From Cell–Cell Interaction to Stochastic and Deterministic Descriptions of a Cancer–Immune System Competition Model
Abstract
1. Introduction
2. Descriptions of Cancer and Immune System Competition at Three Different Scales
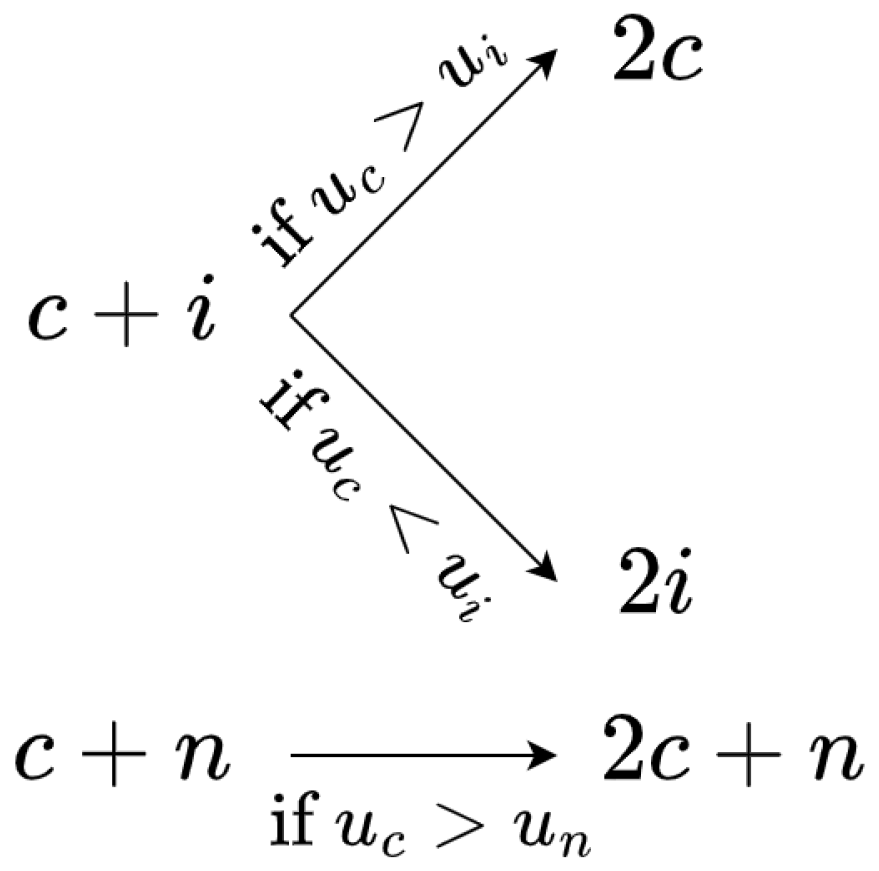
2.1. A Model at Cell Scale
2.2. Thermostatted Kinetic Theory Framework
2.3. Stochastic Description by a Master Equation
2.4. Derivation of the Macroscopic Equations from the Kinetic Equations
3. Results
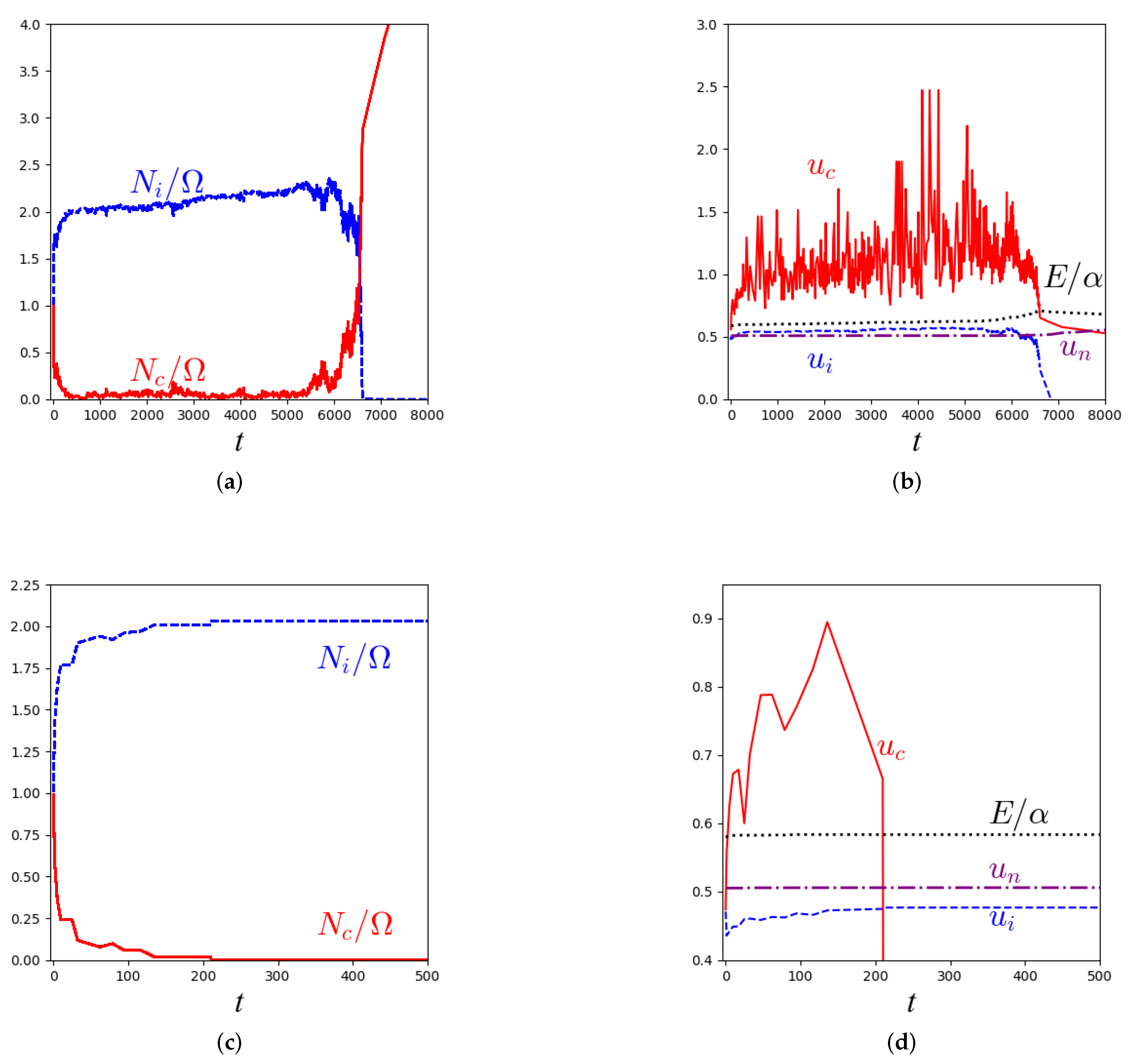
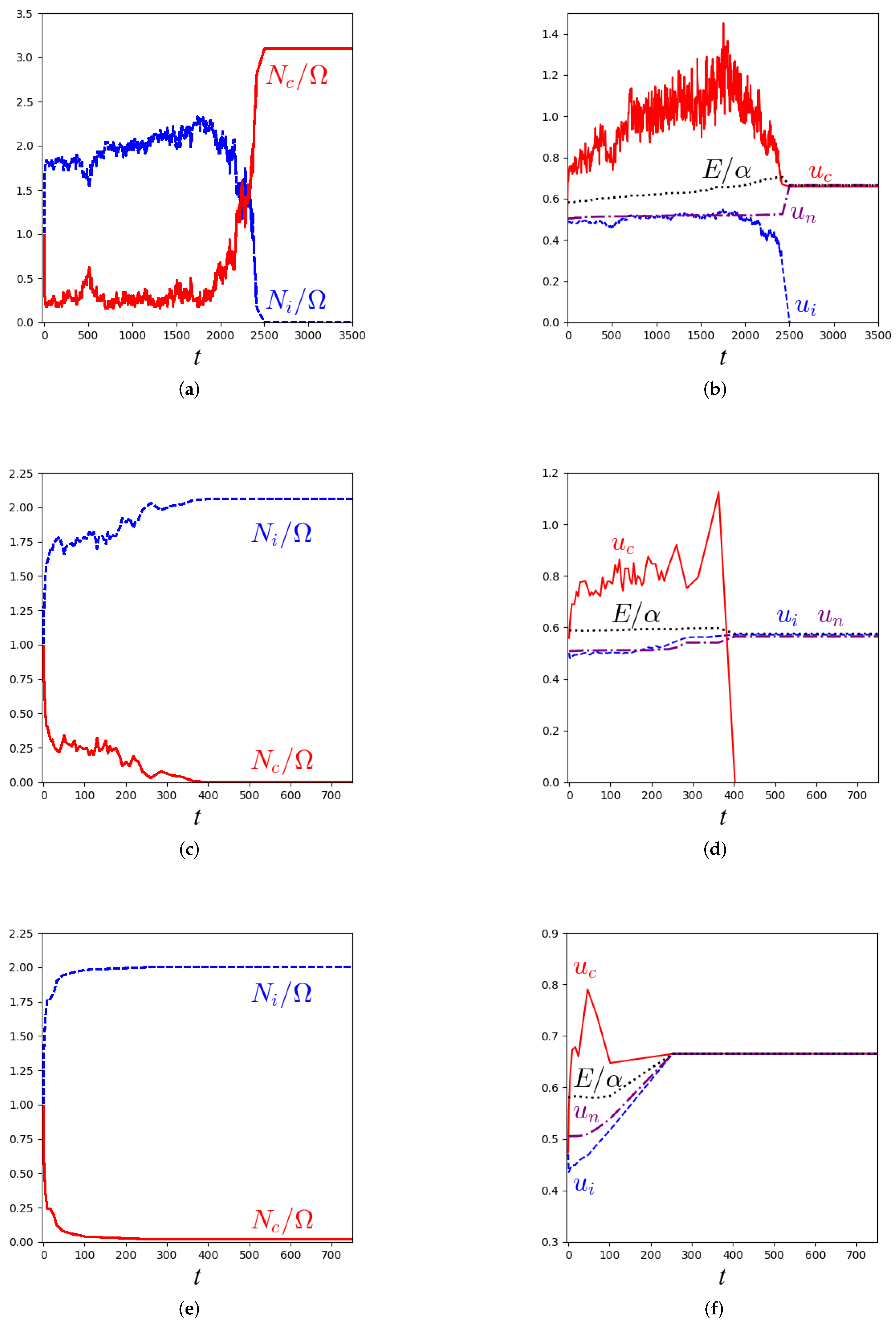
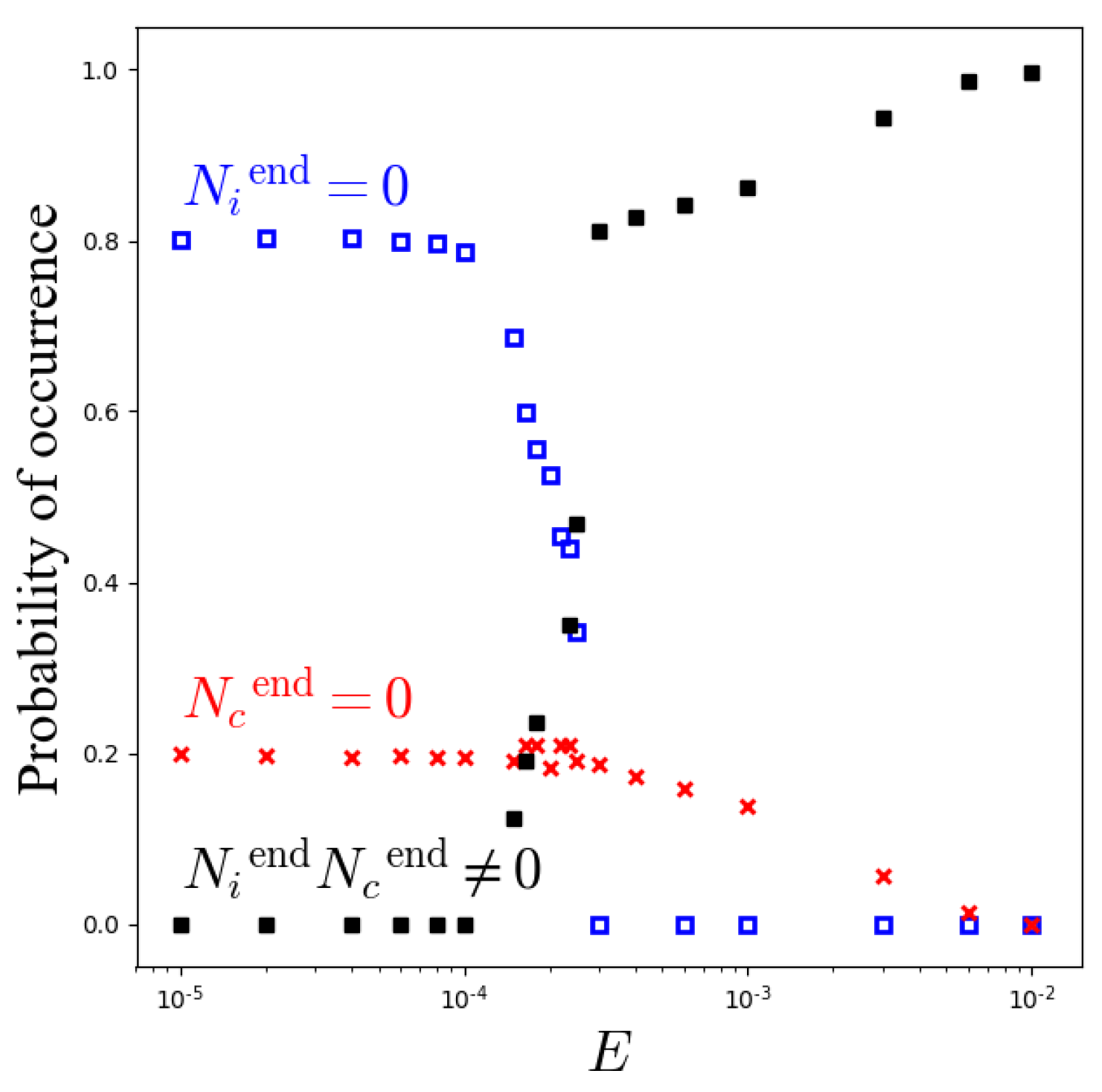
3.1. Kinetic Monte Carlo Simulations of the Master Equation
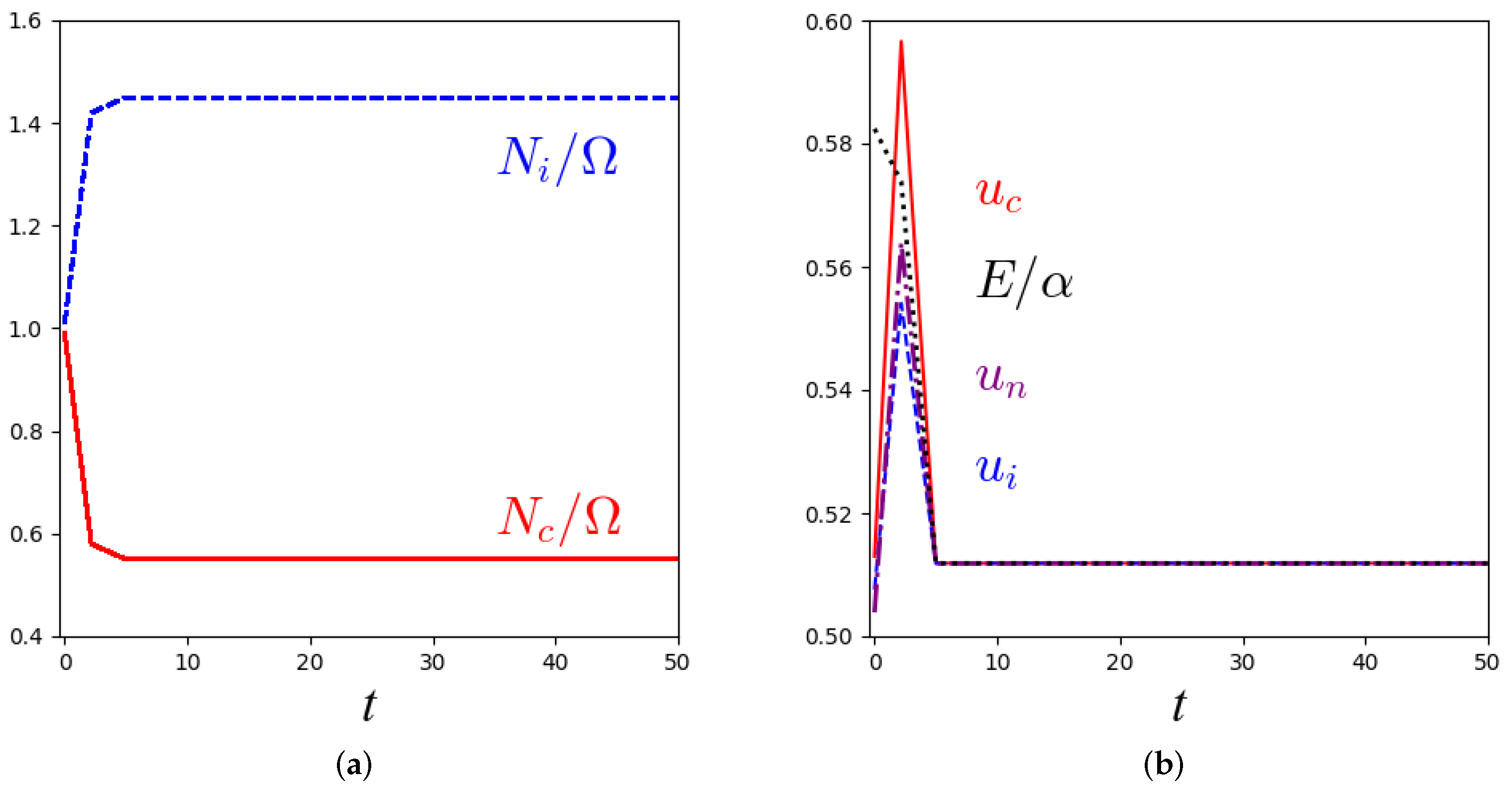
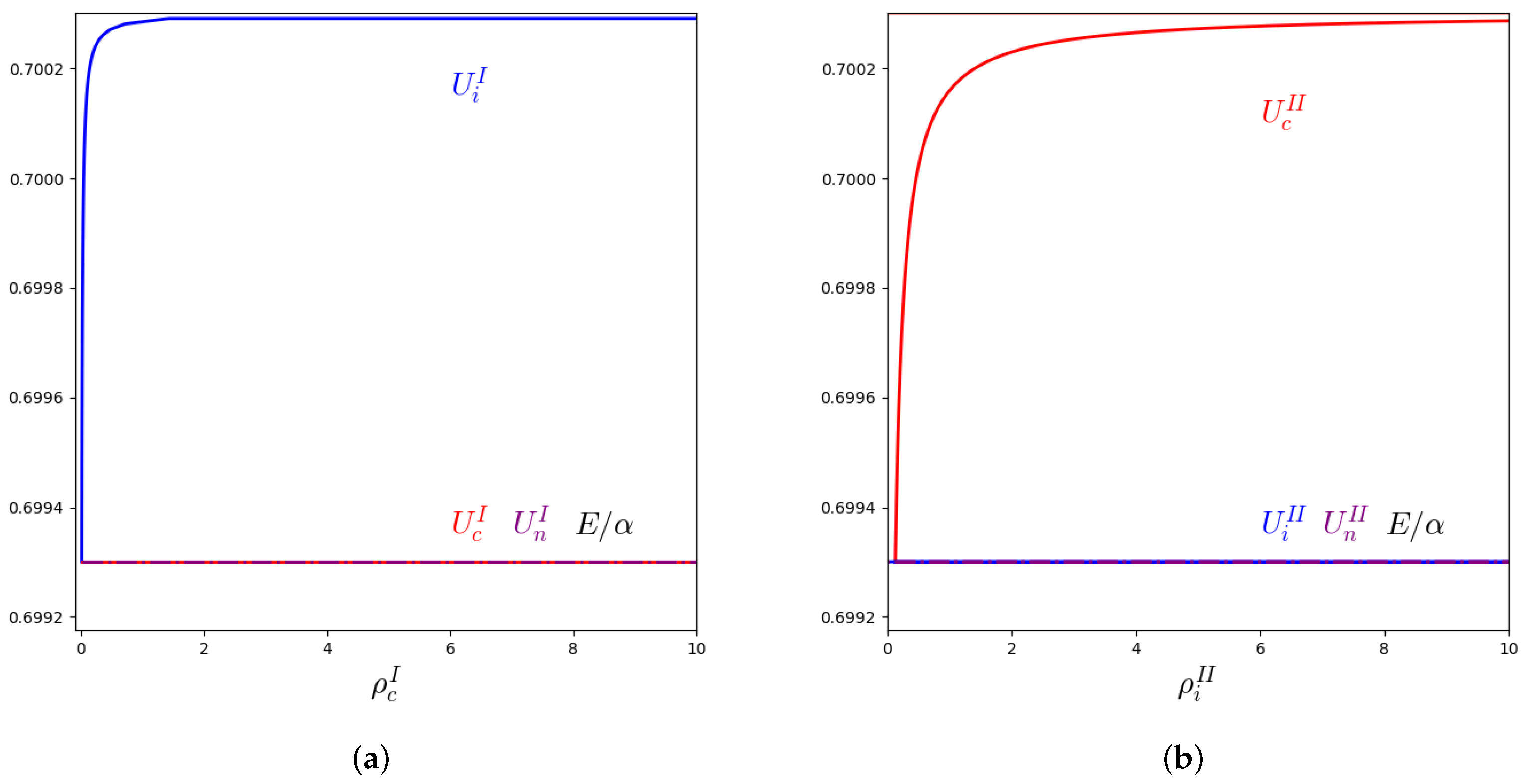
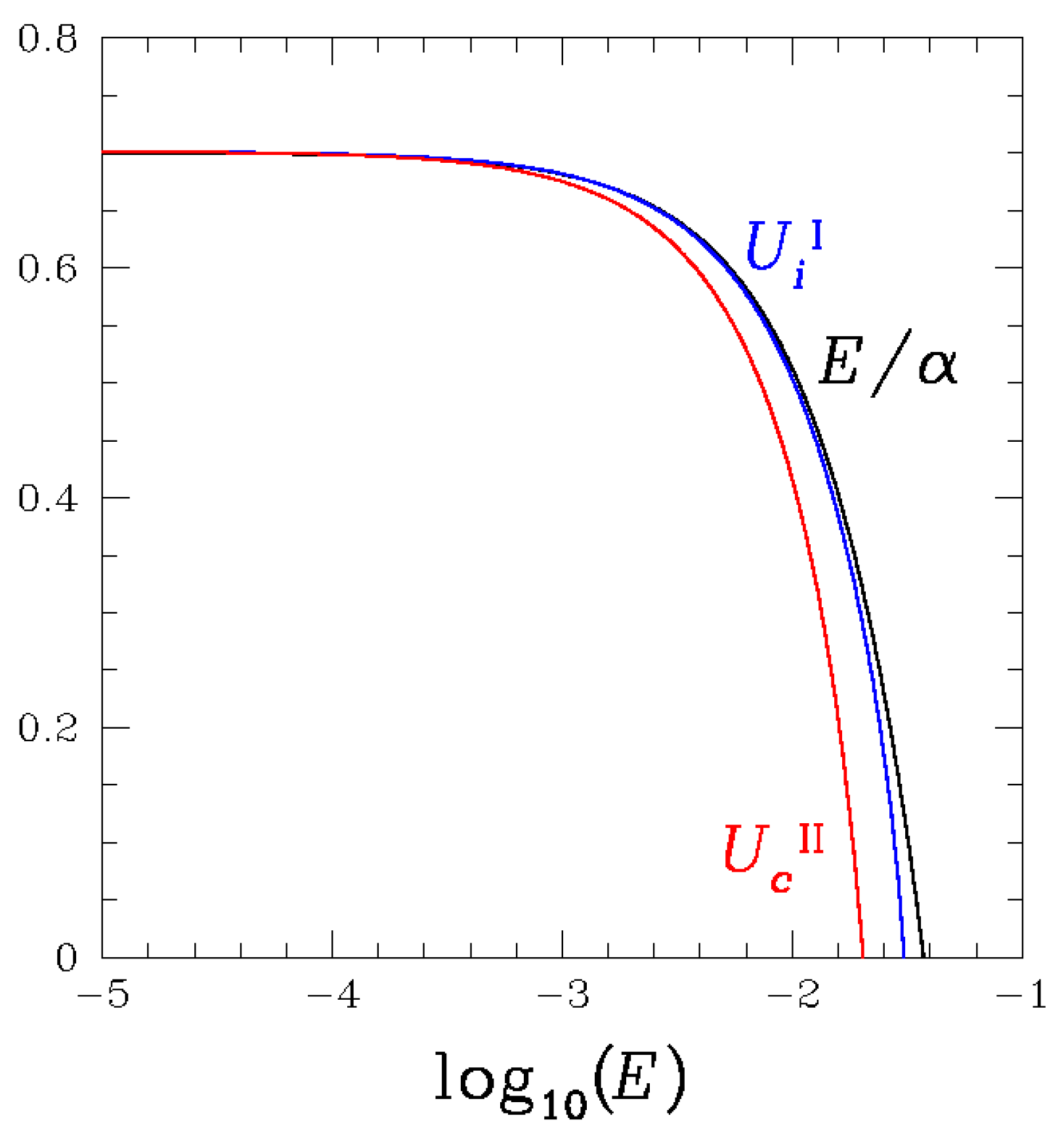
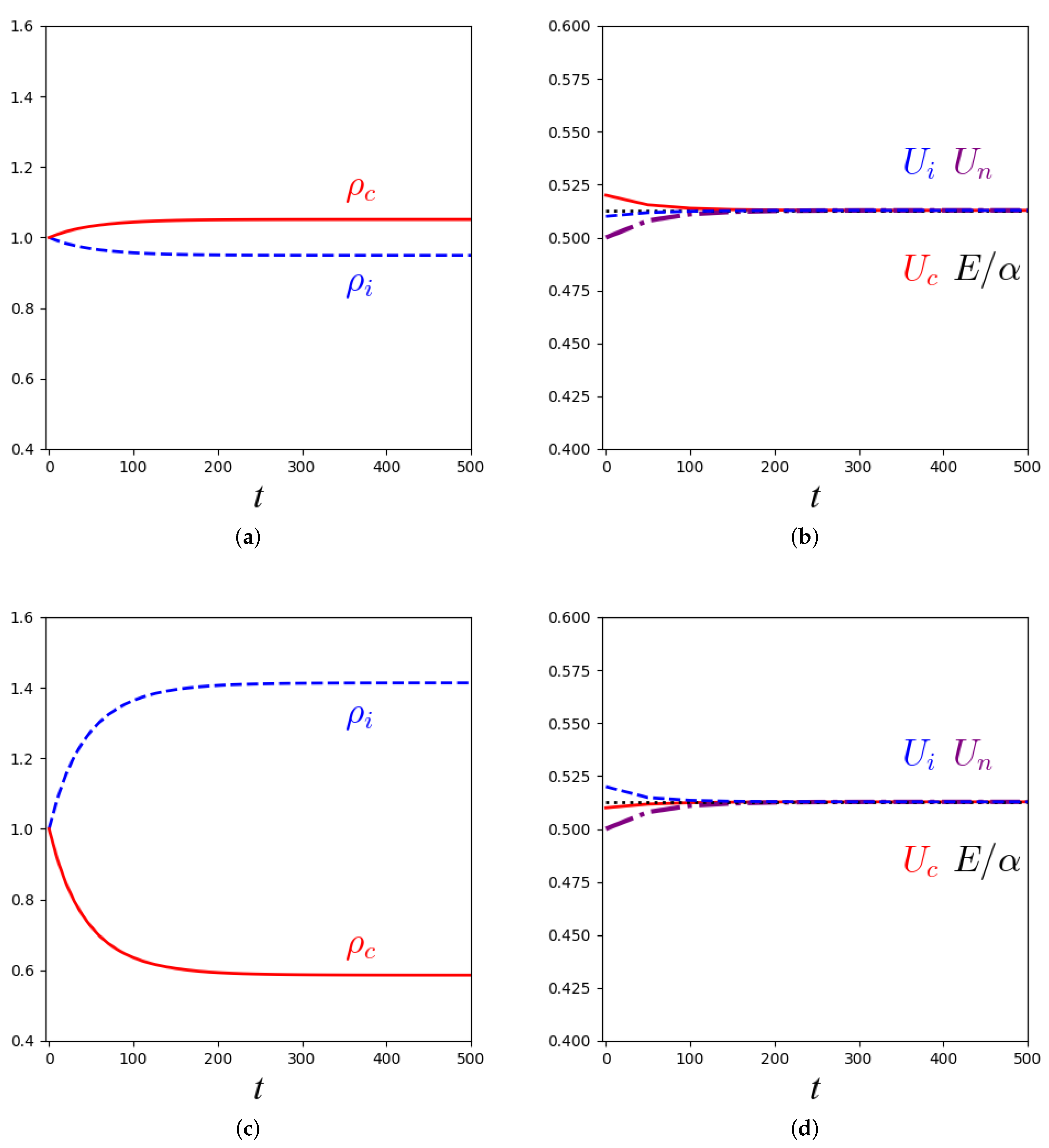
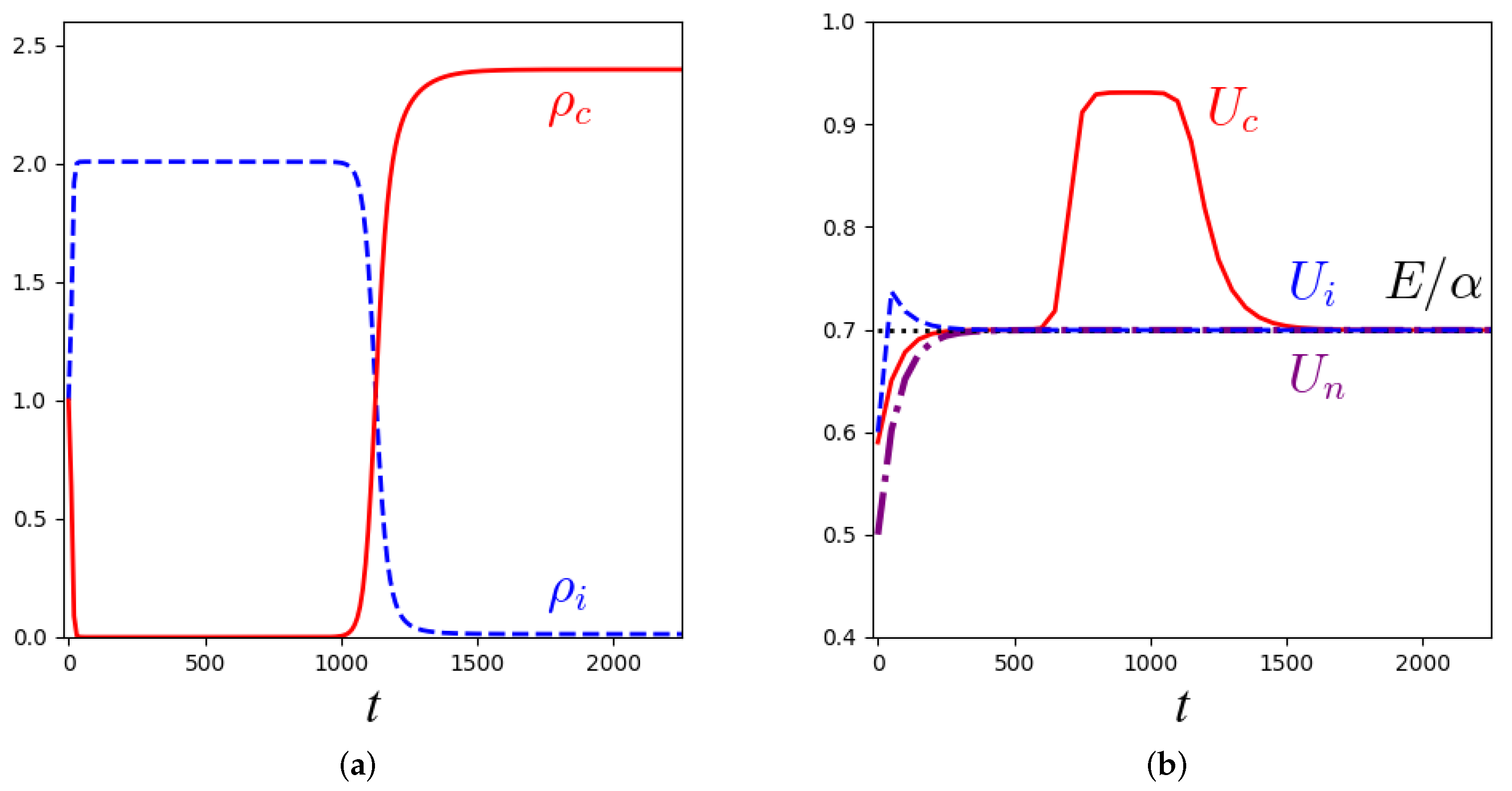
3.2. Stability Analysis and Numerical Solutions for the Macroscopic Equations
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| FKPP | Fisher–Kolmogorov–Petrosky–Piskunov |
| DSMC | Direct Simulation Monte Carlo |
| CRP | C-reactive protein |
References
- Chen, D.S.; Mellman, I. Oncology Meets Immunology: The Cancer-Immunity Cycle. Immunity 2013, 39, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Farkona, S.; Diamandis, E.P.; Blasutig, I.M. Cancer immunotherapy: The beginning of the end of cancer? BMC Med. 2016, 14, 73. [Google Scholar] [CrossRef] [PubMed]
- Oiseth, S.J.; Aziz, M.S. Cancer immunotherapy: A brief review of the history, possibilities, and challenges ahead. J. Cancer Metastasis Treat. 2017, 3, 250–261. [Google Scholar] [CrossRef]
- Waldman, A.; Fritz, J.; Lenardo, M. A guide to cancer immunotherapy: From T cell basic science to clinical practice. Nat. Rev. Immunol. 2020, 20, 651–668. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Hu-Lieskovan, S.; Wargo, J.A.; Ribas, A. Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy. Cell 2017, 168, 707–723. [Google Scholar] [CrossRef]
- Dunn, G.P.; Bruce, A.T.; Ikeda, H.; Old, L.J.; Schreiber, R.D. Cancer immunoediting: From immunosurveillance to tumor escape. Nat. Immunol. 2002, 3, 991–998. [Google Scholar] [CrossRef]
