics and coexisting multistable firing patterns in 3D autonomous Morris–Lecar model and microcontroller-based validations. Int. J. Bifurc. Chaos 2019, 29, 1950134. [Google Scholar] [CrossRef]
- Hu, X.; Liu, C.; Liu, L.; Ni, J.; Li, S. An electronic implementation for Morris–Lecar neuron model. Nonlinear Dyn. 2016, 84, 2317–2332. [Google Scholar] [CrossRef]
- Shi, M.; Wang, Z. Abundant bursting patterns of a fractional-order Morris–Lecar neuron model. Commun. Nonlinear Sci. Numer. Simul. 2014, 19, 1956–1969. [Google Scholar] [CrossRef]
- Wu, K.; Luo, T.; Lu, H.; Wang, Y. Bifurcation study of neuron firing activity of the modified Hindmarsh–Rose model. Neural Comput. Appl. 2016, 27, 739–747. [Google Scholar] [CrossRef]
- Wang, S.; He, S.; Rajagopal, K.; Karthikeyan, A.; Sun, K. Route to hyperchaos and chimera states in a network of modified Hindmarsh-Rose neuron model with electromagnetic flux and external excitation. Eur. Phys. J. Spec. Top. 2020, 229, 929–942. [Google Scholar] [CrossRef]
- Zhang, X.; Min, F.; Dou, Y.; Xu, Y. Bifurcation analysis of a modified FitzHugh-Nagumo neuron with electric field. Chaos Solitons Fractals 2023, 170, 113415. [Google Scholar] [CrossRef]
- Lv, M.; Ma, J. Multiple modes of electrical activities in a new neuron model under electromagnetic radiation. Neurocomputing 2016, 205, 375–381. [Google Scholar] [CrossRef]
- Lin, H.; Wang, C.; Deng, Q.; Xu, C.; Deng, Z.; Zhou, C. Review on chaotic dynamics of memristive neuron and neural network. Nonlinear Dyn. 2021, 106, 959–973. [Google Scholar] [CrossRef]
- Xu, Q.; Wang, K.; Chen, M.; Parastesh, F.; Wang, N. Bursting and spiking activities in a Wilson neuron circuit with memristive sodium and potassium ion channels. Chaos Solitons Fractals 2024, 181, 114654. [Google Scholar] [CrossRef]
- Wang, C.; Tang, D.; Lin, H.; Yu, F.; Sun, Y. High-dimensional memristive neural network and its application in commercial data encryption communication. Expert Syst. Appl. 2024, 242, 122513. [Google Scholar] [CrossRef]
- Deng, Q.; Wang, C.; Lin, H. Memristive Hopfield neural network dynamics with heterogeneous activation functions and its application. Chaos Solitons Fractals 2024, 178, 114387. [Google Scholar] [CrossRef]
- Lin, H.; Wang, C.; Yu, F.; Hong, Q.; Xu, C.; Sun, Y. A triple-memristor Hopfield neural network with space multistructure attractors and space initial-offset behaviors. IEEE Trans. Comput.-Aided Des. Integr. Circuits Syst. 2023, 42, 4948–4958. [Google Scholar] [CrossRef]
- Xu, Q.; Wang, Y.; Wu, H.; Chen, M.; Chen, B. Periodic and chaotic spiking behaviors in a simplified memristive Hodgkin-Huxley circuit. Chaos Solitons Fractals 2024, 179, 114458. [Google Scholar] [CrossRef]
- Chen, X.; Wang, N.; Wang, Y.; Wu, H.; Xu, Q. Memristor initial-offset boosting and its bifurcation mechanism in a memristive FitzHugh-Nagumo neuron model with hidden dynamics. Chaos Solitons Fractals 2023, 174, 113836. [Google Scholar] [CrossRef]
- Lin, H.; Wang, C.; Sun, Y.; Yao, W. Firing multistability in a locally active memristive neuron model. Nonlinear Dyn. 2020, 100, 3667–3683. [Google Scholar] [CrossRef]
- Martínez-Guerra, R.; Gómez-Cortés, G.; Pérez-Pinacho, C. Synchronization of integral and fractional order chaotic systems. In A Differential Algebraic and Differential Geometric Approach with Selected Applications in Real-Time; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Wu, T.; Zhang, X.; Liu, Z. Understanding the mechanisms of brain functions from the angle of synchronization and complex network. Front. Phys. 2022, 17, 31504. [Google Scholar] [CrossRef]
- Jiruska, P.; De Curtis, M.; Jefferys, J.G.; Schevon, C.A.; Schiff, S.J.; Schindler, K. Synchronization and desynchronization in epilepsy: Controversies and hypotheses. J. Physiol. 2013, 591, 787–797. [Google Scholar] [CrossRef] [PubMed]
- Penn, Y.; Segal, M.; Moses, E. Network synchronization in hippocampal neurons. Proc. Natl. Acad. Sci. USA 2016, 113, 3341–3346. [Google Scholar] [CrossRef]
- Andreev, A.V.; Maksimenko, V.A.; Pisarchik, A.N.; Hramov, A.E. Synchronization of interacted spiking neuronal networks with inhibitory coupling. Chaos Solitons Fractals 2021, 146, 110812. [Google Scholar] [CrossRef]
