Nonlinear Dynamics of a General Stochastic SIR Model with Behavioral and Physical Changes: Analysis and Application to Zoonotic Tuberculosis
Abstract
1. Introduction
- denotes the susceptible individuals who maintain their behavior unchanged in response to the epidemic.
- represents the susceptible individuals who alter their behavior due to various interventions, such as media campaigns or governmental measures.
- I signifies the count of infected individuals within the population.
- C stands for the individuals who have recovered from the infection and acquired complete immunity.
- r represents the rate of population influx, encompassing births, immigration, or any other form of population input.
- and indicate the transmission rates of the epidemic, reflecting the likelihood of infection spread within the population.
- represents the natural death rate of the population.
- c signifies the rate at which susceptible individuals adjust their behavior and transition to the second class.
- denotes the recovery rate of infected individuals.
- represents the mortality rate attributed to infection.
2. Model Formulation
- (A0): The functions and are uniformly continuous and such that
- .
- .
- .
- , where indicates the index at which the maximum value is reached.
- , where indicates the index at which the minimum value is reached.
- .
- .
- .
- : , and .
- : such that .
3. Results
3.1. Evaluating the Mathematical Sufficiency of Stochastic System (2)
3.2. Threshold Analysis of Stochastic System (2)
- The exponential extinction of the epidemic if .
- The stochastic continuation of the epidemic if .
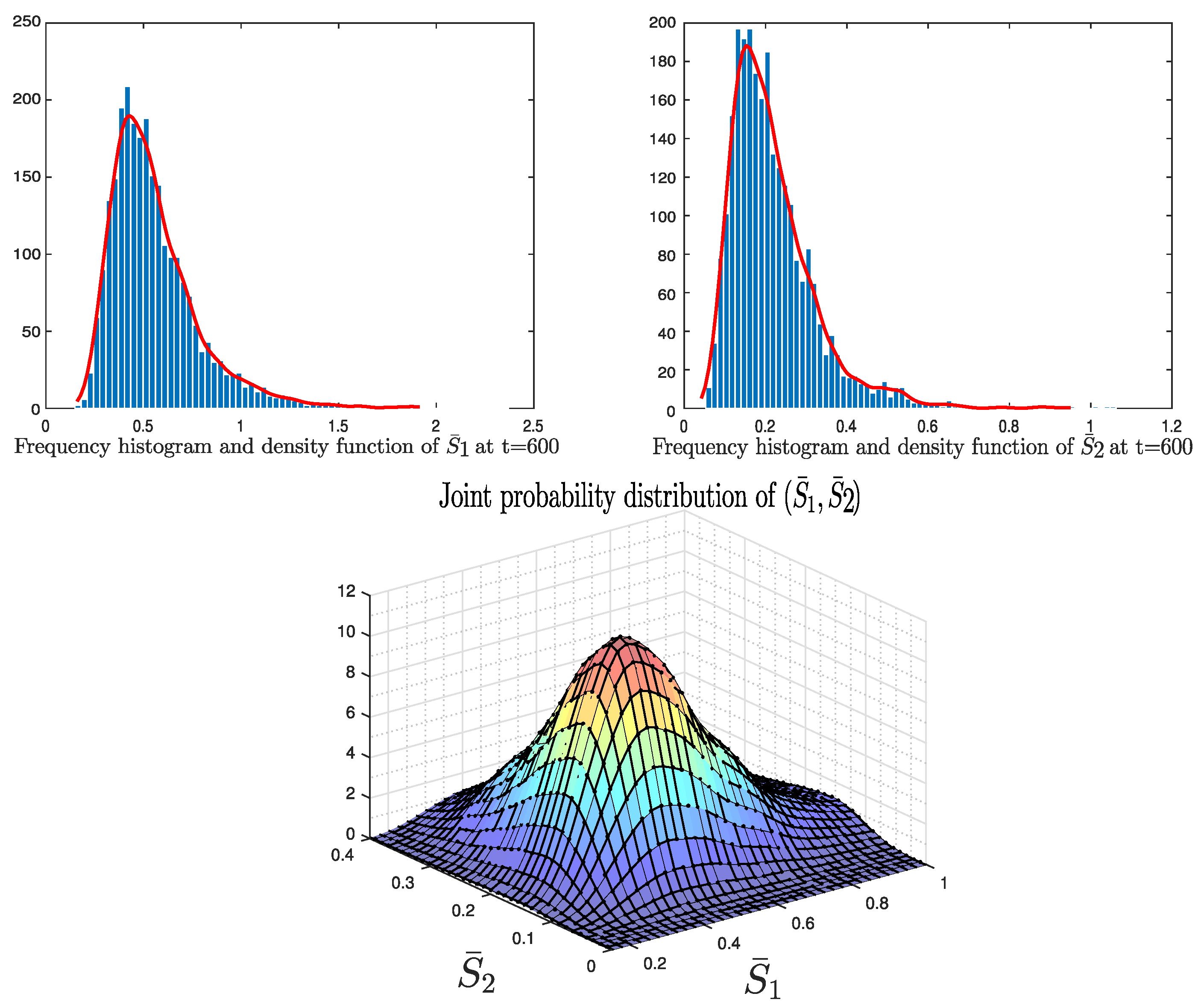
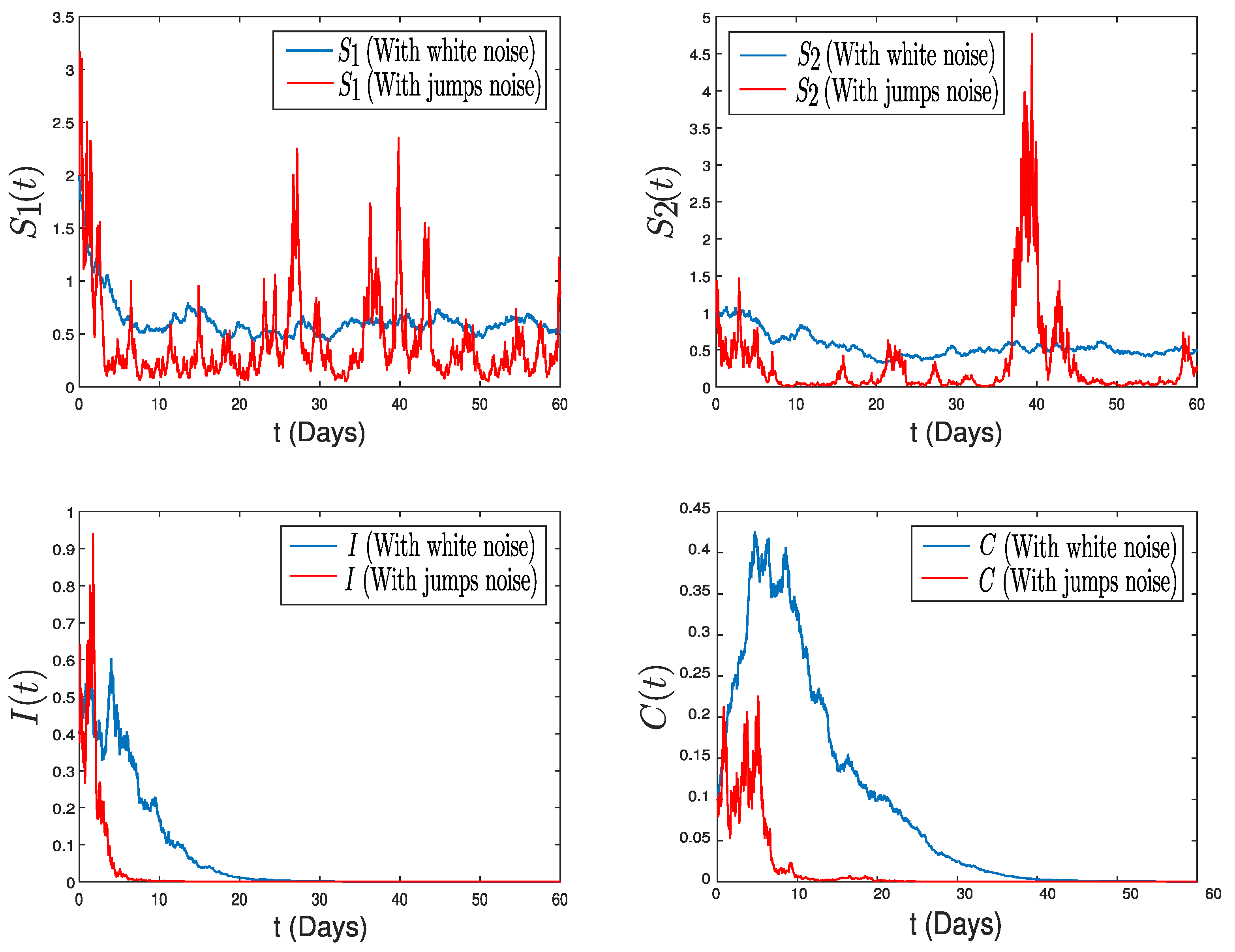
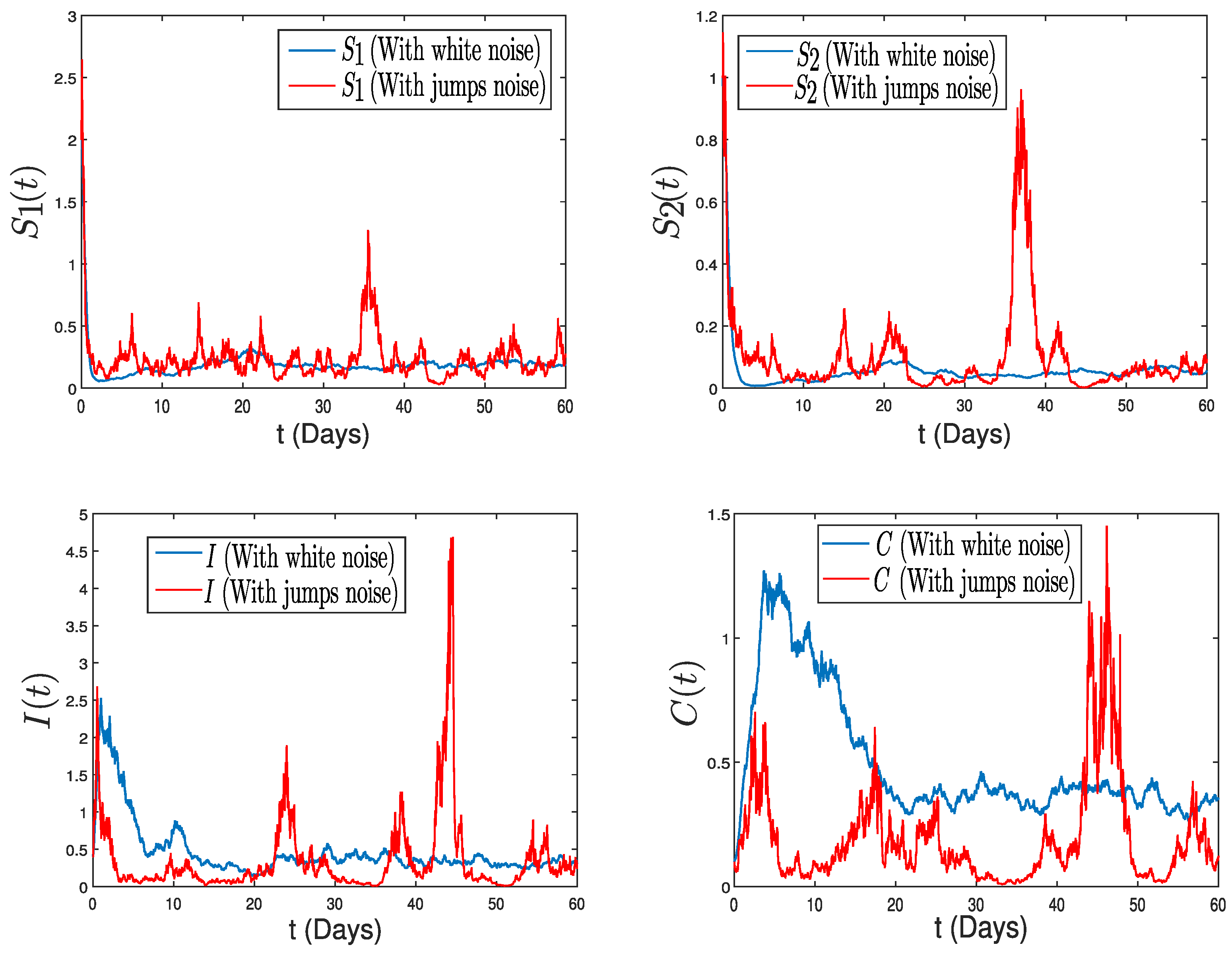
4. Numerical Application: Zoonotic Tuberculosis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Capasso, V. Mathematical Structures of Epidemic Systems; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2008; Volume 97. [Google Scholar]
- Brauer, F.; Castillo-Chavez, C. Mathematical Models in Population Biology and Epidemiology; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013; Volume 40. [Google Scholar]
- Kermack, W.O.; McKendrick, A.G. Contributions to the mathematical theory of epidemics–I. 1927. Bull. Math. Biol. 1991, 53, 33–55. [Google Scholar]
- Nguyen, D.T.; Du, N.H.; Nguyen, S.L. Asymptotic behavior for a stochastic behavioral change SIR model. J. Math. Anal. Appl. 2024, 538, 128361. [Google Scholar] [CrossRef]
- Frieswijk, K.; Zino, L.; Ye, M.; Rizzo, A.; Cao, M. A mean-field analysis of a network behavioral-epidemic model. IEEE Control Syst. Lett. 2022, 6, 2533–2538. [Google Scholar] [CrossRef]
- Osi, A.; Ghaffarzadegan, N. Parameter estimation in behavioral epidemic models with endogenous societal risk-response. PLoS Comput. Biol. 2024, 20, e1011992. [Google Scholar] [CrossRef]