- Dunn, G.P.; Old, L.J.; Schreiber, R.D. The Three Es of Cancer Immunoediting. Annu. Rev. Immunol. 2004, 22, 329–360. [Google Scholar] [CrossRef]
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. Molecular Biology of the Cell; Garland: New York, NY, USA, 2002. [Google Scholar]
- Yizhak, K.; Chaneton, B.; Gottlieb, E.; Ruppin, E. Modeling cancer metabolism on a genome scale. Mol. Syst. Biol. 2015, 11, 817. [Google Scholar] [CrossRef]
- Sivapatham, S.; Selvaraj, L. Currently available molecular analyses for personalized tumor therapy (Review). Biomed. Rep. 2022, 17, 95. [Google Scholar] [CrossRef]
- El-Hachem, M.; McCue, S.W.; Simpson, M.J. Travelling wave analysis of cellular invasion into surrounding tissues. Phys. D Nonlinear Phenom. 2021, 428, 133026. [Google Scholar] [CrossRef]
- Pozzi, G.; Grammatica, B.; Chaabane, L.; Catucci, M.; Mondino, A.; Zunino, P.; Ciarletta, P. T cell therapy against cancer: A predictive diffuse-interface mathematical model informed by pre-clinical studies. J. Theor. Biol. 2022, 547, 111172. [Google Scholar] [CrossRef] [PubMed]
- Topalian, S.L.; Taube, J.M.; Pardoll, D.M. Neoadjuvant checkpoint blockade for cancer immunotherapy. Science 2020, 367, eaax0182. [Google Scholar] [CrossRef]
- Huntington, N.D.; Cursons, J.; Rautela, J. The cancer-natural killer cell immunity cycle. Nat. Rev. Cancer 2020, 20, 437–454. [Google Scholar] [CrossRef] [PubMed]
- Sové, R.J.; Jafarnejad, M.; Zhao, C.; Wang, H.; Ma, H.; Popel, A.S. QSP-IO: A Quantitative Systems Pharmacology Toolbox for Mechanistic Multiscale Modeling for Immuno-Oncology Applications. CPT Pharmacomet. Syst. Pharmacol. 2020, 9, 484–497. [Google Scholar] [CrossRef]
- Lejeune, O.; Chaplain, M.A.J.; Akili, I.E. Oscillations and Bistability in the Dynamics of Cytotoxic Reactions Mediated by the Response of Immune Cells to Solid Tumours. Math. Comput. Model. Int. J. 2008, 47, 649–662. [Google Scholar] [CrossRef]
- D’Onofrio, A.; Gatti, F.; Cerrai, P.; Freschi, L. Delay-Induced Oscillatory Dynamics of Tumour-Immune System Interaction. Math. Comput. Model. Int. J. 2010, 51, 572–591. [Google Scholar] [CrossRef]
