Analysis of the In-Host Dynamics of Tuberculosis and SARS-CoV-2 Coinfection
Abstract
1. Introduction
2. TB and SARS-CoV-2 Coinfection Model with Immunity
3. Properties of Solutions
- (1)
- The healthy equilibrium always exists;
- (2)
- The Mtb mono-infection equilibrium with inactive CTLs exists if ;
- (3)
- The SARS-CoV-2 mono-infection equilibrium with inactive CTLs exists if ;
- (4)
- The Mtb mono-infection equilibrium with active CTLs exists if ;
- (5)
- The SARS-CoV-2 mono-infection equilibrium with active CTLs exists if ;
- (6)
- The Mtb and SARS-CoV-2 coinfection equilibrium with active CTLs exists if , , , , and .
- (1)
- The healthy equilibrium , which always exists.
- (2)
- The Mtb mono-infection equilibrium with inactive CTLs . The components are computed as follows:where . We see that , while , , and are positive if . Therefore, is defined when . The parameter is a threshold that determines the initiation of Mtb infection in the absence of CTL immunity.
- (3)
- The SARS-CoV-2 mono-infection equilibrium with inactive CTLs . Its components are formalized as follows:where . Notably, , while and if . Thus, exists if . The parameter is a threshold that sets the start of the SARS-CoV-2 infection, where the immune response is not active.
- (4)
- The Mtb mono-infection equilibrium with active CTLs . The components take the following form:where . We note that , , , and are always positive, while if . This implies that exists if . The parameter is a threshold that specifies the start of CTL immunity against Mtb-infected epithelial cells.
- (5)
- The SARS-CoV-2 mono-infection equilibrium with active CTLs . The components are defined aswhere . We observe that , while if . Hence, exists when . Here, is a threshold that determines the activation state of CTL immunity against SARS-CoV-2-infected epithelial cells.
- (6)
- The Mtb and SARS-CoV-2 coinfection equilibrium with active CTLs . The components are given by the following formulas:We see that , , and are positive if , while and are positive if , and if . In addition, we need the two conditions and . Hence, exists and the coinfection occurs when these conditions are hold.
4. Global Properties
5. Numerical Simulations
- Set I
- : .
- Set II
- : .
- Set III
- : .
- (1)
- We select , , and . This yields and . This result implies that the equilibrium is GAS (Figure 1), which is compatible with the result of Theorem 3. This point simulates the case of a healthy individual who does not have TB and SARS-CoV-2.
- (2)
- We choose , , and to obtain , , and . This is consistent with the result of Theorem 4 that the equilibrium (20,800, 9480, 7584, 0, 729,231, 0, 0) is GAS (Figure 2). In this case, the patient suffers from TB mono-infection while the CTL immunity is not active.
- (3)
- We consider , , and . This yields , , and . These values lead to the global asymptotic stability of the equilibrium (8571.43, 0, 0, 391,429, 0, , 0), which agrees with the result of Theorem 5 (Figure 3). In this situation, the patient has SARS-CoV-2 mono-infection, where the CTL immunity is not present.
- (4)
- We select , , and . This gives and . This implies that the equilibrium ( 117,514, 7062.15, 1000, 0, 96,153.8, 0, 4.64972) is GAS (Figure 4), which is in complete agreement with Theorem 6. At this point, the CTL immune response is activated to eliminate TB disease from the body. As a result, the concentrations of Mtb-infected cells and Mtb particles are decreased, while the concentration of healthy cells is increased.
- (5)
- We choose , , and . This generates the values and . In agreement with Theorem 7, the equilibrium (31,578.9, 0, 0, , 0, , 0.05368) is GAS (Figure 5). This point simulates the case of a COVID-19 patient with active CTL immunity that works to extract SARS-CoV-2 from the body.