- Marsudi, T.; Suryanto, A.; Darti, I. Global stability and optimal control of an HIV/AIDS epidemic model with behavioral change and treatment. Eng. Lett. 2021, 29, 575–591. [Google Scholar]
- Li, J.; Wang, X.; Lin, X. Impact of behavioral change on the epidemic characteristics of an epidemic model without vital dynamics. Math. Biosci. Eng. 2018, 15, 1425–1434. [Google Scholar] [CrossRef]
- Ward, C.; Deardon, R.; Schmidt, A.M. Bayesian modeling of dynamic behavioral change during an epidemic. Infect. Dis. Model. 2023, 8, 947–963. [Google Scholar] [CrossRef]
- Li, S. SIR epidemic model with general nonlinear incidence rate and Lévy jumps. Mathematics 2024, 12, 215. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, G.; Chen, T.; Li, Z. Threshold analysis of a stochastic SIRS epidemic model with logistic birth and nonlinear incidence. Mathematics 2023, 11, 1737. [Google Scholar] [CrossRef]
- Zhao, Y.; Jiang, D. The behavior of an SVIR epidemic model with stochastic perturbation. Abstr. Appl. Anal. 2014, 2014, 742730. [Google Scholar] [CrossRef]
- Sabbar, Y.; Khan, A.; Din, A.; Tilioua, M. New method to investigate the impact of independent quadratic alpha stable Poisson jumps on the dynamics of a disease under vaccination strategy. Fractal Fract. 2023, 7, 226. [Google Scholar] [CrossRef]
- Kiouach, D.; Sabbar, Y.; El-idrissi, S.E.A. New results on the asymptotic behavior of an SIS epidemiological model with quarantine strategy, stochastic transmission, and Lévy disturbance. Math. Meth. Appl. Sci. 2021, 44, 13468–13492. [Google Scholar] [CrossRef]