- Liu, D.; Ruan, S.; Zhu, D. Stable periodic oscillations in a two-stage cancer model of tumor and immune system interactions. Math. Biosci. Eng. 2012, 9, 347–368. [Google Scholar] [CrossRef] [PubMed]
- Bi, P.; Ruan, S.; Zhang, X. Periodic and chaotic oscillations in a tumor and immune system interaction model with three delays. Chaos 2014, 24, 023101. [Google Scholar] [CrossRef]
- Li, W.; Jin, W. Uncovering the underlying mechanism of cancer tumorigenesis and development under an immune microenvironment from global quantification of the landscape. J. R. Soc. Interface 2017, 14, 20170105. [Google Scholar] [CrossRef]
- Dritschel, H.; Waters, S.; Roller, A.; Byrne, H. A mathematical model of cytotoxic and helper T cell interactions in a tumour microenvironment. Lett. Biomath. 2018, 5, S36–S68. [Google Scholar] [CrossRef]
- Belgaid, Y.; Helal, M.; Venturino, E. Mathematical analysis of a B-cell chronic lymphocytic leukemia model with immune response. Appl. Math. Nonlinear Sci. 2019, 4, 551–558. [Google Scholar] [CrossRef]
- Benítez, L.; Barberis, L.; Condat, C. Modeling tumorspheres reveals cancer stem cell niche building and plasticity. Physica A Stat. Mech. Its Appl. 2019, 533, 121906. [Google Scholar] [CrossRef]
- Taloni, A.; Amar, M.B.; Zapperi, S.; Porta, C.L. The role of pressure in cancer growth. Eur. Phys. J. Plus 2015, 130, 224. [Google Scholar] [CrossRef]
- Tabdanov, E.D.; Puram, V.V.; Win, Z.; Alamgir, A.; Alford, P.W.; Provenzano, P.P. Bimodal sensing of guidance cues in mechanically distinct microenvironments. Nat. Commun. 2018, 9, 4891. [Google Scholar] [CrossRef]
- Büscher, T.; Ganai, N.; Gompper, G.; Elgeti, J. Tissue evolution: Mechanical interplay of adhesion, pressure, and heterogeneity. New J. Phys. 2019, 22, 033048. [Google Scholar] [CrossRef]
- Roy, M.; Finle, S.D. Metabolic reprogramming dynamics in tumor spheroids: Insights from a multicellular, multiscale model. PLoS Comput. Biol. 2019, 15, e1007053. [Google Scholar] [CrossRef] [PubMed]