- (6)
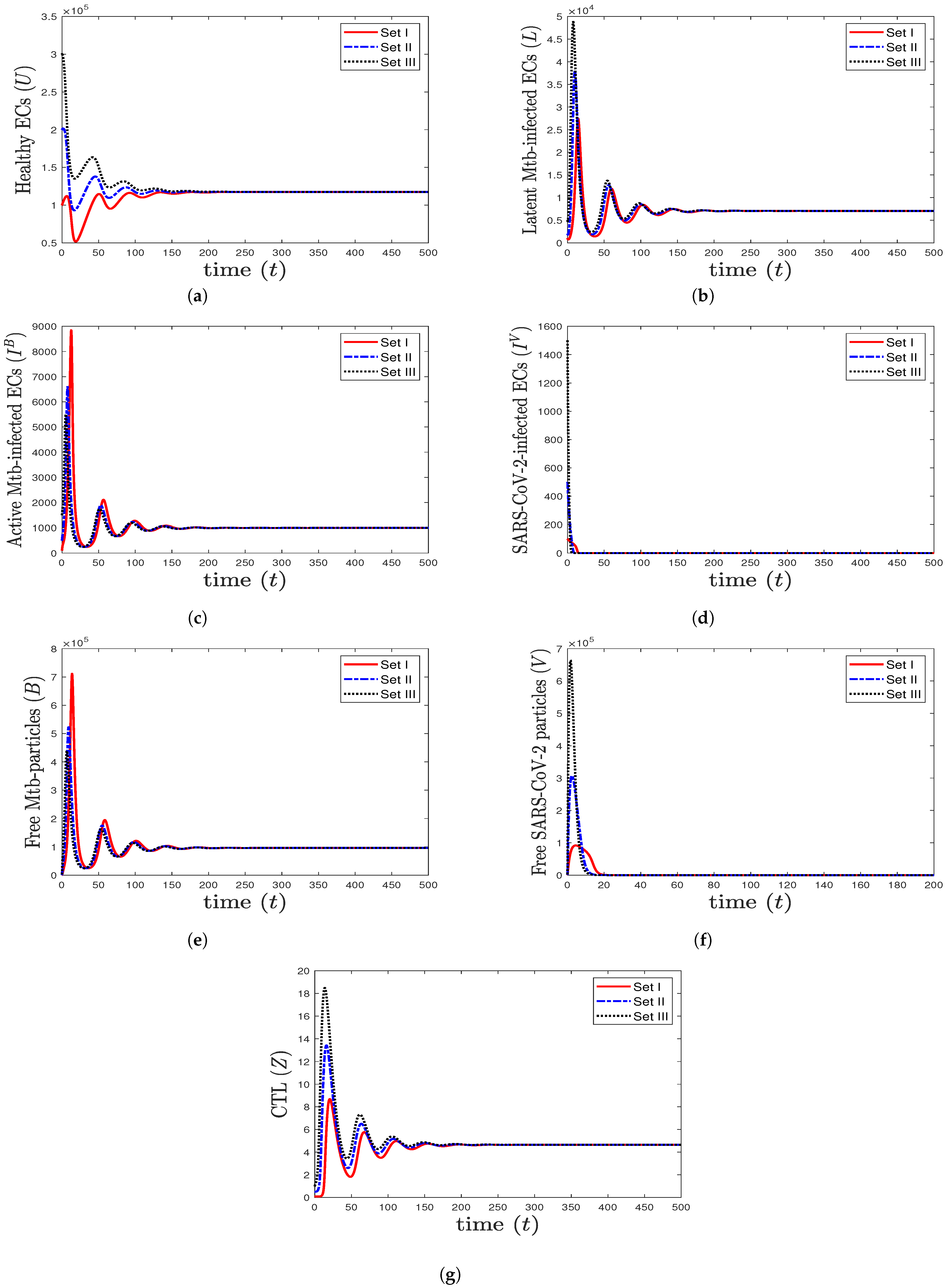
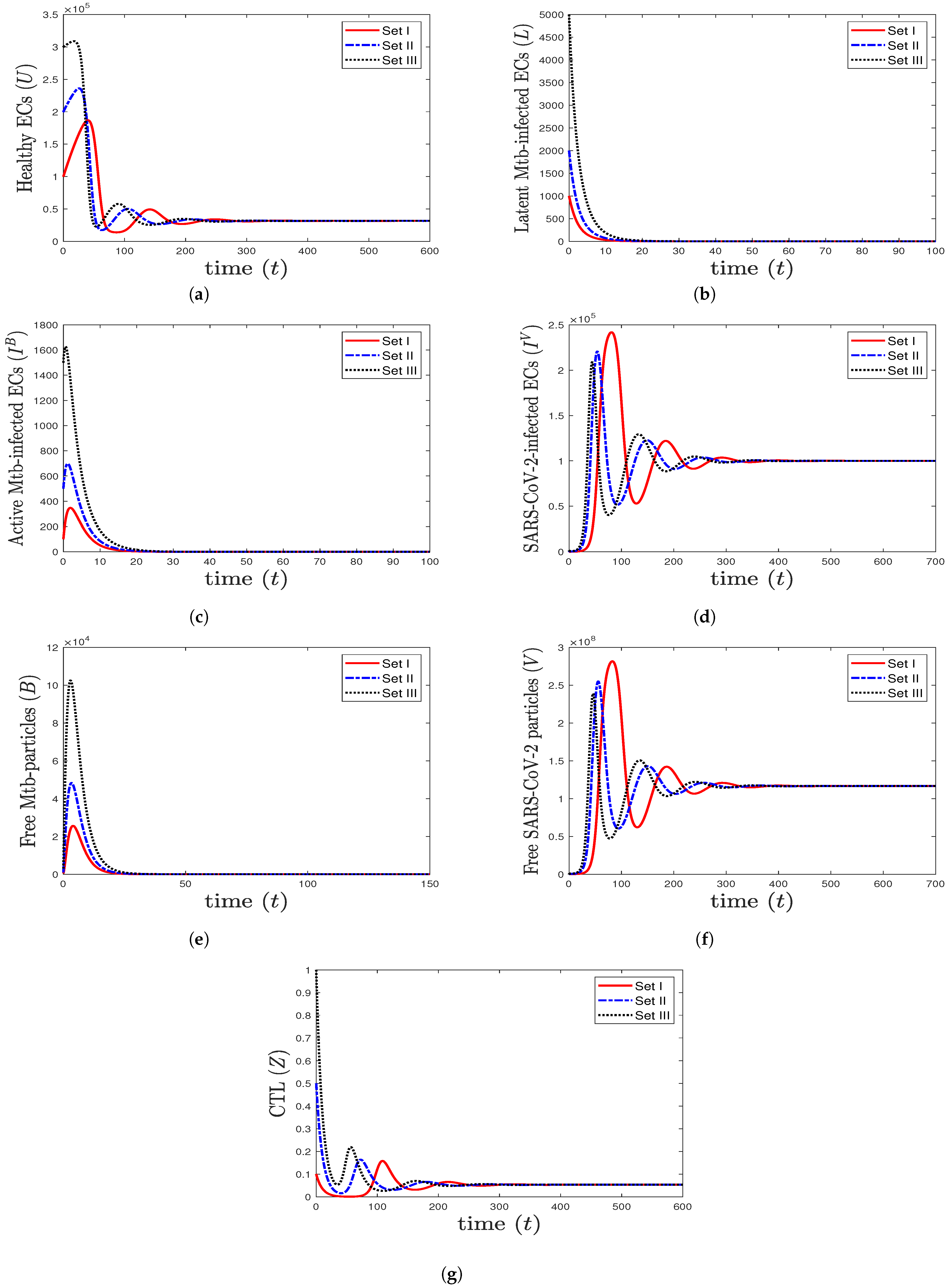
- We take , , and . These values give , , , and . This leads to the global asymptotic stability of the equilibrium ( 27,125.6, 5712.6, 4380.44, 45,619.6, 421,196, , 0.043), which is compatible with the result of Theorem 8 (Figure 6). In this case, the patient suffers from TB and SARS-CoV-2 coinfection in the presence of CTL immunity.
| Parameter | Description | Value | Reference |
|---|---|---|---|
| Recruitment rate of healthy ECs | [39] | ||
| Infection rate constant of ECs by Mtb | Varied | – | |
| Infection rate constant of ECs by SARS-CoV-2 | Varied | – | |
| a | Activation rate constant of latent Mtb-infected ECs | 0.4 | [15] |
| Killing rate constant of infected ECs by CTLs | 0.5 | [14] | |
| Number of Mtb particles released per Mtb-infected cell | 100 | [16] | |
| Production rate constant of SARS-CoV-2 by SARS-CoV-2 infected ECss | 700 | [39] | |
| Impact of lymphopenia on CTL immunity | – | ||
| Stimulation rate constant of CTLs | Varied | – | |
| Death rate constant of ECs | 0.01 | [39] | |
| Death rate constant of Mtb-infected ECs | 0.5 | [16] | |
| Death rate constant of SARS-CoV-2-infected ECs | 0.01 | [39] | |
| Death rate constant of Mtb | 0.52 | [18] | |
| Death rate constant of SARS-CoV-2 | 0.6 | [26] | |
| Death rate constant of CTLs | 0.1 | [39] |
The Effect of Lymphopenia on SARS-CoV-2
6. Discussion and Future Works
- (1)
- The healthy equilibrium always exists. It is GAS if and . This state simulates the situation of a healthy person who does not suffer from TB and SARS-CoV-2 infections.
