Computational Analysis of Hemodynamic Indices Based on Personalized Identification of Aortic Pulse Wave Velocity by a Neural Network
Abstract
1. Introduction
2. Materials and Methods
2.1. Coronary Circulation Model
2.2. Hemodynamic Indices Calculation
2.3. Datasets
2.3.1. Synthetic Database
2.3.2. PWV Dataset
2.3.3. FFR Dataset
2.4. PWV Estimation with Neural Network and Other Machine Learning Methods
2.4.1. Error Estimation
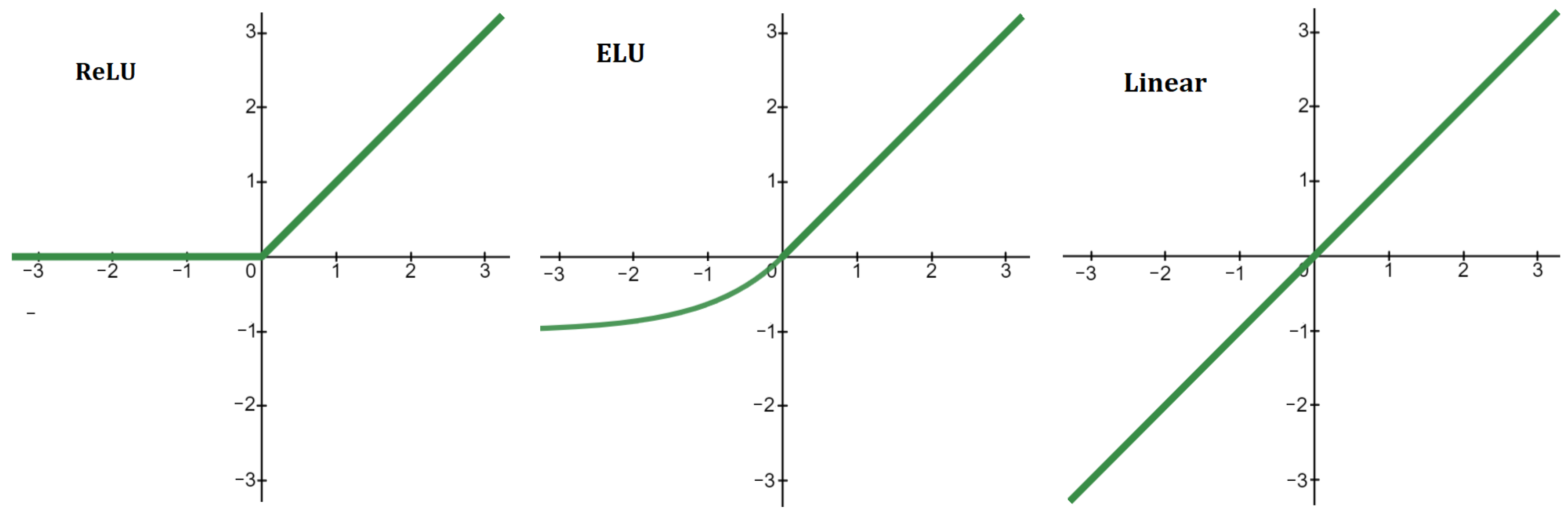
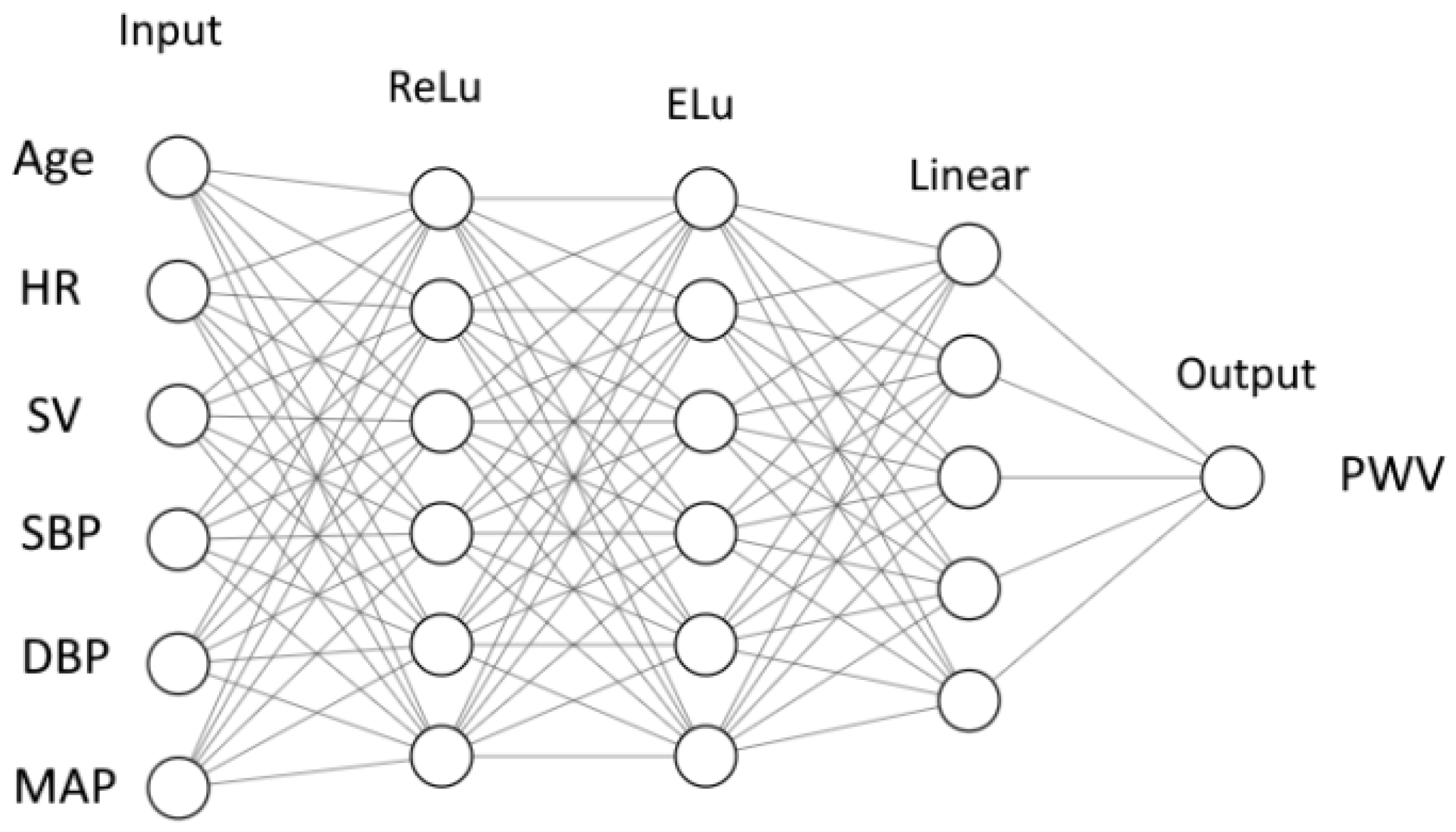
2.4.2. Neural Network
2.4.3. Other Methods
3. Results
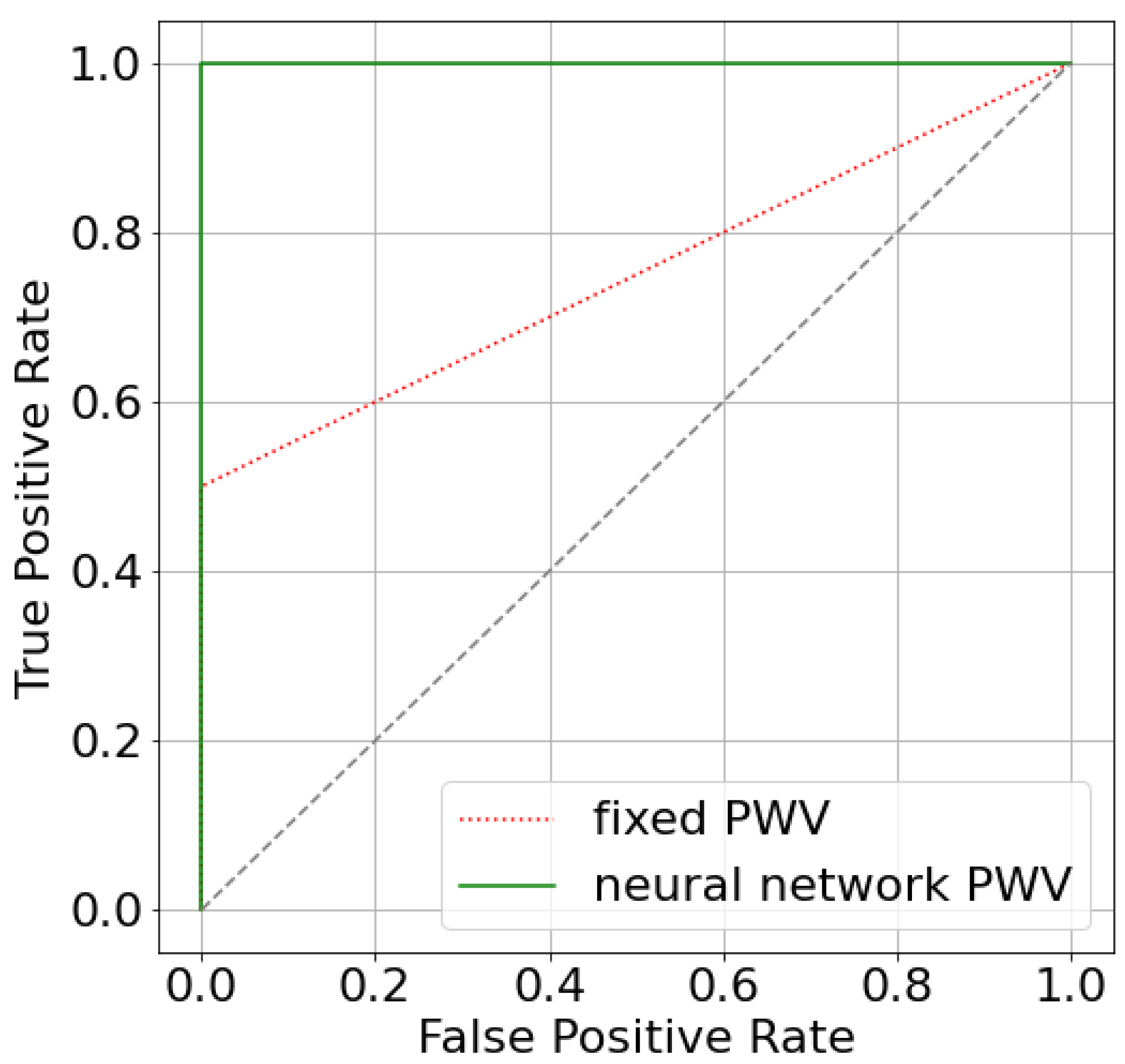
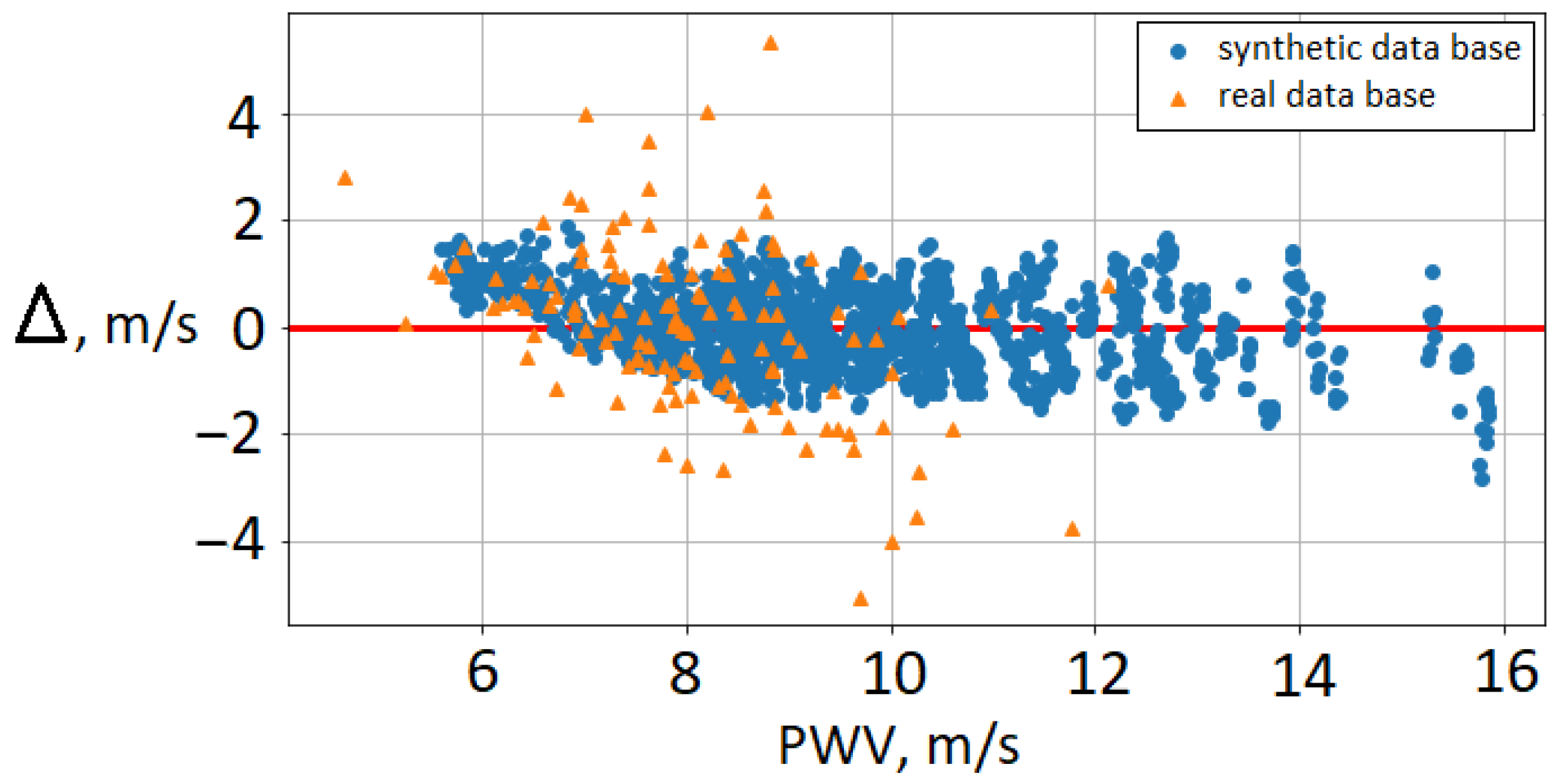
3.1. AoPWV Estimation
- 1.
- Estimating carotid-femoral PWV from the radial pressure wave using machine learning algorithms [48]. The study population was the Twins UK cohort, containing 3082 subjects aged from 18 to 110 years. The authors used Gaussian process regression and a recurrent neural network to estimate carotid-femoral PWV from the entire radial pressure wave. They report errors of 17–19%.
- 2.
- Estimating carotid-femoral PWV from one carotid waveform measured by tonometry and few clinical variables (age, blood pressure, heart rate, etc.) [48]. The study population included 5050 subjects in the age range of 20 to 69. The authors use the newly developed Intrinsic Frequency algorithm together with neural networks and bootstrap averaging. This algorithm uses an uncalibrated noisy waveform with few additional parameters. The reported error is 14%.
- 3.
- Estimating aortic PWV with ridge regression and a deep neural network from two sets of inputs: a basic set of predictors (age, sex, height, weight, heart rate, systolic and diastolic blood pressure) and an expanded set of predictors (HbA1c, total cholesterol, use of antihypertensive, antidiabetic or cholesterol-lowering medication and smoking status in addition to basic set) [49]. A total of 2254 participants from the Netherlands Epidemiology of Obesity study were included (age 45–65 years). The reported error is 18–22%.