- Alarcon, T.; Byrne, H.M.; Maini, P.K. Towards whole-organ modelling of tumour growth. Prog. Biophys. Mol. Biol. 2004, 85, 451–472. [Google Scholar] [CrossRef]
- Cappuccio, A.; Tieri, P.; Castiglione, F. Multiscale modelling in immunology: A review. Briefings Bioinform. 2015, 17, 408–418. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsov, M.; Clairambault, J.; Volpert, V. Improving cancer treatments via dynamical biophysical models. Phys. Life Rev. 2021, 39, 1–48. [Google Scholar] [CrossRef]
- Bellomo, N.; Bianca, C.; Delitala, M. Complexity analysis and mathematical tools towards the modelling of living systems. Phys. Life Rev. 2009, 6, 144–175. [Google Scholar] [CrossRef]
- Bianca, C. Onset of nonlinearity in thermostatted active particles models for complex systems. Nonlinear Anal. Real World Appl. 2012, 13, 2593–2608. [Google Scholar] [CrossRef]
- Bianca, C.; Dogbe, C. Kinetic models coupled with Gaussian thermostats: Macroscopic frameworks. Nonlinearity 2014, 27, 2771–2803. [Google Scholar] [CrossRef]
- Bianca, C.; Dogbe, C.; Lemarchand, A. The Role of Nonconservative Interactions in the Asymptotic Limit of Thermostatted Kinetic Models. Acta Appl. Math. 2015, 139, 1–24. [Google Scholar] [CrossRef]
- Bianca, C.; Lemarchand, A. Density evolution by the low-field limit of kinetic frameworks with thermostat and mutations. Commun. Nonlinear Sci. Numer. Simul. 2015, 20, 14–23. [Google Scholar] [CrossRef]
- Bianca, C.; Lemarchand, A. Miming the cancer-immune system competition by kinetic Monte Carlo simulations. J. Chem. Phys. 2016, 145, 154108. [Google Scholar] [CrossRef]
- Masurel, L.; Bianca, C.; Lemarchand, A. On the learning control effects in the cancer-immune system competition. Phys. A Stat. Mech. Its Appl. 2018, 506, 462–475. [Google Scholar] [CrossRef]
- Masurel, L.; Bianca, C.; Lemarchand, A. Space-velocity thermostatted kinetic theory model of tumor growth. Math. Biosci. Eng. 2021, 18, 5525–5551. [Google Scholar] [CrossRef]
- Bird, G.A. Molecular Gas Dynamics and Direct Simulation of Gas Flows; Clarendon: Oxford, UK, 1994. [Google Scholar]