- (2)
- The Mtb mono-infection equilibrium with inactive CTLs exists if , while it is GAS if and . The patient here has Mtb mono-infection with inactive CTL immunity.
- (3)
- The SARS-CoV-2 mono-infection equilibrium with inactive CTLs exists if . It is GAS if and . In this state, the patient has SARS-CoV-2 mono-infection with inactive CTL immunity.
- (4)
- The Mtb mono-infection equilibrium with active CTLs exists if , while it is GAS if . In this situation, the CTL immune response is activated to extract Mtb infection from the body.
- (5)
- The SARS-CoV-2 mono-infection equilibrium with active CTLs exists if , and it is GAS if . This imitates the situation of a patient with SARS-CoV-2 mono-infection and active CTL immunity.
- (6)
- The Mtb and SARS-CoV-2 coinfection equilibrium with active CTLs exists, and it is GAS if , , , , and . The patient here suffers from TB and SARS-CoV-2 coinfection and its consequences.
- (i)
- Estimating the parameters’ values of model (1) by fitting with real data,
- (ii)
- Evaluating the proposed model with real data,
- (iii)
- Adding one compartment that shows the role of the antibody immune response in eliminating Mtb or SARS-CoV-2 particles,
- (iv)
- Inserting the effect of some treatments on coinfection, and
- (v)
- Considering the effect of CTL immunity with different killing and stimulation rates.
7. Conclusions
- (1)
- The mono-infection state where the person has either TB or SARS-CoV-2 infection.
- (2)
- The coinfection state where the person has TB and SARS-CoV-2 infections.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Coronavirus Disease (COVID-19), Weekly Epidemiological Update (8 January 2023). World Health Organization (WHO). 2023. Available online: https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19—11-january-2023 (accessed on 12 January 2023).
- Song, W.M.; Zhao, J.Y.; Zhang, Q.Y.; Liu, S.Q.; Zhu, X.H.; An, Q.Q.; Xu, T.T.; Li, S.J.; Liu, J.Y.; Tao, N.N.; et al. COVID-19 and Tuberculosis coinfection: An overview of case reports/case series and meta-analysis. Front. Med. 2021, 8, 1–13. [Google Scholar] [CrossRef]
- Tuberculosis, Fact Sheets. World Health Organization (WHO). 2022. Available online: https://www.who.int/news-room/fact-sheets/detail/tuberculosis (accessed on 12 January 2023).
- Shah, T.; Shah, Z.; Yasmeen, N.; Baloch, Z.; Xia, X. Pathogenesis of SARS-CoV-2 and Mycobacterium tuberculosis coinfection. Front. Immunol. 2022, 13, 1–17. [Google Scholar] [CrossRef]
- Luke, E.; Swafford, K.; Shirazi, G.; Venketaraman, V. TB and COVID-19: An exploration of the characteristics and resulting complications of co-infection. Front. Biosci. Sch. 2022, 14, 1–11. [Google Scholar] [CrossRef]
- Gatechompol, S.; Avihingsanon, A.; Putcharoen, O.; Ruxrungtham, K.; Kuritzkes, D.R. COVID-19 and HIV infection co-pandemics and their impact: A review of the literature. AIDS Res. Ther. 2021, 18, 28. [Google Scholar] [CrossRef]
- Shariq, M.; Sheikh, J.; Quadir, N.; Sharma, N.; Hasnain, S.; Ehtesham, N. COVID-19 and tuberculosis: The double whammy of respiratory pathogens. Eur. Respir. Rev. 2022, 31, 1–8. [Google Scholar] [CrossRef]
- Tapela, K.; Olwal, C.O.; Quaye, O. Parallels in the pathogenesis of SARS-CoV-2 and M. tuberculosis: A synergistic or antagonistic alliance? Future Microbiol. 2020, 15, 1691–1695. [Google Scholar] [CrossRef]