| Description | Error |
|---|---|
| Brachial–ankle PWV with a neural network trained on synthetic data (Table 3) | 16% |
| Carotid–femoral PWV with machine learning using peripheral pulse waves [48] | 17–19% |
| Carotid–femoral PWV with a neural network using carotid waveform [50] | 14% |
| Aortic PWV with a neural network [49] | 18–22% |
| The difference between two occasionally different measurements of brachial–ankle PWV by one observer [42] | 10% |
| Repeatability of carotid–femoral PWV measurements [41] | 3.4–9.5% |
3.2. FFR Estimation with Predicted AoPWV
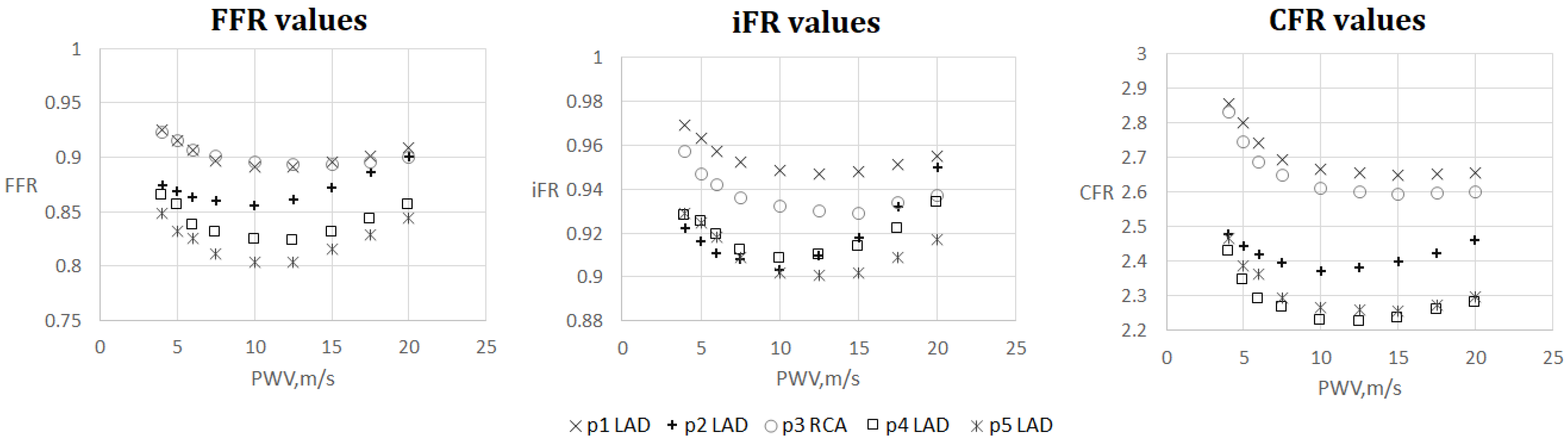
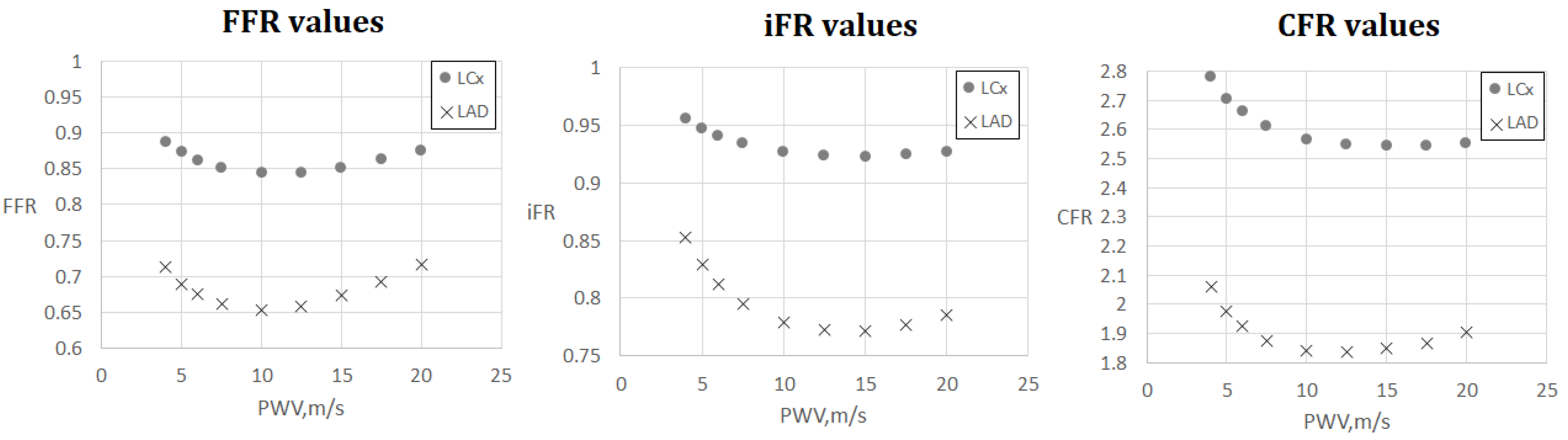
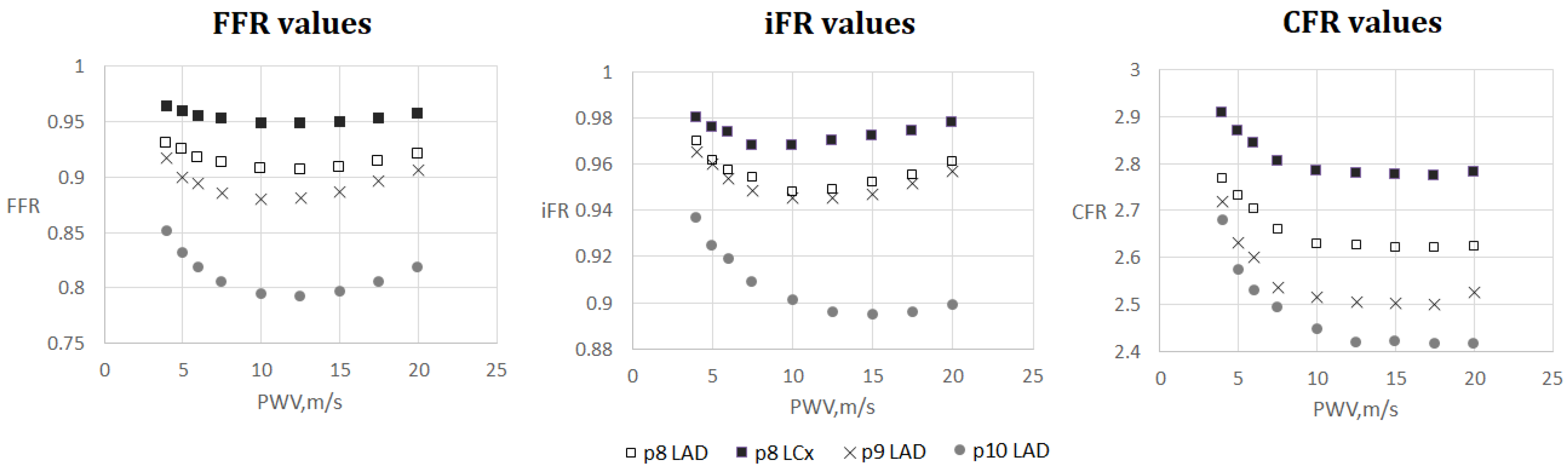
3.3. FFR, iFR and CFR Sensitivity to AoPWV
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| PWV | Pulse wave velocity |
| RMSE | Root mean square error |
| FFR | Fractional flow reserve |
| AoPWV | Aortic pulse wave velocity |
| CFR | Coronary flow reserve |
| iFR | Instantaneous wave-free ratio |
| CAD | Coronary artery disease |
| LCA | Left coronary artery |
| RCA | Right coronary artery |
| SBP | Systolic blood pressure |
| DBP | Diastolic blood pressure |
| MBP | Mean blood pressure |
| ECG | Electrocardiogram |
| BMI | Body mass index |
| CoPWV | Coronary vessels pulse wave velocity |
| LAD | Left anterior descending artery |
| LADp | Proximal part of the left anterior descending artery |
| LADd | Distal part of the left anterior descending artery |
| DA | Diagonal artery |
| LCX | Circumflex branch of left coronary artery |
Appendix A
| Number of Layers | RMSE, m/s | Percentage Error |
|---|---|---|
| 2 layers | 1.55 ± 0.41 | 19% ± 5% |
| 3 layers | 1.31 ± 0.19 | 16% ± 2% |
| 4 layers | 1.32 ± 0.22 | 16% ± 3% |
| 5 layers | 1.37 ± 0.19 | 17% ± 2% |
References
- Götberg, M.; Christiansen, E.H.; Gudmundsdottir, I.J.; Sandhall, L.; Danielewicz, M.; Jakobsen, L.; Olsson, S.E.; Öhagen, P.; Olsson, H.; Omerovic, E.; et al. Instantaneous wave-free ratio versus fractional flow reserve to guide PCI. N. Engl. J. Med. 2019, 376, 1813–1823. [Google Scholar] [CrossRef] [PubMed]
- Gould, K.L.; Kirkeeide, R.L.; Buchi, M. Coronary flow reserve as a physiologic measure of stenosis severity. J. Am. Coll. Cardiol. 1990, 15, 459–474. [Google Scholar] [CrossRef] [PubMed]