- Alexander, F.J.; Garcia, A.L. The Direct Simulation Monte Carlo Method. Comput. Phys. 1997, 11, 588–593. [Google Scholar] [CrossRef]
- Lemarchand, A.; Nowakowski, B. Perturbation of particle velocity distribution in a bistable chemical system. Phys. A Stat. Mech. Its Appl. 1999, 271, 87–101. [Google Scholar] [CrossRef]
- Nowakowski, B.; Lemarchand, A. Thermal explosion near bifurcation: Stochastic features of ignition. Phys. A Stat. Mech. Its Appl. 2002, 311, 80–96. [Google Scholar] [CrossRef]
- Lemarchand, A.; Nowakowski, B. Enhanced sensitivity of a thermochemical system to microscopic perturbations. Phys. A Stat. Mech. Its Appl. 2004, 331, 409–421. [Google Scholar] [CrossRef]
- Gillespie, D.T. Exact stochastic simulation of coupled chemical reactions. J. Phys. Chem. 1977, 81, 2340–2361. [Google Scholar] [CrossRef]
- van der Bruggen, P.; Traversari, C.; Chomez, P.; Lurquin, C.; Plaen, E.D.; den Eynde, B.V.; Knuth, A.; Boon, T. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science 1992, 254, 1643–1647. [Google Scholar] [CrossRef] [PubMed]
- Guermonprez, P.; Valladeau, J.; Zitvogel, L.; Théry, C.; Amigorena, S. Antigen presentation and T cell stimulation by dendritic cells. Annu. Rev. Immunol. 2002, 20, 621–667. [Google Scholar] [CrossRef] [PubMed]
- Cuccu, A.; Francescangeli, F.; Angelis, M.L.D.; Bruselles, A.; Giuliani, A.; Zeuner, A. Analysis of Dormancy-Associated Transcriptional Networks Reveals a Shared Quiescence Signature in Lung and Colorectal Cancer. Int. J. Mol. Sci. 2022, 23, 9869. [Google Scholar] [CrossRef] [PubMed]
- Huang, S. Reconciling Non-Genetic Plasticity with Somatic Evolution in Cancer. Trends Cancer 2021, 7, 309–322. [Google Scholar] [CrossRef] [PubMed]
- Francescangeli, F.; Contavalli, P.; Angelis, M.D.; Careccia, S.; Signore, M.; Haas, T.L.; Salaris, F.; Baiocchi, M.; Boe, A.; Giuliani, A.; et al. A pre-existing population of ZEB2+ quiescent cells with stemness and mesenchymal features dictate chemoresistance in colorectal cancer. J. Exp. Clin. Cancer Res. 2022, 39, 2. [Google Scholar] [CrossRef]
- Weiss, F.; Lauffenburger, D.; Friedl, P. Towards targeting of shared mechanisms of cancer metastasis and therapy resistance. Nat. Rev. Cancer 2022, 22, 157–173. [Google Scholar] [CrossRef]