- Inagaki, T.; Inaba, K.; Leleu, T.; Honjo, T.; Ikuta, T.; Enbutsu, K.; Umeki, T.; Kasahara, R.; Aihara, K.; Takesue, H. Collective and synchronous dynamics of photonic spiking neurons. Nat. Commun. 2021, 12, 2325. [Google Scholar] [CrossRef]
- Mehrabbeik, M.; Jafari, S.; Perc, M. Synchronization in simplicial complexes of memristive Rulkov neurons. Front. Comput. Neurosci. 2023, 17, 1248976. [Google Scholar] [CrossRef]
- Parastesh, F.; Jafari, S.; Azarnoush, H.; Shahriari, Z.; Wang, Z.; Boccaletti, S.; Perc, M. Chimeras. Phys. Rep. 2021, 898, 1–114. [Google Scholar] [CrossRef]
- Majhi, S.; Bera, B.K.; Ghosh, D.; Perc, M. Chimera states in neuronal networks: A review. Phys. Life Rev. 2019, 28, 100–121. [Google Scholar] [CrossRef] [PubMed]
- Dudkowski, D.; Maistrenko, Y.; Kapitaniak, T. Different types of chimera states: An interplay between spatial and dynamical chaos. Phys. Rev. E 2014, 90, 032920. [Google Scholar] [CrossRef] [PubMed]
- Schöll, E. Synchronization patterns and chimera states in complex networks: Interplay of topology and dynamics. Eur. Phys. J. Spec. Top. 2016, 225, 891–919. [Google Scholar] [CrossRef]
- Hussain, I.; Jafari, S.; Perc, M.; Ghosh, D. Chimera states in a multi-weighted neuronal network. Phys. Lett. A 2022, 424, 127847. [Google Scholar] [CrossRef]
- Bera, B.K.; Ghosh, D.; Banerjee, T. Imperfect traveling chimera states induced by local synaptic gradient coupling. Phys. Rev. E 2016, 94, 012215. [Google Scholar] [CrossRef]
- Mishra, A.; Saha, S.; Roy, P.K.; Kapitaniak, T.; Dana, S.K. Multicluster oscillation death and chimeralike states in globally coupled Josephson Junctions. Chaos 2017, 27, 023110. [Google Scholar] [CrossRef]
- Omel’chenko, O. Traveling chimera states. J. Phys. A 2019, 52, 104001. [Google Scholar] [CrossRef]
- Min, F.; Zhai, G.; Yin, S.; Zhong, J. Switching bifurcation of a Rulkov neuron system with ReLu-type memristor. Nonlinear Dyn. 2024, 112, 5687–5706. [Google Scholar] [CrossRef]
- Chen, X.; Wang, N.; Wang, K.; Chen, M.; Parastesh, F.; Xu, Q. Coupling dynamics in an FHN bi-neuron model coupled via ReLU function-based locally active memristor. Nonlinear Dyn. 2024, 112, 20365–20379. [Google Scholar] [CrossRef]
- Gu, Y.; Hu, F.; Zhang, X.; Min, F. Complex firing activities and bifurcations in memristor-coupled Hindmarsh–Rose neuron. AIP Adv. 2024, 14, 015353. [Google Scholar] [CrossRef]
- Xu, Q.; Chen, X.; Wu, H.; Iu, H.H.-C.; Parastesh, F.; Wang, N. ReLU Function-Based Locally Active Memristor and Its Application in Generating Spiking Behaviors. IEEE Trans. Circuits Syst. II 2024. [Google Scholar] [CrossRef]
- Chen, C.; Min, F.; Zhang, Y.; Bao, H. ReLU-type Hopfield neural network with analog hardware implementation. Chaos Solitons Fractals 2023, 167, 113068. [Google Scholar] [CrossRef]
- Chen, C.; Min, F. ReLU-type memristor-based Hopfield neural network. Eur. Phys. J. Spec. Top. 2022, 231, 2979–2992. [Google Scholar] [CrossRef]
- Bao, H.; Yu, X.; Xu, Q.; Wu, H.; Bao, B. Three-dimensional memristive Morris–Lecar model with magnetic induction effects and its FPGA implementation. Cognit. Neurodyn. 2023, 17, 1079–1092. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almatroud, O.A.; Pham, V.-T.; Rajagopal, K. A Rectified Linear Unit-Based Memristor-Enhanced Morris–Lecar Neuron Model. Mathematics 2024, 12, 2970. https://doi.org/10.3390/math12192970
Almatroud OA, Pham V-T, Rajagopal K. A Rectified Linear Unit-Based Memristor-Enhanced Morris–Lecar Neuron Model. Mathematics. 2024; 12(19):2970. https://doi.org/10.3390/math12192970
Chicago/Turabian StyleAlmatroud, Othman Abdullah, Viet-Thanh Pham, and Karthikeyan Rajagopal. 2024. "A Rectified Linear Unit-Based Memristor-Enhanced Morris–Lecar Neuron Model" Mathematics 12, no. 19: 2970. https://doi.org/10.3390/math12192970
APA StyleAlmatroud, O. A., Pham, V.-T., & Rajagopal, K. (2024). A Rectified Linear Unit-Based Memristor-Enhanced Morris–Lecar Neuron Model. Mathematics, 12(19), 2970. https://doi.org/10.3390/math12192970