- Petrone, L.; Petruccioli, E.; Vanini, V.; Cuzzi, G.; Gualano, G.; Vittozzi, P.; Nicastri, E.; Maffongelli, G.; Grifoni, A.; Sette, A.; et al. Coinfection of tuberculosis and COVID-19 limits the ability to in vitro respond to SARS-CoV-2. Int. J. Infect. Dis. 2021, 113, S82–S87. [Google Scholar] [CrossRef]
- Blower, S.M.; Mclean, A.R.; Porco, T.C.; Small, P.M.; Hopewell, P.C.; Sanchez, M.A.; Moss, A.R. The intrinsic transmission dynamics of tuberculosis epidemics. Nat. Med. 1995, 1, 815–821. [Google Scholar] [CrossRef]
- Castillo-Chavez, C.; Feng, Z. To treat or not to treat: The case of tuberculosis. J. Math. Biol. 1997, 35, 629–656. [Google Scholar] [CrossRef]
- Feng, Z.; Castillo-Chavez, C.; Capurro, A. A model for tuberculosis with exogenous reinfection. Theor. Popul. Biol. 2000, 57, 235–247. [Google Scholar] [CrossRef]
- Castillo-Chavez, C.; Song, B. Dynamical models of tuberculosis and their applications. Math. Biosci. Eng. 2004, 1, 361–404. [Google Scholar] [CrossRef]
- Du, Y.; Wu, J.; Heffernan, J. A simple in-host model for Mycobacterium tuberculosis that captures all infection outcomes. Math. Popul. Stud. 2017, 24, 37–63. [Google Scholar] [CrossRef]
- He, D.; Wang, Q.; Lo, W. Mathematical analysis of macrophage-bacteria interaction in tuberculosis infection. Discret. Contin. Dyn. Syst. Ser. B 2018, 23, 3387–3413. [Google Scholar] [CrossRef]
- Yao, M.; Zhang, Y.; Wang, W. Bifurcation analysis for an in-host Mycobacterium tuberculosis model. Discret. Contin. Dyn. Syst. Ser. B 2021, 26, 2299–2322. [Google Scholar] [CrossRef]
- Zhang, W. Analysis of an in-host tuberculosis model for disease control. Appl. Math. Lett. 2020, 99, 1–7. [Google Scholar] [CrossRef]
- Ibarguen-Mondragon, E.; Esteva, L.; Burbano-Rosero, E. Mathematical model for the growth of Mycobacterium tuberculosis in the granuloma. Math. Biosci. Eng. 2018, 15, 407–428. [Google Scholar]
- Liang, K. Mathematical model of infection kinetics and its analysis for COVID-19, SARS and MERS. Infect. Genet. Evol. 2020, 82, 104306. [Google Scholar] [CrossRef]
- Krishna, M.V. Mathematical modelling on diffusion and control of COVID-19. Infect. Dis. Model. 2020, 5, 588–597. [Google Scholar] [CrossRef]
- Ivorra, B.; Ferrandez, M.R.; Vela-Perez, M.; Ramos, A.M. Mathematical modeling of the spread of the coronavirus disease 2019 (COVID-19) taking into account the undetected infections. The case of China. Commun. Nonlinear Sci. Numer. Simul. 2020, 88, 105303. [Google Scholar] [CrossRef]
- Yang, C.; Wang, J. A mathematical model for the novel coronavirus epidemic in Wuhan, China. Math. Biosci. Eng. 2020, 17, 2708–2724. [Google Scholar] [CrossRef]
- Krishna, M.V.; Prakash, J. Mathematical modelling on phase based transmissibility of Coronavirus. Infect. Dis. Model. 2020, 5, 375–385. [Google Scholar] [CrossRef]
- Rajagopal, K.; Hasanzadeh, N.; Parastesh, F.; Hamarash, I.I.; Jafari, S.; Hussain, I. A fractional-order model for the novel coronavirus (COVID-19) outbreak. Nonlinear Dyn. 2020, 101, 711–718. [Google Scholar] [CrossRef]
- Chen, T.M.; Rui, J.; Wang, Q.P.; Zhao, Z.Y.; Cui, J.A.; Yin, L. A mathematical model for simulating the phase-based transmissibility of a novel coronavirus. Infect. Dis. Poverty 2020, 9, 1–8. [Google Scholar] [CrossRef]