- Nicolis, G.; Prigogine, I. Self-Organization in Nonequilibrium Systems; Wiley: New York, NY, USA, 1977. [Google Scholar]
- Lemarchand, A.; Jullien, L. Tuning a reaction using two-site catalysis and far-from-equilibrium conditions. Phys. Chem. Chem. Phys. 2004, 6, 398–405. [Google Scholar] [CrossRef]
- Murray, J.D. Mathematical Biology I: An Introduction; Springer: New York, NY, USA, 2011. [Google Scholar]
- Morgado, G.; Nowakowski, B.; Lemarchand, A. Fisher-Kolmogorov-Petrovskii-Piskunov wave front as a sensor of perturbed diffusion in concentrated systems. Phys. Rev. E 2019, 99, 022205. [Google Scholar] [CrossRef]
- Morgado, G.; Nowakowski, B.; Lemarchand, A. Stochastic approach to Fisher and Kolmogorov, Petrovskii, and Piskunov wave fronts for species with different diffusivities in dilute and concentrated solutions. Phys. A Stat. Mech. Appl. 2020, 558, 124954. [Google Scholar] [CrossRef]
- Bellouquid, A.; Delitala, M. Mathematical Modeling of Complex Biological Systems—A Kinetic Theory Approach; Birkhäuser: Boston, MA, USA, 2006. [Google Scholar] [CrossRef]
- Lillo, S.D.; Delitala, M.; Salvatori, M.C. Modelling epidemics and virus mutations by methods of the mathematical kinetic theory for active particles. In Mathematical Models and Methods in Applied Sciences; Bellomo, N., Brezzi, F., Eds.; World Scientific: Singapore, 2009; Volume 19, pp. 1405–1425. [Google Scholar] [CrossRef]
- Tsukahara, T.; Kawaguchi, S.; Torigoe, T.; Asanuma, H.; Nakazawa, E.; Shimozawa, K.; Nabeta, Y.; Kimura, S.; Kaya, M.; Nagoya, S.; et al. Prognostic significance of HLA class I expression in osteosarcoma defined by anti-pan HLA class I monoclonal antibody, EMR8-5. Cancer Sci. 2006, 97, 1374–1380. [Google Scholar] [CrossRef] [PubMed]
- McQuarrie, D.A. Statistical Mechanics; Harper and Row: New York, NY, USA, 1975. [Google Scholar]
- Gandhi, S.A.; Kampp, J. Skin Cancer Epidemiology, Detection, and Management. Med. Clin. N. Am. 2015, 99, 1323–1335. [Google Scholar] [CrossRef] [PubMed]