- Carson, J.M.; Pant, S.; Roobottom, C.; Alcock, R.; Blanco, P.J.; Carlos Bulant, C.A.; Vassilevski, Y.; Simakov, S.; Gamilov, T.; Pryamonosov, R.; et al. Non-invasive coronary CT angiography-derived fractional flow reserve: A benchmark study comparing the diagnostic performance of four different computational methodologies. Int. J. Numer. Methods Biomed. Eng. 2019, 35, e3235. [Google Scholar] [CrossRef] [PubMed]
- Carson, J.M.; Roobottom, C.; Alcock, R.; Nithiarasu, P. Computational instantaneous wave-free ratio (IFR) for patient-specific coronary artery stenoses using 1D network models. Int. J. Numer. Methods Biomed. Eng. 2019, 35, e3255. [Google Scholar] [CrossRef]
- Simakov, S.; Gamilov, T.; Liang, F.; Kopylov, P. Computational analysis of haemodynamic indices in synthetic atherosclerotic coronary netwroks. Mathematics 2021, 9, 2221. [Google Scholar] [CrossRef]
- Gognieva, D.; Mitina, Y.; Gamilov, T.; Pryamonosov, R.; Vasilevskii, Y.; Simakov, S.; Liang, F.; Ternovoy, S.; Serova, N.; Tebenkova, E.; et al. Noninvasive assessment of the fractional flow reserve with the CT FFRc 1D method: Final results of a pilot study. Glob. Heart 2020, 16, 837. [Google Scholar] [CrossRef]
- Zheng, D.; Weiwei, C.; Hao, G.; Xilan, Y.; Xiaoyu, L.; Nicholas, A.H. A One-Dimensional Hemodynamic Model of the Coronary Arterial Tree. Front. Physiol. 2019, 10, 853. [Google Scholar]
- Mynard, J.P.; Penny, D.J.; Smolich, J.J. Scalability and in vivo validation of a multiscale numerical model of the left coronary circulation. Am. J. Physiol. Heart Circ. 2014, 306, H517–H528. [Google Scholar] [CrossRef]
- Kamangar, S.; Badruddin, I.A.; Govindaraju, K.; Nik-Ghazali, N.; Badarudin, A.; Viswanathan, G.N.; Ahmed, N.; Khan, T. Patient-specific 3D hemodynamics modelling of left coronary artery under hyperemic conditions. Med. Biol. Eng. Comput. 2017, 55, 1451–1461. [Google Scholar] [CrossRef]
- Lu, M.T.; Ferencik, M.; Roberts, R.S.; Lee, K.L.; Ivanov, A.; Adami, E.; Mark, D.B.; Jaffer, F.A.; Leipsic, J.A.; Douglas, P.S.; et al. Noninvasive FFR Derived From Coronary CT Angiography: Management and Outcomes in the PROMISE Trial. JACC Cardiovasc. Imaging 2017, 10, 1350–1358. [Google Scholar] [CrossRef]
- Charlton, P.H.; Mariscal Harana, J.; Vennin, S.; Li, Y.; Chowienczyk, P.; Alastruey, J. Modeling arterial pulse waves in healthy aging: A database for in silico evaluation of hemodynamics and pulse wave indexes. Am. J.-Physiol.-Heart Circ. Physiol. 2019, 317, H1062–H1085. [Google Scholar] [CrossRef] [PubMed]
- Charlton, P.H. Pulse Wave Database. Available online: https://peterhcharlton.github.io/pwdb/pwdb.html (accessed on 23 February 2023).