- Almocera, A.S.; Quiroz, G.; Hernandez-Vargas, E.A. Stability analysis in COVID-19 within-host model with immune response. Commun. Nonlinear Sci. Numer. Simul. 2020, 95, 105584. [Google Scholar] [CrossRef]
- Hernandez-Vargas, E.A.; Velasco-Hernandez, J.X. In-host mathematical modeling of COVID-19 in humans. Annu. Rev. Control 2020, 50, 448–456. [Google Scholar] [CrossRef]
- Li, C.; Xu, J.; Liu, J.; Zhou, Y. The within-host viral kinetics of SARS-CoV-2. Math. Biosci. Eng. 2020, 17, 2853–2861. [Google Scholar] [CrossRef]
- Agha, A.D.A.; Elaiw, A.M. Global dynamics of SARS-CoV-2/malaria model with antibody immune response. Math. Biosci. Eng. 2022, 19, 8380–8410. [Google Scholar] [CrossRef]
- Pinky, L.; Dobrovolny, H.M. SARS-CoV-2 coinfections: Could influenza and the common cold be beneficial? J. Med. Virol. 2020, 92, 2623–2630. [Google Scholar] [CrossRef]
- Elaiw, A.M.; Agha, A.D.A.; Azoz, S.A.; Ramadan, E. Global analysis of within-host SARS-CoV-2/HIV coinfection model with latency. Eur. Phys. J. Plus 2022, 137, 1–22. [Google Scholar] [CrossRef]
- Ahmed, I.; Goufo, E.F.D.; Yusuf, A.; Kumam, P.; Chaipanya, P.; Nonlaopon, K. An epidemic prediction from analysis of a combined HIV-COVID-19 co-infection model via ABC-fractional operator. Alex. Eng. J. 2021, 60, 2979–2995. [Google Scholar] [CrossRef]
- Omame, A.; Abbas, M.; Abdel-Aty, A. Assessing the impact of SARS-CoV-2 infection on the dynamics of dengue and HIV via fractional derivative. Chaos Solitons Fractals 2022, 162, 1–22. [Google Scholar] [CrossRef]
- Mekonen, K.; Balcha, S.; Obsu, L.; Hassen, A. Mathematical modeling and analysis of TB and COVID-19 coinfection. J. Appl. Math. 2022, 2022, 1–20. [Google Scholar] [CrossRef]
- Bandekar, S.; Ghosh, M. A co-infection model on TB—COVID-19 with optimal control and sensitivity analysis. Math. Comput. Simul. 2022, 200, 1–31. [Google Scholar] [CrossRef]
- Marimuthu, Y.; Nagappa, B.; Sharma, N.; Basu, S.; Chopra, K. COVID-19 and tuberculosis: A mathematical model based forecasting in Delhi, India. Indian J. Tuberc. 2020, 67, 177–181. [Google Scholar] [CrossRef]
- Rwezaura, H.; Diagne, M.L.; Omame, A.; de Espindola, A.L.; Tchuenche, J.M. Mathematical modeling and optimal control of SARS-CoV-2 and tuberculosis co-infection: A case study of Indonesia. Model. Earth Syst. Environ. 2022, 8, 5493–5520. [Google Scholar] [CrossRef]
- Khalil, H.K. Nonlinear Systems; Prentice-Hall: Hoboken, NJ, USA, 1996. [Google Scholar]
- Sumi, T.; Harada, K. Immune response to SARS-CoV-2 in severe disease and long COVID-19. iScience 2022, 25, 1–18. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elaiw, A.M.; Agha, A.D.A. Analysis of the In-Host Dynamics of Tuberculosis and SARS-CoV-2 Coinfection. Mathematics 2023, 11, 1104. https://doi.org/10.3390/math11051104
Elaiw AM, Agha ADA. Analysis of the In-Host Dynamics of Tuberculosis and SARS-CoV-2 Coinfection. Mathematics. 2023; 11(5):1104. https://doi.org/10.3390/math11051104
Chicago/Turabian StyleElaiw, Ahmed M., and Afnan D. Al Agha. 2023. "Analysis of the In-Host Dynamics of Tuberculosis and SARS-CoV-2 Coinfection" Mathematics 11, no. 5: 1104. https://doi.org/10.3390/math11051104
APA StyleElaiw, A. M., & Agha, A. D. A. (2023). Analysis of the In-Host Dynamics of Tuberculosis and SARS-CoV-2 Coinfection. Mathematics, 11(5), 1104. https://doi.org/10.3390/math11051104