- Lucas, Z.; Mukherjee, A.; Chia§, S.; Veytsman, I. Metastasis of Laryngeal Squamous Cell Carcinoma to Bilateral Thigh Muscles. Case Rep. Oncol. Med. 2014, 2014, 424568. [Google Scholar] [CrossRef] [PubMed]
- Whiteley, A.E.; Price, T.T.; Cantelli, G.; Sipkins, D.A. Leukaemia: A model metastatic disease. Nat. Rev. Cancer 2021, 21, 461–475. [Google Scholar] [CrossRef]
- Turnquist, C.; Ryan, B.M.; Horikawa, I.; Harris, B.T.; Harris, C.C. Cytokine Storms in Cancer and COVID-19. Cancer Cell 2020, 38, 598–601. [Google Scholar] [CrossRef] [PubMed]
- Goldbeter, A. Biochemical Oscillations and Cellular Rhythms; Cambridge University Press: New York, NY, USA, 2010. [Google Scholar] [CrossRef]
- Westera, L.; Drylewicz, J.; Den Braber, I.; Mugwagwa, T.; Van Der Maas, I.; Kwast, L.; Volman, T.; Van De Weg-Schrijver, E.H.; Bartha, I.; Spierenburg, G.; et al. Closing the Gap between T-Cell Life Span Estimates from Stable Isotope-Labeling Studies in Mice and Humans. Blood 2013, 122, 2205–2212. [Google Scholar] [CrossRef]
- Macallan, D.C.; Borghans, J.A.M.; Asquith, B. Human T Cell Memory: A Dynamic View. Vaccines 2017, 5, 5. [Google Scholar] [CrossRef]
- Wennberg, B.; Wondmagegne, Y. The Kac Equation with a Thermostatted Force Field. J. Stat. Phys. 2006, 124, 859–880. [Google Scholar] [CrossRef]
- Wennberg, B.; Wondmagegne, Y. Stationary States for the Kac Equation with a Gaussian Thermostat. Nonlinearity 2004, 17, 633–648. [Google Scholar] [CrossRef]
- Tanimoto, J. Sociophysics Approach to Epidemics; Springer: Singapore, 2021. [Google Scholar]
- Radha, G.; Lopus, M. The spontaneous remission of cancer: Current insights and therapeutic significance. Transl. Oncol. 2021, 14, 101166. [Google Scholar] [CrossRef] [PubMed]
- Sawamura, M.; Yamaguchi, S.; Murakami, H.; Kitahara, T.; Itoh, K.; Maehara, T.; Kawada, E.; Matsushima, T.; Tamura, J.; Naruse, T.; et al. Cyclic Haemopoiesis at 7- or 8-Day Intervals. Br. J. Haematol. 1994, 88, 215–218. [Google Scholar] [CrossRef]