- Reavette, R.M.; Sherwin, S.J.; Tang, M.; Weinberg, P.D. Comparison of arterial wave intensity analysis by pressure-velocity and diameter-velocity methods in a virtual population of adult subjects. Proc. Inst. Mech. Eng. H 2020, 234, 1260–1276. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.; Parr, J.; Nithiarasu, P.; Pant, S. A physiologically realistic virtual patient database for the study of arterial haemodynamics. Int. J. Numer. Method Biomed. Eng. 2021, 37, e3497. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Jin, W.; Liang, F.; Alastruey, J. Machine Learning–Based Pulse Wave Analysis for Early Detection of Abdominal Aortic Aneurysms Using In Silico Pulse Waves. Symmetry 2021, 13, 804. [Google Scholar] [CrossRef]
- Carson, J.M.; Chakshu, N.K.; Sazonov, I.; Nithiarasu, P. Artificial intelligence approaches to predict coronary stenosis severity using non-invasive fractional flow reserve. Proc. Inst. Mech. Eng. H 2020, 234, 1337–1350. [Google Scholar] [CrossRef]
- Fossan, F.E.; Müller, L.O.; Sturdy, J.; Barten, A.T.; Jørgensen, A.; Wiseth, R.; Hellevik, L.R. Machine learning augmented reduced-order models for FFR-prediction. Comput. Methods Appl. Mech. Eng. 2021, 384, 113892. [Google Scholar] [CrossRef]
- Danilov, A.; Ivanov, Yu.; Pryamonosov, R.; Vassilevski, Yu. Methods of graph network reconstruction in personalized medicine. Int. J. Numer. Methods Biomed. Eng. 2016, 32, e02754. [Google Scholar] [CrossRef]
- Vassilevski, Y.V.; Salamatova, V.Y.; Simakov, S.S. On the elasticity of blood vessels in one-dimensional problems of haemodynamics. Comput. Math. Math. Phys. 2015, 55, 1567–1578. [Google Scholar] [CrossRef]
- Simakov, S.; Gamilov, T.; Liang, F.; Gognieva, D.G.; Gappoeva, M.K.; Kopylov, P.Y. Numerical evaluation of the effectiveness of coronary revascularization. Russ. J. Num. Anal. Math. Mod. 2021, 36, 303–312. [Google Scholar] [CrossRef]
- Matthys, K.S.; Alastruey, J.; Peiro, J.; Khir, A.W.; Segers, P.; Verdonck, P.R.; Parker, K.H.; Sherwin, S.J. Pulse wave propagation in a model human arterial network: Assessmentof 1D numerical simulations against in-vitro measurements. J. Biomech. 2007, 40, 3476–3486. [Google Scholar] [CrossRef]
- Milan, A.; Zocaro, G.; Leone, D.; Tosello, F.; Buraioli, I.; Schiavone, D.; Veglio, F. Current assessment of pulse wave velocity: Comprehensive review of validation studies. J. Hypertens. 2019, 37, 1547–1557. [Google Scholar] [CrossRef] [PubMed]
- Pereira, T.; Correia, C.; Cardoso, J. Novel Methods for Pulse Wave Velocity Measurement. J. Med. Biol. Eng. 2015, 35, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Filip, C.; Cirstoveanu, C.; Bizubac, M.; Berghea, E.C.; Căpitănescu, A.; Bălgrădean, M.; Pavelescu, C.; Nicolescu, A.; Ionescu, M.D. Pulse Wave Velocity as a Marker of Vascular Dysfunction and Its Correlation with Cardiac Disease in Children with End-Stage Renal Disease (ESRD). Diagnostics 2021, 12, 71. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, R.; Shankar, A.; Amier, R.; Nijveldt, R.; Westenberg, J.J.M.; de Roos, A.; Lelieveldt, B.P.F.; van der Geest, R.J. Quantification of aortic pulse wave velocity from a population based cohort: A fully automatic method. J. Cardiovasc. Magn. Reson. 2019, 21, 27. [Google Scholar] [CrossRef]
- Van Hout, M.J.; Dekkers, I.A.; Westenberg, J.J.; Schalij, M.J.; Widya, R.L.; de Mutsert, R.; Rosendaal, F.R.; de Roos, A.; Jukema, J.W.; Scholte, A.J.; et al. Normal and reference values for cardiovascular magnetic resonance-based pulse wave velocity in the middle-aged general population. J. Cardiovasc. Magn. Reson. 2021, 23, 46. [Google Scholar] [CrossRef]
- Aguado-Sierra, J.; Parke, K.H.; Davies, J.E.; Francis, D.; Hughes, A.D.; Mayet, J. Arterial pulse wave velocity in coronary arteries. In Proceedings of the 2006 International Conference of the IEEE Engineering in Medicine and Biology Society, New York, NY, USA, 30 August–3 September 2006; pp. 867–870. [Google Scholar]
- Harbaoui, B.; Courand, P.-Y.; Cividjian, A.; Lantelme, P. Development of Coronary Pulse Wave Velocity: New Pathophysiological Insight Into Coronary Artery Disease. J. Am. Heart Assoc. 2017, 6, e004981. [Google Scholar] [CrossRef]
- Barret, K.; Brooks, H.; Boitano, S.; Barman, S. Ganong’s Review of Medical Physiology, 23rd ed.; The McGraw-Hill: New York, NY, USA, 2010. [Google Scholar]