- Rosinski, J. Tempering stable processes. Stoch. Process. Their Appl. 2007, 117, 677–707. [Google Scholar] [CrossRef]
- Koponen, I. Analytic approach to the problem of convergence of truncated Lévy flights towards the gaussian stochastic process. Phys. Rev. E 1995, 52, 1197–1199. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yang, Q. Threshold behavior in a stochastic SVIR model with general incidence rates. Appl. Math. Lett. 2021, 121, 107403. [Google Scholar] [CrossRef]
- Zhou, B.; Zhang, X.; Jiang, D. Dynamics and density function analysis of a stochastic SVI epidemic model with half saturated incidence rate. Chaos Solit. Fract. 2020, 137, 109865. [Google Scholar] [CrossRef]
- Mao, X. Stochastic Differential Equations and Applications; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Karatzas, I.; Shreve, S.E. Brownian Motion and Stochastic Calculus; Springer: Berlin/Heidelberg, Germany, 1998. [Google Scholar]
- Sabbar, Y.; Kiouach, D.; Rajasekar, S.P.; El-Idrissi, S.E.A. The influence of quadratic Lévy noise on the dynamic of an SIC contagious illness model: New framework, critical comparison and an application to COVID-19 (SARS-CoV-2) case. Chaos Solit. Fract. 2022, 159, 112110. [Google Scholar] [CrossRef] [PubMed]
- Øksendal, B.K.; Sulem, A. Applied Stochastic Control of Jump Diffusions; Springer: Berlin/Heidelberg, Germany, 2007; Volume 498. [Google Scholar]