- Coventry, B.J.; Ashdown, M.L.; Quinn, M.A.; Markovic, S.N.; Yatomi-Clarke, S.L.; Robinson, A.P. CRP Identifies Homeostatic Immune Oscillations in Cancer Patients: A Potential Treatment Targeting Tool? J. Transl. Med. 2009, 7, 102. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, F.A.; Rivera, N.I. The Role of C-Reactive Protein as a Prognostic Indicator in Advanced Cancer. Curr. Oncol. Rep. 2002, 4, 250–255. [Google Scholar] [CrossRef]
- Fahimi, M.; Nouri, K.; Torkzadeh, L. Chaos in a stochastic cancer model. Phys. A Stat. Mech. Appl. 2020, 545, 123810. [Google Scholar] [CrossRef]
- Gillespie, D.T. The chemical Langevin equation. J. Chem. Phys. 2000, 113, 297–306. [Google Scholar] [CrossRef]
- Pikovsky, A.S.; Kurths, J. Coherence Resonance in a Noise-Driven Excitable System. Phys. Rev. Lett. 1997, 78, 775–778. [Google Scholar] [CrossRef]
- Lemarchand, A.; Nowakowski, B. Internal fluctuations in a thermochemical system: Excitability, oscillations and coherence resonances. J. Phys. Condens. Matter 2007, 19, 065130. [Google Scholar] [CrossRef]
- Kolbus, A.; Lemarchand, A.; Kawczynski, A.L.; Nowakowski, B. Coherence resonances in an excitable thermochemical system with multiple stationary states. Phys. Chem. Chem. Phys. 2010, 12, 13224–13231. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morgado, G.; Lemarchand, A.; Bianca, C. From Cell–Cell Interaction to Stochastic and Deterministic Descriptions of a Cancer–Immune System Competition Model. Mathematics 2023, 11, 2188. https://doi.org/10.3390/math11092188
Morgado G, Lemarchand A, Bianca C. From Cell–Cell Interaction to Stochastic and Deterministic Descriptions of a Cancer–Immune System Competition Model. Mathematics. 2023; 11(9):2188. https://doi.org/10.3390/math11092188
Chicago/Turabian StyleMorgado, Gabriel, Annie Lemarchand, and Carlo Bianca. 2023. "From Cell–Cell Interaction to Stochastic and Deterministic Descriptions of a Cancer–Immune System Competition Model" Mathematics 11, no. 9: 2188. https://doi.org/10.3390/math11092188
APA StyleMorgado, G., Lemarchand, A., & Bianca, C. (2023). From Cell–Cell Interaction to Stochastic and Deterministic Descriptions of a Cancer–Immune System Competition Model. Mathematics, 11(9), 2188. https://doi.org/10.3390/math11092188