- Gamilov, T.; Kopylov, P.; Serova, M.; Syunaev, R.; Pikunov, A.; Belova, S.; Liang, F.; Alastruey, J.; Simakov, S. Computational analysis of coronary blood flow: The role of asynchronous pacing and arrhythmias. Mathematics 2020, 8, 1205. [Google Scholar] [CrossRef]
- Magomedov, K.M.; Kholodov, A.S. Grid–Characteristic Numerical Methods; Nauka: Moscow, Russia, 2018. (In Russian) [Google Scholar]
- Ernest, W.L.; Menezes, L.J.; Torii, R. On outflow boundary conditions for CT-based computation of FFR: Examination using PET images. Med. Eng. Phys. 2020, 76, 79–87. [Google Scholar]
- Pijls, N.H.J.; de Bruyne, B.; Peels, K.; van der Voort, P.H.; Bonnier, H.J.R.M.; Bartunek, J.; Koolen, J.J. Measurement of Fractional Flow Reserve to Assess the Functional Severity of Coronary-Artery Stenoses. N. Engl. J. Med. 1996, 334, 1703–1708. [Google Scholar] [CrossRef]
- Nijjer, S.S.; Sen, S.; Petraco, R.; Sachdeva, R.; Cuculi, F.; Escaned, J.; Broyd, C.; Foin, N.; Hadjiloizou, N.; Foale, R.A.; et al. Improvement in coronary haemodynamics after percutaneous coronary intervention: Assessment using instantaneous wave-free ratio. Heart 2013, 99, 1740–1748. [Google Scholar] [CrossRef]
- Sen, S.; Escaned, J.; Malik, I.S.; Mikhail, G.W.; Foale, R.A.; Mila, R.; Tarkin, J.; Petraco, R.; Broyd, C.; Jabbou, R.; et al. Development and validation of a new adenosine–independent index of stenosis severity from coronary wave-intensity analysis: Results of the ADVISE (ADenosine Vasodilator Independent Stenosis Evaluation) study. J. Am. Coll. Cardiol. 2012, 59, 1392–1402. [Google Scholar] [CrossRef] [PubMed]
- Carson, J.; Pant, S.; Roobottom, C.; Alcock, R.; Blanco, P.J.; Bulant, C.A.; Vassilevski, Y.; Simakov, S.; Gamilov, T.; Pryamonosov, R.; et al. Supplementary Material. 2019. Available online: https://doi.org/10.6084/m9.figshare.8047742.v2 (accessed on 23 February 2023). [CrossRef]
- Laurent, S.; Boutouyrie, P.; Asmar, R.; Gautier, I.; Laloux, B.; Guize, L.; Ducimetiere, P.; Benetos, A. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension 2001, 37, 1236–1241. [Google Scholar] [CrossRef] [PubMed]
- Munakata, M. Brachial-Ankle Pulse Wave Velocity: Background, Method, and Clinical Evidence. Pulse 2016, 3, 195–204. [Google Scholar] [CrossRef]
- Collis, T.; Devereux, R.B.; Roman, M.J.; de Simone, G.; Yeh, J.; Howard, B.V.; Fabsitz, R.R.; Welty, T.K. Relations of stroke volume and cardiac output to body composition: The strong heart study. Circulation 2001, 103, 820–825. [Google Scholar] [CrossRef] [PubMed]
- Schmidhuber, J. Deep learning in neural networks: An overview. Neural Netw. 2015, 61, 85–117. [Google Scholar] [CrossRef]
- Grillo, A.; Parati, G.; Rovina, M.; Moretti, F.; Salvi, L.; Gao, L.; Baldi, C.; Sorropago, G.; Faini, A.; Millasseau, S.; et al. Short-Term Repeatability of Noninvasive Aortic Pulse Wave Velocity Assessment: Comparison Between Methods and Devices. Am. J. Hypertens. 2018, 31, 80–88. [Google Scholar] [CrossRef]
- Yamashina, A.; Tomiyama, H.; Takeda, K.; Tsuda, H.; Arai, T.; Hirose, K.; Koji, Y.; Hori, S.; Yamamoto, Y. Validity, reproducibility, and clinical significance of noninvasive brachial-ankle pulse wave velocity measurement. Hypertens. Res. 2002, 25, 359–364. [Google Scholar] [CrossRef]
- Kang, J.; Kim, H.L.; Lim, W.H.; Seo, J.B.; Zo, J.H.; Kim, M.A.; Kim, S.H. Relationship between brachial-ankle pulse wave velocity and invasively measured aortic pulse pressure. J. Clin. Hypertens. 2018, 20, 462–468. [Google Scholar] [CrossRef]
- Sugawara, J.; Hayashi, K.; Yokoi, T.; Cortez-Cooper, M.Y.; DeVan, A.E.; Anton, M.A.; Tanaka, H. Brachial–ankle pulse wave velocity: An index of central arterial stiffness? J. Hum. Hypertens 2005, 19, 401–406. [Google Scholar] [CrossRef]
- Mahesh, B. Machine Learning Algorithms—A Review. Int. J. Sci. Res. 2020, 9, 381–386. [Google Scholar]
- Dietterich, T. An Experimental Comparison of Three Methods for Constructing Ensembles of Decision Trees: Bagging, Boosting, and Randomization. Mach. Learn. 2000, 40, 139–157. [Google Scholar] [CrossRef]
- Rossi, A.; Bonapace, S.; Cicoira, M.; Conte, L.; Anselmi, A.; Vassanelli, C. Aortic stiffness: An old concept for new insights into the pathophysiology of functional mitral regurgitation. Heart Vessel. 2013, 28, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Chowienczyk, P.; Alastruey, J. Estimating pulse wave velocity from the radial pressure wave using machine learning algorithms. PLoS ONE 2021, 16, e0245026. [Google Scholar] [CrossRef] [PubMed]