- Privault, N.; Wang, L. Stochastic SIR Lévy jump model with heavy tailed increments. J. Nonlinear Sci. 2021, 31, 15. [Google Scholar] [CrossRef]
- El Attouga, S.; Bouggar, D.; El Fatini, M.; Hilbert, A.; Pettersson, R. Lévy noise with infinite activity and the impact on the dynamic of an SIRS epidemic model. Physica A 2023, 618, 128701. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabbar, Y.; Izadi, M.; Raezah, A.A.; Adel, W. Nonlinear Dynamics of a General Stochastic SIR Model with Behavioral and Physical Changes: Analysis and Application to Zoonotic Tuberculosis. Mathematics 2024, 12, 1974. https://doi.org/10.3390/math12131974
Sabbar Y, Izadi M, Raezah AA, Adel W. Nonlinear Dynamics of a General Stochastic SIR Model with Behavioral and Physical Changes: Analysis and Application to Zoonotic Tuberculosis. Mathematics. 2024; 12(13):1974. https://doi.org/10.3390/math12131974
Chicago/Turabian StyleSabbar, Yassine, Mohammad Izadi, Aeshah A. Raezah, and Waleed Adel. 2024. "Nonlinear Dynamics of a General Stochastic SIR Model with Behavioral and Physical Changes: Analysis and Application to Zoonotic Tuberculosis" Mathematics 12, no. 13: 1974. https://doi.org/10.3390/math12131974
APA StyleSabbar, Y., Izadi, M., Raezah, A. A., & Adel, W. (2024). Nonlinear Dynamics of a General Stochastic SIR Model with Behavioral and Physical Changes: Analysis and Application to Zoonotic Tuberculosis. Mathematics, 12(13), 1974. https://doi.org/10.3390/math12131974