- Van Hout, M.J.; Dekkers, I.A.; Lin, L.; Westenberg, J.J.; Schalij, M.J.; Jukema, J.W.; Widya, R.L.; Boone, S.C.; de Mutsert, R.; Rosendaal, F.R.; et al. Estimated pulse wave velocity (ePWV) as a potential gatekeeper for MRI-assessed PWV: A linear and deep neural network based approach in 2254 participants of the Netherlands Epidemiology of Obesity study. Int. J. Cardiovasc. Imaging 2022, 38, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Tavallali, P.; Razavi, M.; Pahlevan, N.M. Artificial Intelligence Estimation of Carotid-Femoral Pulse Wave Velocity using Carotid Waveform. Sci. Rep. 2018, 8, 1014. [Google Scholar] [CrossRef]
- Sutton-Tyrrell, K.; Mackey, R.H.; Holubkov, R.; Vaitkevicius, P.V.; Spurgeon, H.A.; Lakatta, E.G. Measurement variation of aortic pulse wave velocity in the elderly. Am. J. Hypertens. 2001, 14, 463–468. [Google Scholar] [CrossRef]
- Yong, A.S.C.; Javadzadegan, A.; Fearon, W.F.; Moshfegh, A.; Lau, J.K.; Nicholls, S.; Ng, M.K.C.; Kritharides, L. The relationship between coronary artery distensibility and fractional flow reserve. PLoS ONE 2017, 12, e0181824. [Google Scholar] [CrossRef]
- Cividjian, A.; Harbaoui, B.; Chambonnet, C.; Bonnet, J.M.; Paquet, C.; Courand, P.Y.; Lantelme, P. Comprehensive assessment of coronary pulse wave velocity in anesthetized pigs. Physiol. Rep. 2020, 8, e14424. [Google Scholar] [CrossRef]


| Database | PWV Dataset | Synthetic Database |
|---|---|---|
| Subjects | 102 | 4374 |
| Age, years | 58 ± 15 | 50 ± 17 |
| Heart rate, bpm | 68 ± 12 | 76 ± 9 |
| Stroke volume, ml | 54.6 ± 19.6 | 60.4 ± 12.3 |
| SBP (brachial), mmHg | 104.0 ± 17.1 | 119.1 ± 11.4 |
| DBP (brachial), mmHg | 86.7 ± 12.2 | 72.6 ± 7.2 |
| MBP (brachial), mmHg | 92.5 ± 12.9 | 93.8 ± 6.75 |
| AoPWV (brachial–ankle), m/s | 8.0 ± 1.4 | 9.4 ± 2.1 |
| Method | RMSE, m/s | Percentage Error |
|---|---|---|
| K-nearest neighbors | 1.96 ± 0.09 | 24% ± 1% |
| Decision tree | 1.88 ± 0.21 | 23% ± 3% |
| Random forest | 1.73 ± 0.14 | 22% ± 2% |
| Neural network | 1.31 ± 0.19 | 16% ± 2% |
| Patient | Vessel ID | d, mm | Degree | ||||
|---|---|---|---|---|---|---|---|
| 1 | LAD | 1.9 | 46% | 0.89 | 0.90 | 9.7 | 0.89 |
| 2 | LAD | 3.3 | 61% | 0.86 | 0.87 | 6.4 | 0.87 |
| 3 | RCA | 3.0 | 61% | 0.88 | 0.91 | 13.5 | 0.89 |
| 4 | LAD | 2.5 | 48% | 0.82 | 0.83 | 8.8 | 0.83 |
| 5 | LAD | 1.6 | 55% | 0.82 | 0.81 | 7.3 | 0.81 |
| 6 | LADp | 2.4 | 38% | 0.90 | 0.92 | 9.1 | 0.91 |
| 6 | LADd | 2.4 | 28% | 0.82 | 0.86 | 9.1 | 0.85 |
| 6 | DA | 1.9 | 58% | 0.81 | 0.84 | 9.1 | 0.83 |
| 7 | LAD | 1.5 | 57% | 0.75 | 0.66 | 7.4 | 0.66 |
| 7 | LCX | 1.9 | 32% | 0.84 | 0.85 | 7.4 | 0.85 |
| 8 | LAD | 2.3 | 56% | 0.88 | 0.91 | 7.8 | 0.91 |
| 8 | LCX | 3.1 | 58% | 0.89 | 0.95 | 7.8 | 0.95 |
| 9 | LAD | 2.0 | 48% | 0.83 | 0.89 | 6.6 | 0.89 |
| 10 | LAD | 1.9 | 63% | 0.72 | 0.81 | 14.2 | 0.79 |
| 2.2 ± 0.5 | 51% ± 11% | 0.84 ± 0.05 | 0.86± 0.07 | 9.1 ± 2.6 | 0.85 ± 0.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gamilov, T.; Liang, F.; Kopylov, P.; Kuznetsova, N.; Rogov, A.; Simakov, S. Computational Analysis of Hemodynamic Indices Based on Personalized Identification of Aortic Pulse Wave Velocity by a Neural Network. Mathematics 2023, 11, 1358. https://doi.org/10.3390/math11061358
Gamilov T, Liang F, Kopylov P, Kuznetsova N, Rogov A, Simakov S. Computational Analysis of Hemodynamic Indices Based on Personalized Identification of Aortic Pulse Wave Velocity by a Neural Network. Mathematics. 2023; 11(6):1358. https://doi.org/10.3390/math11061358
Chicago/Turabian StyleGamilov, Timur, Fuyou Liang, Philipp Kopylov, Natalia Kuznetsova, Artem Rogov, and Sergey Simakov. 2023. "Computational Analysis of Hemodynamic Indices Based on Personalized Identification of Aortic Pulse Wave Velocity by a Neural Network" Mathematics 11, no. 6: 1358. https://doi.org/10.3390/math11061358
APA StyleGamilov, T., Liang, F., Kopylov, P., Kuznetsova, N., Rogov, A., & Simakov, S. (2023). Computational Analysis of Hemodynamic Indices Based on Personalized Identification of Aortic Pulse Wave Velocity by a Neural Network. Mathematics, 11(6), 1358. https://doi.org/10.3390/math11061358






